Differential Efficacy of Olanzapine for Deficit and Nondeficit Negative Symptoms in Schizophrenia
Abstract
OBJECTIVE: Atypical antipsychotic medications have generally been found to be more effective than conventional antipsychotics in the treatment of negative symptoms. Whether the benefits derived from the atypical agents are the result of improvements in primary versus secondary negative symptoms is unclear. The authors examined the effects of olanzapine on primary and secondary negative symptoms for patients with severe negative symptoms who did or did not have the deficit syndrome.METHOD: Thirty-nine outpatients with schizophrenia and severe negative symptoms were assessed for the presence of the deficit syndrome and entered into a 12-week, open-label study of olanzapine. Positive and negative symptoms, extrapyramidal side effects, quality of life, and level of functioning of the patients were assessed at baseline and endpoint.RESULTS: All 39 patients completed the 12-week protocol; 13 of the patients had deficit negative symptoms, and 26 had nondeficit negative symptoms. Patients who had nondeficit negative symptoms demonstrated improvements in positive and negative symptoms, level of functioning, and extrapyramidal side effects over baseline. In contrast, patients meeting criteria for the deficit syndrome improved significantly over baseline only in extrapyramidal side effects.CONCLUSIONS: The results of this study suggest that olanzapine is efficacious for secondary negative symptoms in schizophrenia but fail to support the contention that olanzapine has a direct beneficial effect on primary negative symptoms.
The negative symptoms of schizophrenia are deficiencies in vital areas of human endeavor, including motivation, verbal and nonverbal communication, experiencing pleasure, interest in socialization, and expression of affect. Negative symptoms are consistently found to be predictors of community functioning, especially compared with positive symptoms (1, 2). The increased recognition of the central role of negative symptoms has led to the routine inclusion of instruments that measure negative symptoms, such as the Scale for the Assessment of Negative Symptoms (SANS) (3) and the Positive and Negative Syndrome Scale (4), in pharmacological and psychosocial treatment studies of schizophrenia.
Several studies comparing atypical antipsychotic medications with conventional antipsychotics have found the atypical agents to be superior for the treatment of negative symptoms (5). However, this conclusion is limited by concerns as to whether the atypical agents have a direct effect on primary negative symptoms (i.e., those negative symptoms intrinsic to schizophrenia) or an indirect effect mediated by an improvement in the putative causes of secondary negative symptoms, such as medication side effects (e.g., akinesia), inadequate social stimulation, unrecognized depression, and/or the intrusion of positive symptoms (5).
There have been a few attempts to address these concerns. One strategy has been the use of path analysis, a statistical technique based on multiple regression analysis in which relationships among variables can be direct (i.e., A to C) or indirect (i.e., A to B to C). This statistical approach has been used in studies demonstrating direct effects on negative symptoms with risperidone (6) and olanzapine (7). In these studies, the relationships included in the model were the drug’s direct effects on negative symptoms as well as indirect effects mediated by improvement in positive symptoms, depressive symptoms, and extrapyramidal side effects. Changes in ratings of negative symptoms during treatment that could be linked to (i.e., mediated by) improvements in positive symptoms, depression, and extrapyramidal side effects were subtracted from the total change score. The remaining proportion of variance unexplained by these factors was interpreted as a direct effect of the drug on primary negative symptoms.
The path analysis method, however, is limited by how completely the model identifies relevant variables and direct and indirect relationships between them (8, 9). Previous work has not explicitly distinguished between primary and secondary negative symptoms. Because no direct measure of secondary negative symptoms was available, attributions of direct and indirect effects on negative symptoms had to be based on estimates derived from the presumed causes of secondary negative symptoms. The validity of this path analytic method rests on the assumption that all of the relevant “causes” have been included.
It seems unlikely that positive symptoms, depression, and extrapyramidal side effects are the only causes of secondary negative symptoms in schizophrenia. Other causes may be relevant to the study of negative symptoms, such as lack of environmental stimulation, demoralization, and anxiety (10). Additionally, the causes of secondary negative symptoms can exacerbate each other. For instance, extrapyramidal symptoms can worsen anxiety, depression, and positive symptoms, each of which, in turn, can elevate negative symptoms (11).
