Female Sexual Dysfunction Associated With Antidepressant Administration: A Randomized, Placebo-Controlled Study of Pharmacologic Intervention
Abstract
OBJECTIVE: Few controlled trials of pharmacologic intervention in women with antidepressant-associated sexual dysfunction have been reported, and there is uncertainty about the usefulness of putative treatments and the assessment methodologies. The authors evaluated the efficacy of buspirone and amantadine in the treatment of sexual dysfunction associated with fluoxetine administration. METHOD: Women who had been successfully treated with fluoxetine for at least 8 weeks and who had reported a deterioration in sexual function not present before the initiation of fluoxetine entered a 4-week assessment period. After assessment they were randomly assigned to an 8-week treatment trial with buspirone (N=19), amantadine (N=18), or placebo (N=20). Outcomes were assessed by using a patient-rated daily diary and a clinician-rated structured interview. RESULTS: While the amantadine-treated women did report significantly greater improvements in energy levels than women in the placebo group, all treatment groups experienced improvement in overall sexual function as well as in most individual measures. There were no statistically significant differences among the three groups. CONCLUSIONS: Neither buspirone nor amantadine was more effective than placebo in ameliorating antidepressant-associated sexual dysfunction. All groups experienced marked nonspecific improvement during treatment, which suggests the importance of placebo-controlled trials for this condition.
Many classes of antidepressants, including selective serotonin reuptake inhibitors (SSRIs), have been reported to affect sexual function (1), but adequate, systematic studies of this phenomenon, particularly in women, have not been reported. Depression is itself associated with sexual dysfunction, and successful treatment is associated with improvement. Additionally, assessment of sexual functioning is complex. As a result, available estimates of the magnitude of antidepressant-associated sexual problems are unreliable.
Women with alterations in sexual function that are associated with antidepressant administration are an important population in which to test intervention strategies and assessment methodologies. The issue is clinically important, since current data suggest that patients should continue antidepressant treatment well after acute symptoms resolve and interest and energy for intimate relationships have been restored (2, 3). From the perspective of study design, the association of symptoms and antidepressant treatment can be established, and within a given class of medication, mechanisms related to sexual effects are likely to be common to most patients. Further, hypothesis-based treatment strategies can be generated on the basis of known properties of particular antidepressant drugs.
The physiology of SSRI effects on sexual function are unknown but could relate to increased serotonergic tone inhibiting dopamine-related activation of sexual response. Potential strategies suggested for intervention have included manipulation of various serotonin receptors as well as attempts to potentiate dopamine activity. Both buspirone (which has 5-HT1A agonist properties) and amantadine (thought to increase dopamine availability) have been proposed as potential ameliorating agents, and each has been reported to be effective in uncontrolled case reports (4–8). However, to our knowledge, no controlled trials of either agent have been reported. Indeed, few controlled intervention trials of any sort for female antidepressant-associated sexual dysfunction have been undertaken, and methodological issues, including the magnitude of placebo response and the performance of instruments to assess severity and change, are poorly understood.
We conducted a randomized, placebo-controlled trial of buspirone and amantadine in the treatment of patient-reported sexual impairment associated with fluoxetine therapy. We report the results of this study here.
METHOD
Subjects
Subjects were recruited by referral and advertisement at three centers. Women 50 years of age or younger who had been taking a stable dose of fluoxetine for at least 8 weeks before study entry and who reported either impaired orgasm or sexual arousal (defined operationally as vaginal lubrication) that first occurred after initiation of fluoxetine therapy were eligible to participate in the study. All participants were involved in a stable relationship in which regular (at least twice a month) sexual activity was occurring. Participants were all premenopausal or on a regimen of estrogen replacement, had no other potential etiologies for their sexual dysfunction, and had responded satisfactorily to fluoxetine treatment. The study was approved by the ethical review board for each site, and after the procedures of the study had been fully explained, written informed consent was obtained from each subject before entry into the study.