The studies mentioned failed to include these variables in the model. Moreover, neither the risperidone (6) nor the olanzapine (7) study made any attempt to include measurement and psychometric sources of variance in the model, such as interrater reliability, validity of assessment instruments, or time lag between improvements in mediating variables (e.g., positive symptoms) and the therapeutic effect on negative symptoms. Ultimately, concluding that statistical variance unexplained by some limited set of mediating variables necessarily represents a direct effect on negative symptoms is at best an extrapolation of the data (12).
A more straightforward approach to distinguishing between direct treatment effects on primary negative symptoms and indirect effects mediated by improvements in the causes of secondary negative symptoms is to separately categorize these two types of negative symptoms by applying the deficit-nondeficit distinction (13). In this formulation, the deficit syndrome refers to a subtype of negative symptoms that are prominent, primary, and enduring features of a patient’s clinical presentation. In contrast, nondeficit negative symptoms are transient and secondary to depression, anxiety, extrapyramidal side effects, social deprivation, or positive symptoms. The deficit/nondeficit categorization requires an intensive evaluation of the individual patient to make a distinction between primary versus secondary negative symptoms as well as enduring versus transient negative symptoms. This distinction can be derived only from a longitudinal assessment of the relevant parameters. The Schedule for the Deficit Syndrome is a rating scale designed for such an assessment (14).
In a preliminary report from a study that prospectively assessed deficit versus nondeficit negative symptoms, clozapine had no beneficial effect on deficit symptoms while significantly improving nondeficit negative symptoms (15). However, in the final report from that study (16), there was no evidence of clozapine’s efficacy on either deficit or nondeficit negative symptoms. Another research group (7), using a proxy measure to define a putative deficit syndrome subgroup, reported that olanzapine was an effective treatment for patients with the deficit syndrome. These results have been questioned because the investigators did not compare the clinical features of the putative deficit and nondeficit groups with those of groups diagnosed with the full Schedule for the Deficit Syndrome, leaving in doubt the validity of the proxy determinations (12).
The present study is an attempt to replicate and extend studies of the effects of olanzapine on primary and secondary negative symptoms, using patients prospectively assessed with the full Schedule for the Deficit Syndrome. The research question was whether patients with negative symptoms of similar severity but different types (i.e., deficit versus nondeficit) would show divergent responsiveness to olanzapine. We hypothesized that patients with the deficit syndrome would be relatively nonresponsive to olanzapine, but patients with nondeficit negative symptoms would respond to olanzapine, showing an overall decrease in negative symptoms as well as a modest improvement in level of functioning and quality of life.
Method
Subjects
Clinicians at the San Fernando Mental Health Center were asked to refer clinically stable outpatients with a chart diagnosis of chronic schizophrenia, no psychiatric hospitalizations for 1 year, and severe negative symptoms for a study of the effects of olanzapine on negative symptoms. Fifty-one patients were referred for the study. After a complete description of the study was given to the patients, written informed consent was obtained.
A diagnosis of chronic schizophrenia was confirmed by using the Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P) (17). They were then evaluated with the SANS (3) as a screening tool. The global subscale scores of the SANS were added (range=5–25), and the 40 patients with a combined global subscale score of 18 or higher were determined to have severe negative symptoms and were thus eligible for the treatment phase of the study. None of the participants met criteria for any other current axis I diagnosis. One of the patients was subsequently dropped from the study because of uncertainty in the deficit/nondeficit categorization (see Results section).
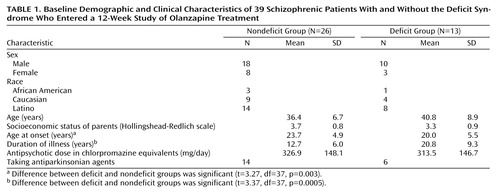
Twenty-eight (72%) of the 39 patients were men; the mean age of the patients was 37.8 years (SD=7.1). Four individuals (10%) were African American, 13 (33%) were Caucasian, and 22 (56%) were of Latino heritage. Twenty-seven participants (69%) lived with their families, and 12 (31%) lived in residential care homes in the community. The mean age at illness onset was 22.5 years (SD=5.1), and the mean duration of illness was 15.4 years (SD=7.1).
Procedures
At baseline, demographic and clinical history information was collected, including age, sex, ethnicity, socioeconomic status of parents, age at illness onset, duration of illness, current dose of antipsychotic medication, and use of antiparkinsonian medication. The participants were also rated for positive and negative symptoms, level of functioning, extrapyramidal side effects, and quality of life. Also at baseline, the patients were characterized into deficit and nondeficit subgroups, the key distinction in this study.