Study Design
This was a 12-week study that consisted of a 4-week initial assessment period followed by an 8-week treatment period. Patients continued fluoxetine treatment at their entry dose throughout the 12-week study. Visits were biweekly, and patients kept a daily diary in which they used visual analogue scales to rate their mood, energy level, interest in sex, and awareness of sexual thoughts/feelings, as well as whether sexual activity occurred. On days when sexual activity occurred, patients used visual analogue scales to rate vaginal lubrication, orgasm, pleasure in sex, and any discomfort related to sex. Patient diaries were collected at each visit. At weeks 4 and 12, patients were also evaluated with the Interviewer Rating of Sexual Function, a semistructured interview developed in Edinburgh in conjunction with the Kinsey Institute at Indiana University (9, 10); the interview has been studied and found to be reliable (11). The frequency of sexual intercourse was established for the previous month, and the interviewer rated the proportion of occasions where sexual intercourse involved self-initiation, mutual initiation, sexual arousal, orgasm, vaginal dryness, speed of vaginal lubrication, pain and type of pain, and feeling close and comfortable with the partner during sexual intercourse. Frequency of sexual thoughts, occasions when sexual approach was refused by the partner, and masturbation were also assessed.
Impairment of sexual interest, arousal (vaginal lubrication), orgasm, and overall sexual function was assessed by means of clinician- and patient-rated global impressions (5-point range, 1=no impairment and 5=severely impaired), which were completed at each visit. The 17-item Hamilton Depression Rating Scale (12) was administered at entry to assess current depressive symptoms, and the Beck Depression Inventory (13) and State-Trait Anxiety Inventory for Adults (14) were administered at each visit.
To enter the study, the patient’s overall sexual function had to have a clinician-rated global impression of at least 3 (mildly impaired), and patients had to report impairment of either vaginal lubrication or orgasm and have a Hamilton depression scale score of 10 or less. After the 4-week initial assessment period, patients who continued to meet the entry criteria and who had reported at least 2 episodes of sexual activity since the initial visit were randomly assigned to treatment with either amantadine, 50 mg/day (50 mg in the morning and a dummy capsule in the evening); buspirone, 20 mg/day (10 mg in the morning and in the evening); or placebo (one capsule twice a day). After 4 weeks of treatment, patients whose overall sexual function continued to have a clinician-rated global impression of at least 3 had their doses increased (amantadine, 100 mg/day, administered as 50 mg twice a day; buspirone, 30 mg/day, administered as 15 mg twice a day; patients receiving placebo treatment were still given one capsule twice a day). Patients and efficacy raters were blinded to treatment assignment and to the criteria for study entry and dose adjustments.
Statistical Analysis
Patient demographic and baseline clinical characteristics were compared among groups by means of analysis of variance (ANOVA) for continuous variables and Pearson’s chi-square test for discrete variables. Efficacy was assessed in all patients who had at least 4 weeks of diary data available after randomization.
For continuous diary measures, mean ratings for the initial 4 weeks and the final 4 weeks of the study were calculated for each item. Change in each item was calculated as the difference of these means, and group mean change scores were compared by using two-way ANOVA with terms for treatment, center, and treatment-by-center interaction included in the model. Overall sexual functioning was derived from the sum of the mean scores for the interest item (completed daily) and the lubrication, orgasm, and pleasure items (completed when activity occurred). Changes from baseline (week 4) to endpoint (week 12) in other continuous measures were compared between treatment groups in the same fashion.
For the Interviewer Rating of Sexual Function, measures analogous to the diary categories were computed as the sum of the relevant individual questions. Higher scores indicate better functioning. Changes from baseline (week 4) to endpoint (week 12) were compared in the same fashion as the diary measures.
Within treatment groups, baseline and endpoint measures for continuous variables were compared by using Wilcoxon’s signed-rank test for nonzero change from baseline. Adverse event reports were compared among groups by using Pearson’s chi-square test. Correlation between partner variables and change in overall sexual function in the structured interview were analyzed by Pearson’s correlation analysis.