Before participating in the study, all of the patients were receiving oral preparations of conventional antipsychotic medications prescribed by their clinic psychiatrist. After confirmation that they met study criteria (SCID-P diagnosis of schizophrenia and a total score on the global subscale items of the SANS of 18 or higher), patients began a 2-day washout period during which their antipsychotic and antiparkinsonian medications were discontinued.
After the washout, patients began treatment with 10 mg of olanzapine at bedtime and continued to receive this medication for 12 weeks. An open-label design was used because the study was not a comparison between medication conditions but, rather, a comparison of olanzapine’s efficacy on two negative symptom subtypes (i.e., deficit versus nondeficit). Because the key variable in the study was the negative symptom subtype, the clinic psychiatrists and the raters were kept blind to the patients’ subtype designations. The 12-week duration was selected because previous studies of olanzapine suggested that efficacy for both positive and negative symptoms would be apparent within this time frame (7).
Over the first 4 weeks of the study, the dose of olanzapine was titrated by the clinic psychiatrist, using clinical judgment to maximize efficacy or to minimize side effects in a range from 5 to 30 mg. Clinic psychiatrists did not use any formal symptom or side effect rating scales. After week 4, the olanzapine dose was fixed for the remainder of the 12-week trial. At the end of the 12 weeks, patients were again rated for positive and negative symptoms, level of functioning, extrapyramidal side effects, and quality of life.
Throughout the course of the study, treatment was provided to the patients by their usual clinic psychiatrist, who at no time was informed of the patient’s negative symptom subtype. The clinic psychiatrists were asked not to administer adjunctive agents (e.g., antidepressants, anxiolytics, sedative-hypnotics, or antiparkinsonian drugs) prophylactically. Instead, they were asked to use these medications when clinically indicated and to record their reasons. Compliance was assessed weekly by a pill count and medication review by the clinic psychiatrist. In addition, all patients had a compliance plan that consisted of medication checks by family members and/or mental health care providers who had extensive contact with them. All 39 patients were judged by their psychiatrists to have taken 80% or more of their olanzapine doses.
Measures
The patients were categorized into deficit or nondeficit subgroups by using the Schedule for the Deficit Syndrome (14), a semistructured interview that defines the deficit syndrome as having at least two negative symptoms of at least moderate severity present for the preceding 12 months even during periods of clinical stability and in the absence of factors such as anxiety, drug effect, positive symptoms, mental retardation, and depression. Besides self-report, confirming information was obtained from the referring clinicians and family members. One of us (A.K.) received formal training in the use of the Schedule for the Deficit Syndrome from Brian Kirkpatrick, M.D., its first author. Using the training materials of the Maryland Psychiatric Research Center, A.K. trained K.T. on the Schedule for the Deficit Syndrome to high interrater agreement on the global categorization (kappa=0.84). After this training, all study participants were categorized independently by A.K. and K.T. Neither of the Schedule for the Deficit Syndrome raters served as the clinic psychiatrist for any of the study participants.
Positive symptoms were calculated by summing the scores on the four positive symptom items of the Brief Psychiatric Rating Scale (BPRS) (18): conceptual disorganization, hallucinations, unusual thought content, and delusions. Each of these items is rated on a 7-point severity scale (1=absent; 7=extremely severe). Negative symptoms were assessed with the SANS (3), which permits ratings of the severity of negative symptoms on five 0–5-point subscales (affective flattening or blunting, alogia, avolition-apathy, anhedonia-asociality, and attention). Each of the subscales includes a global item that serves as a summary measure of that subscale. The total SANS rating was derived by adding the patients’ scores on the global items for each of the five subscales (maximum=25). The SANS and BPRS were administered by one of us (R.Z.), who was blind to the participants’ negative symptom subtype. This rater was trained to excellent reliability (kappa=0.81) at the Diagnostic and Psychopathology Unit of the University of California, Los Angeles, Research Center for Treatment and Rehabilitation of Psychosis.
Additionally, each patient was rated for level of functioning with the Clinical Global Impression (CGI) scale (19) and quality of life with the Quality of Life Scale (20). Trained master’s-level research associates who were blind to the participants’ negative symptom subtype administered the CGI and Quality of Life Scale. Intraclass correlation coefficients for the CGI and Quality of Life Scale were 0.78 and 0.84, respectively. The Simpson-Angus Rating Scale (21) was used to assess extrapyramidal symptoms and was administered by R.Z. The SANS, BPRS, CGI, Quality of Life Scale, and Simpson-Angus Rating Scale were administered at baseline and at the end of the 12-week trial of olanzapine.
Statistical Analyses
Two-tailed t tests were used to determine the significance of within-group and between-group differences. Chi-square tests of distribution were also used to test significance. Effect sizes were calculated by using Cohen’s d (22) to determine if lack of significance could be due to low statistical power. A regression equation was used, as was analysis of variance.
Results
The raters agreed on the deficit/nondeficit categorization for 39 of the 40 patients: 13 patients met criteria for the deficit syndrome and 26 patients did not. The patient on whom there was disagreement was not included in the analyses. All 26 participants who were categorized as having nondeficit negative symptoms did not meet criteria for the deficit syndrome because they had not had persistent negative symptoms for the previous 12 months.
Group comparisons were made on demographic and clinical characteristics as depicted in Table 1; two-tailed t tests or chi-square tests of distribution were used to test significance. No significant differences between the groups were found in age, sex, ethnicity, or socioeconomic status; nor were there any significant differences between the groups before their study participation in terms of dose of conventional antipsychotic medication or number of patients taking antiparkinsonian medication. The deficit patients were significantly younger at onset of illness and had a longer duration of illness (Table 1).
All patients in both groups continued to take olanzapine for the full 12 weeks of treatment and completed both the baseline and posttreatment assessments. Endpoint doses of olanzapine and the percentage of participants who received adjunctive medications in each group were compared; these comparisons are shown in Table 2. The purpose of these analyses was to examine possible group differences in medication regimens that could account for differential treatment effects on positive and negative symptoms. According to t tests, there were no significant differences in olanzapine dose or use of adjunctive medications between the deficit and nondeficit patients.
To determine if there were pretreatment group differences in any of the outcome variables, independent group t tests were conducted. At baseline, deficit and nondeficit patients did not differ significantly on positive psychotic symptoms (t=0.38, df=37, p=0.70) or extrapyramidal side effects (t=1.88, df=37, p=0.07). However, deficit patients did have more severe negative symptoms (t=3.37, df=37, p=0.002, Cohen’s d=1.10), a lower level of functioning (t=3.63, df=37, p=0.001, Cohen’s d=1.20), and a poorer quality of life (t=2.17, df=37, p=0.04, Cohen’s d=1.20) than their nondeficit counterparts.
To determine if there were differences between the groups after olanzapine treatment, independent group t tests were conducted on each of the outcome variables (Table 3). Participants were not randomly assigned to groups at pretreatment; rather, assignment was determined by negative symptom subtype (i.e., deficit versus nondeficit), which formed the basis for the main research question. Other pretreatment differences in the outcome variables could be integral to or derive directly from this grouping variable of interest; therefore, no attempt was made to control for baseline differences statistically. All measures were coded as continuous variables. Also, effect sizes were calculated by using Cohen’s d (22) to determine if lack of significance could be due to low statistical power.
After treatment with olanzapine, the nondeficit group had significantly lower levels of negative symptoms and positive symptoms as well as higher levels of functioning and quality of life than the deficit group (Table 3). There was no statistically significant difference between the groups in extrapyramidal side effects after 12 weeks of treatment with olanzapine.
To control for type 1 error, Bonferroni corrections (based on k=5) were conducted on the variables that showed significant group differences. After applying these corrections (adjusted significance level: p≤0.01), differences in negative symptoms, positive symptoms, and level of functioning remained statistically significant, but differences in quality of life did not. We also repeated the between-groups analysis using pre- to postintervention changes (rather than absolute values) as the dependent variable. The results were essentially the same.
Within-group comparisons using paired t tests also demonstrated differential effects of olanzapine on deficit versus nondeficit patients. From baseline to posttreatment, participants with deficit negative symptoms showed no statistically significant changes in positive symptoms, quality of life, negative symptoms, or level of functioning (Table 3). Participants with deficit symptoms did demonstrate a significant improvement in extrapyramidal symptoms (Table 3). In contrast, participants with nondeficit negative symptoms improved significantly, with large effect sizes not only for extrapyramidal symptoms but also for positive symptoms, negative symptoms, and level of functioning (Table 3). Participants with nondeficit negative symptoms did not improve significantly in quality of life.