RESULTS
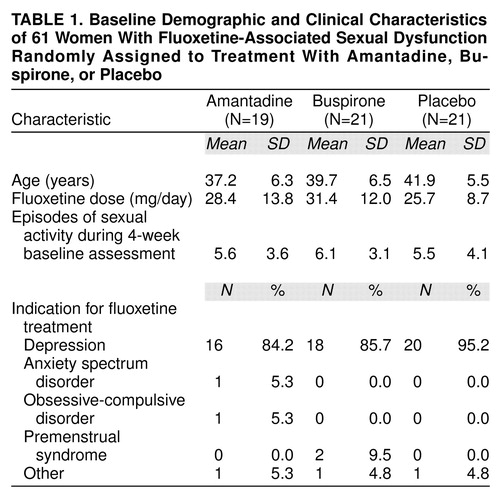
Baseline characteristics are summarized in table 1. Of the 67 women initially enrolled in the study, 61 continued to meet the criteria after 4 weeks and were randomly assigned to treatment. Of these, 57 (buspirone N=19, amantadine N=18, placebo N=20) had evaluatable data and were included in the analyses. The amantadine-treated group was slightly younger than the placebo-treated group. Mean dose of fluoxetine, the indications for which fluoxetine had been prescribed, and the frequency of sexual activity were similar for all groups.
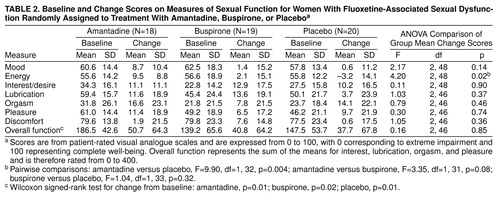
Diary results are summarized in table 2. At baseline, pleasure and lubrication were rated as moderately impaired, while sexual interest and orgasm were more severely impaired. There was a modest imbalance between groups in initial ratings of interest/desire, pleasure, and overall function. Women in the buspirone and placebo groups rated these items as slightly more impaired than did women in the amantadine group. Overall sexual function improved significantly in each group, and the magnitude of mean improvement was similar for all three treatments. Most individual items showed a similar degree of improvement, however discomfort was not prominent at baseline and did not increase appreciably during the study. The degree of improvement from baseline on individual items was generally in the range of 20%–50%. There were no statistically significant differences between treatments and no statistically significant treatment-by-center interactions for any diary measure of change from baseline. The groups were not significantly different from one another at baseline or endpoint with respect to mean frequency of sexual activity, and there was no significant change in frequency of sexual activity during the course of the study within any group.
In addition, structured interviews determined that there were no statistically significant differences among groups or statistically significant treatment-by-center interactions on change from baseline in any measure. There was no statistically significant relationship between partner variables and change in overall sexual function.
There were no statistically significant differences in the clinician-rated global impressions at endpoint between treatment groups on any measure. All groups showed improvement over the course of the study, and at endpoint 27% of patients were given ratings of 1 or 2 (no or minimal impairment).
On mood and anxiety measures, patients in the amantadine group reported significantly greater improvements in energy level at endpoint than the placebo-treated group (table 2), as assessed by mean change in diary ratings. There were no differences among the groups in mean change from randomization to endpoint on either the Beck Depression Inventory or the State-Trait Anxiety Inventory scores, nor were there any significant treatment-by-center interactions on change from baseline.
Augmentation with either buspirone or amantadine was well tolerated. No patients discontinued because of an adverse event, and reports of adverse events were similar among treatment groups.
DISCUSSION
Although case reports have suggested the efficacy of buspirone (8) and amantadine (4) in the treatment of SSRI-associated sexual dysfunction, to our knowledge no controlled studies have been reported, and the extent of placebo response of this condition has not been previously assessed. This study differs from other reports in that patients were carefully characterized before entry, they were then randomly assigned to a treatment group, the design of the study included a placebo arm, and specific aspects of sexual function were systematically assessed during treatment. Our results suggest that neither amantadine nor buspirone is an effective agent for the treatment of any aspect of SSRI-associated sexual dysfunction and that many aspects of sexual function are placebo responsive.
Several factors limit the interpretation of the results of this study. Most importantly, it is possible that higher doses of either buspirone or amantadine would have been effective and that the lack of observed effect is an artifact of not achieving adequate drug exposures. Sampling issues also could have affected outcomes, since women were not randomly selected, and the clinical signs and symptoms with which these women presented may not be representative of the broader group of women who experience changes in sexual function after starting treatment with an SSRI.