To examine the hypothesis that nondeficit negative symptoms are secondary to the effects of positive symptoms, medication side effects, and depression, a regression equation was conducted. The percentages of change in positive symptoms, extrapyramidal symptoms, and the depression item of the BPRS from before to after treatment were entered simultaneously as predictors. The percentage of change in negative symptoms from before to after treatment was the dependent variable. The result of this model was a significant F value (F=3.99, df=3, 22, p=0.02), which accounted for 35% of the variance in negative symptom change. It should be noted that the only single significant predictor was change in depression score (beta=0.69, t=3.22, df=22, p=0.004).
Discussion
The results of this study strike a cautionary note with respect to the inferences that can be drawn from using a path analysis approach to distinguish direct and indirect effects of antipsychotic medications on negative symptoms. We found that only individuals with nondeficit negative symptoms, which appear to be at least in part secondary to factors such as positive symptoms, depression, and extrapyramidal side effects, demonstrated significant improvements in negative symptoms after a 12-week trial of olanzapine. Indeed, 35% of the improvement in negative symptoms experienced by the patients who did not have the deficit syndrome was accounted for by their improvements in positive symptoms, extrapyramidal symptoms, and depression. Of the 26 patients with nondeficit negative symptoms, 24 experienced a decrease in negative symptoms from baseline to posttreatment; 21 of these 24 patients improved in either positive symptoms (N=18) and/or extrapyramidal side effects (N=16). Each of the three remaining patients showed improvements in the depression item of the BPRS. Individuals with deficit symptoms, which are presumed to be intrinsic to the disorder (i.e., primary) and thus the likely target for a direct effect of medication, showed no improvement of negative symptoms with olanzapine. The results of this study stand in contrast to the path analysis study of olanzapine (7), which concluded that olanzapine had a direct salutary effect on negative symptoms.
This study is the second to our knowledge to demonstrate differential results from a psychopharmacological intervention between prospectively assessed groups with distinct negative symptom subtypes. The first such study (15) was a preliminary report and indicated that clozapine had no beneficial effect on deficit symptoms, although it significantly improved nondeficit negative symptoms. However, in the final report from that study (16), there was no evidence of any superior efficacy or long-term effect of clozapine on either deficit or nondeficit negative symptoms. In our study, by contrast, individuals with schizophrenia who manifested nondeficit negative symptoms demonstrated a significant improvement in negative symptoms with olanzapine but those with the deficit syndrome did not.
The conflicting negative symptom results between the Buchanan et al. study (16) and our current study may reflect differences in the medications used (i.e., olanzapine versus clozapine) or differences in the baseline severity of positive and extrapyramidal symptoms. The former explanation is unlikely because clozapine is at least as efficacious as olanzapine for negative symptoms (23). The latter explanation is more likely because patients in the Buchanan et al. study had significant but only modest reductions in positive symptoms and low levels of baseline extrapyramidal symptoms. In the current study, patients with nondeficit negative symptoms had large effect sizes for changes in both positive symptoms and extrapyramidal symptoms. Taken together, these findings suggest that the effects of olanzapine on negative symptoms occur largely in the context of marked improvements in extrapyramidal symptoms and/or positive symptoms.
In addition to improvements in positive symptoms, negative symptoms, and extrapyramidal symptoms, individuals with nondeficit negative symptoms also demonstrated significant improvement in level of functioning after taking olanzapine. Conversely, individuals with deficit negative symptoms demonstrated no improvement in positive symptoms or level of functioning. One explanation for the lack of efficacy of olanzapine for the deficit group is that these patients had the quantitatively greater psychopathology and associated poorer functioning that accompanies chronicity. However, this argument is tempered by the fact that the deficit and nondeficit groups did not differ in positive symptoms at baseline, suggesting that the treatment refractoriness of the deficit group reflects the qualitative differences between individuals with deficit and nondeficit negative symptoms.
There is now mounting evidence to support the subtyping of individuals with schizophrenia who have the deficit syndrome (24–29). Our findings suggest that subtyping has discriminative and predictive validity for both symptomatic and functional improvement following olanzapine treatment. Results from this study, together with neuroanatomical (27–29) and neurocognitive (24) findings, suggest qualitative differences between patients with schizophrenia who do or do not have the deficit syndrome. Specifically, the deficit syndrome may represent a distinct pathophysiological entity with implications for the course and treatment of schizophrenia and perhaps its etiology. The clinical manifestations of the individual with schizophrenia who has the deficit syndrome resemble Kraepelin’s classic description of dementia praecox (30). These patients frequently experience an insidious onset of illness, a chronic deteriorating course, and a poor response to clinical interventions. This course type has been classified as the most severe and unrelenting form of schizophrenia (31, 32).
This study also highlights the need for clinicians to assess the roles of depression, positive symptoms, and extrapyramidal side effects as well as the lack of social interaction (e.g., impoverished environments, institutionalization) in the presence of negative symptoms because they have implications for intervention. First, these factors need to be targeted for treatment because they may be etiologically related to secondary negative symptoms. Second, there is some evidence that these factors may respond to atypical antipsychotic medications (5). Third, assessment of secondary factors would allow identification of those individuals whose clinical state can most effectively be improved by atypical agents or social skills training (33). This is not to suggest, however, that individuals with the deficit syndrome should be bypassed as candidates for treatment. On the contrary, this study stresses the need to find more effective means of treating these individuals instead of labeling them as treatment failures or nonresponders. It is possible that the negative symptoms observed in these individuals require pharmacological strategies apart from antipsychotic agents (34–37).
Although the concept of a deficit-nondeficit dichotomy has been influential over the past decade, another theory has been elucidated (38). The theory, which to date has little empirical support, identifies three separable types of negative symptoms: 1) deficit or primary, enduring negative symptoms that are intrinsic to the disease process, 2) primary, nonenduring negative symptoms that are intrinsic to the disorder but wax and wane like positive symptoms, and 3) secondary negative symptoms that are associated with positive symptoms, extrapyramidal side effects, depression, and lack of environmental stimulation. While recognizing the limitations of the path analysis method for identifying direct effects on primary negative symptoms (12), how do we understand the results of these studies of atypical antipsychotic medications (6, 7)? One possibility is that the unexplained variance, rather than representing a direct effect of atypical antipsychotic medications on primary, enduring (deficit) negative symptoms, may reflect a direct effect on this putative, nonenduring type of primary negative symptom. Further support for this hypothesis is the fact that the regression equation created from the data of the current study, which included positive symptoms, extrapyramidal symptoms, and depressive symptoms, explained only 35% of the variance. Of course, before this hypothesis can be tested, much clinical and basic science research remains to be done to provide support for the existence of primary, nonenduring negative symptoms.
The poorer outcomes observed among patients with the deficit syndrome were not due to group differences in olanzapine dose. Mean dose was actually somewhat higher among deficit patients (Table 2). Although the difference was not significant, deficit patients were more likely (62% versus 35%) to have doses increased from the starting level of 10 mg to 20 mg or more. It is implausible that higher doses in this range were a cause of poorer symptom outcomes. Rather, because doses were determined clinically rather than experimentally, this difference is more likely a reaction to the poorer outcomes in that group than a cause.
As to other possible confounding variables, only three patients in the nondeficit group, and no patients in the deficit group, received antiparkinsonian medications, too small a number for a statistical evaluation. The groups differed in age at onset and consequently in duration of illness (there were no statistical differences in age). Although chronicity could play a role in treatment response, we chose not to control statistically for age at onset because this may be a variable integral to the deficit syndrome subtype. This issue, however, deserves further examination.
All participants in this study manifested very high levels of negative symptoms and moderate levels of positive symptoms before the intervention. Although this constellation of symptoms is not unusual among stable outpatients receiving treatment in a community mental health center, the generalizability of the results may be limited in that patients with lower levels of negative symptoms and higher levels of positive symptoms may have responded differently. Also, the relatively short duration of the study (12 weeks) may not have been long enough to observe the beneficial effects of the medication on deficit negative symptoms and quality of life.
The deficit group comprised only 13 participants, which may represent an additional limitation to the generalizability of the results. Statistical power to detect a conventional medium effect of 0.50 standard deviations was not high (30% at two-tailed alpha=0.05 for the between-groups comparisons), but in general the effects of most interest (positive and negative symptom outcomes [Table 3]) were either very large or statistically quite small. Although the study group was small, taken together these results are consistent with the hypothesis that the beneficial effects of olanzapine for negative symptoms are limited to those patients who have secondary negative symptoms. Further work is needed to replicate these findings in a study with a larger number of subjects and with different atypical agents. Also, an important next step would be to correlate neurobiological and neuropsychological findings regarding negative symptoms with differences in clinical responsiveness to pharmacological interventions.
 |
 |
 |
Received Aug. 9, 1999; revision received Oct. 26, 1999; accepted Oct. 27, 1999. From the Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, School of Medicine. Address correspondence to Dr. Kopelowicz, San Fernando Mental Health Center, 15535 San Fernando Mission Blvd., Mission Hills, CA 91345; [email protected] (e-mail). Supported in part by NIMH grant MH-30911 to the University of California, Los Angeles, Research Center for the Treatment and Rehabilitation of Psychosis (Robert Paul Liberman, M.D., Director). The authors thank Robert Paul Liberman, M.D., and Brian Kirkpatrick, M.D., for their comments on earlier drafts of this manuscript and the staff of the San Fernando Mental Health Center for their clinical and administrative support.
1. Fenton WS, McGlashan TH: Natural history of schizophrenia subtypes, II: positive and negative symptoms and long-term course. Arch Gen Psychiatry 1991; 48:978–986Crossref, Medline, Google Scholar
2. Breier A, Schreiber JL, Dyer J, Pickar D: National Institute of Mental Health longitudinal study of chronic schizophrenia: prognosis and predictors of outcome. Arch Gen Psychiatry 1991; 48:239–246Crossref, Medline, Google Scholar
3. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
4. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Crossref, Medline, Google Scholar
5. Collaborative Working Group on Clinical Trial Evaluations: Assessing the effects of atypical antipsychotics on negative symptoms. J Clin Psychiatry 1998; 59(suppl 12):28–34Google Scholar
6. M�r HJ, M�H, Borison RL, Schooler NR, Chouinard G: A path-analytic approach to differentiate between direct and indirect drug effects in negative symptoms in schizophrenic patient: a re-evaluation of the North American risperidone study. Eur Arch Psychiatry Clin Neurosci 1995; 245:45–49Crossref, Medline, Google Scholar
7. Tollefson GD, Sanger TM: Negative symptoms: a path analytic approach to a double-blind, placebo- and haloperidol-controlled clinical trial with olanzapine. Am J Psychiatry 1997; 154:466–474Link, Google Scholar
8. Kenny DA: Correlation and Causality. New York, John Wiley & Sons, 1979Google Scholar
9. Bollen KA: Structural Equations With Latent Variables. New York, John Wiley & Sons, 1989Google Scholar
10. Whiteford HA, Peabody CA: The differential diagnosis of negative symptoms in chronic schizophrenia. Aust NZ J Psychiatry 1989; 23:491–496Crossref, Medline, Google Scholar
11. Van Putten T, Marder SR: Behavioral toxicity of antipsychotic drugs. J Clin Psychiatry 1987; 48(Sept suppl):13–19Google Scholar
12. Kirkpatrick B, Kopelowicz A, Buchanan RW, Carpenter WT Jr: Assessing the efficacy of treatments for the deficit syndrome of schizophrenia. Neuropsychopharmacology 2000; 22:303–310Crossref, Medline, Google Scholar
13. Carpenter WT Jr, Heinrichs DW, Wagman AMI: Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry 1988; 145:578–583Link, Google Scholar
14. Kirkpatrick B, Buchanan RW, McKinney PD, Alphs LD, Carpenter WT Jr: The Schedule for the Deficit Syndrome: an instrument for research in schizophrenia. Psychiatry Res 1989; 30:119–123Crossref, Medline, Google Scholar
15. Breier A, Buchanan RW, Kirkpatrick B, Davis OR, Irish D, Summerfelt A, Carpenter WT Jr: Effects of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am J Psychiatry 1994; 151:20–26Link, Google Scholar
16. Buchanan RW, Breier A, Kirkpatrick B, Ball P, Carpenter WT Jr: Positive and negative symptom response to clozapine in schizophrenic patients with and without the deficit syndrome. Am J Psychiatry 1998; 155:751–760Link, Google Scholar
17. First MB, Spitzer RL, Gibbon M, Williams JBW: Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition (SCID-P), version 2. New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
18. Ventura J, Green MF, Shaner A, Liberman RP: Training and quality assurance in the use of the Expanded Brief Psychiatric Rating Scale: the “drift busters.” Int J Methods in Psychiatr Res 1993; 3:221–244Google Scholar
19. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 218–222Google Scholar
20. Heinrichs DW, Hanlon TE, Carpenter WT Jr: The Quality of Life Scale: an instrument for rating the schizophrenic deficit syndrome. Schizophr Bull 1984; 10:388–398Crossref, Medline, Google Scholar
21. Simpson GM, Angus JWS: A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 1970; 212:11–19Crossref, Medline, Google Scholar
22. Cohen J: Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Hillsdale, NJ, Lawrence Erlbaum Associates, 1988Google Scholar
23. Henderson DC, Nasrallah RA, Goff DC: Switching from clozapine to olanzapine in treatment-refractory schizophrenia: safety, clinical efficacy, and predictors of response. J Clin Psychiatry 1998; 59:585–588Crossref, Medline, Google Scholar
24. Buchanan RW, Kirkpatrick B, Heinrichs DW, Carpenter WT Jr: Clinical correlates of the deficit syndrome of schizophrenia. Am J Psychiatry 1990; 147:290–294Link, Google Scholar
25. Buchanan RW, Strauss ME, Kirkpatrick B, Holstein C, Breier A, Carpenter WT: Neuropsychological impairments in deficit versus non-deficit forms of schizophrenia. Arch Gen Psychiatry 1994; 51:804–811Crossref, Medline, Google Scholar
26. Fenton WS, McGlashan TH: Antecedents, symptom progression, and long-term outcome of the deficit syndrome in schizophrenia. Am J Psychiatry 1994; 151:351–356Link, Google Scholar
27. Tamminga CA, Thaker GK, Buchanan R, Kirkpatrick B, Alphs LD, Chase TN, Carpenter WT: Limbic system abnormalities identified in schizophrenia using positron emission tomography with fluorodeoxyglucose and neocortical alterations with deficit syndrome. Arch Gen Psychiatry 1992; 49:522–530Crossref, Medline, Google Scholar
28. Buchanan RW, Breier A, Kirkpatrick B, Elkashef A, Munson RC, Gellad F, Carpenter WT Jr: Structural abnormalities in deficit and nondeficit schizophrenia. Am J Psychiatry 1993; 150:59–65Link, Google Scholar
29. Turetsky B, Cowell PE, Gur RC, Grossman RI, Shtasel DL, Gur RE: Frontal and temporal lobe brain volumes in schizophrenia: relationship to symptoms and clinical subtype. Arch Gen Psychiatry 1995; 52:1061–1070Google Scholar
30. Kraepelin E: Dementia Praecox and Paraphrenia. Translated by Barclay RM; edited by Robertson GM. Edinburgh, E & S Livingstone, 1919Google Scholar
31. Ciompi L: Catamnestic long-term study on the course of life and aging of schizophrenics. Schizophr Bull 1980; 6:606–618Crossref, Medline, Google Scholar
32. Harding C: Course types in schizophrenia: an analysis of European and American studies. Schizophr Bull 1988; 14:633–643Crossref, Medline, Google Scholar
33. Kopelowicz A, Liberman RP, Mintz J, Zarate R: Comparison of efficacy of social skills training for deficit and nondeficit negative symptoms in schizophrenia. Am J Psychiatry 1997; 154:424–425Link, Google Scholar
34. Goff DC, Tsai G, Manoach DS, Flood J, Darby DG, Coyle JT: d-Cycloserine added to clozapine for patients with schizophrenia. Am J Psychiatry 1996; 153:1628–1630Google Scholar
35. Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Horowitz A, Kelly D: Double-blind, placebo-controlled, crossover trial of glycine adjuvant therapy for treatment-resistant schizophrenia. Br J Psychiatry 1996; 169:610–617Crossref, Medline, Google Scholar
36. Goff DC, Tsai G, Levitt J, Amico E, Manoach D, Schoenfeld DA, Hayden DL, McCarley R, Coyle JT: A placebo-controlled trial of d-cycloserine added to conventional neuroleptics in patients with schizophrenia. Arch Gen Psychiatry 1999; 56:21–28Crossref, Medline, Google Scholar
37. Heresco-Levy U, Javitt DC, Ermilov M, Mordel C, Silipo G, Lichtenstein M: Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Arch Gen Psychiatry 1999; 56:29–38Crossref, Medline, Google Scholar
38. Greden JF, Tandon R (eds): Negative Schizophrenic Symptoms: Pathophysiology and Clinical Implications. Washington, DC, American Psychiatric Press, 1991Google Scholar