The small, statistically nonsignificant treatment differences could represent true drug effects that were not detected because of the limited sample size. However, overall improvement as assessed by the diary reports was highly comparable among all groups (range of 25%–29%), and even with very much larger samples, such small differences would be unlikely to be statistically or clinically significant. Since the study was limited to women, the potential efficacy of these agents in male sexual dysfunction remains unknown. The buspirone tablets differed in appearance from the amantadine and placebo tablets, which could potentially have compromised the blinding of the study; however, the similarity and consistency of the results among buspirone-treated patients with the other treatment arms suggests that this did not occur or was not an important factor. Fluoxetine was prescribed for several indications other than depression; however, most patients were being treated for depression, and all groups were similar with respect to overall distribution of indications.
Sexual function is a complex phenomenon, and its adequate assessment presents a number of methodological problems. This study controlled for as many variables that impact sexual function as possible. The study required a 4-week initial assessment period, a monogamous relationship, a minimum number of sexual encounters before entry, and impairment of at least moderate severity in either achieving orgasm or vaginal lubrication, since change in sexual interest is likely less specific, more context-dependent, and more subjectively experienced than changes in other spheres. We concentrated on vaginal lubrication as a marker for arousal in the diary reports, but these are not identical, and psychological arousal could potentially be dissociated from physiologic arousal. This distinction was, however, explicitly addressed in the structured interview, which provided no evidence of a specific drug effect on either psychological or physiologic arousal.
Finally, some data suggest that for many women, satisfaction and pleasure associated with sexual activity in a relationship are most highly correlated with attitudes towards the partner (15). Depressive illness can profoundly interfere with family and intimate relationships, and recovery may not repair these impairments immediately or completely, thus potentially affecting sexual functioning. During the structured interview, we inquired about the quality of the relationship with the partner and found no correlation between outcome and partner variables.
This study is, to our knowledge, the first to systematically assess placebo response in SSRI-associated sexual dysfunction. Our results suggest that most aspects of sexual function can be influenced by nonspecific factors and that expectations substantially influence outcomes in intervention studies. The mean change in clinician-rated global impressions of overall sexual function was almost one category (from moderate to minimal), and 27% of patients reported no or minimal symptoms at the end of the study, which suggests that the degree of change observed was clinically meaningful for some patients. The mechanisms underlying this improvement are uncertain but probably relate to the intensive self-monitoring of sexual function and regular clinic visits as well as to nonspecific effects associated with medication administration.
The results of this study do not definitively rule out the possibility that 5-HT1A agonism or increasing dopaminergic activity could improve sexual function in this condition. Buspirone is not an optimal 5-HT1A agonist, and other, more specific and potent agents could be efficacious. Similarly, although amantadine is believed to act through a dopaminergic mechanism, this has not been well characterized, and other dopaminergic agents could have different effects.
This study was not primarily aimed at evaluating the efficacy of the combination of fluoxetine and amantadine or buspirone in treating mood symptoms. However, mood, energy, and anxiety were assessed as a secondary objective in both the diary reports and on specific patient-rated instruments. The diary ratings indicate that compared with placebo amantadine improved energy level and approached statistical significance for mood improvement, a finding consistent with a previous report in patients with multiple sclerosis (16) as well as preclinical studies (17, 18). Amantadine is thought to increase the availability of synaptic dopamine (although this is not well characterized), and manipulation of the dopamine system has been suggested as a potential strategy for augmenting response to conventional antidepressants (19). By contrast, we did not detect differences in mood or anxiety on either the Beck Depression Inventory or the State-Trait Anxiety Inventory, perhaps because these instruments were designed to assess symptoms related to illness rather than subtle changes in a relatively euthymic population. Alternately, our findings could be a chance result.
In summary, these data suggest that neither buspirone nor amantadine is more effective than placebo in the treatment of female sexual dysfunction associated with SSRI administration but rather that nonspecific factors can induce clinically significant improvements in sexual function.
Presented in part at the 11th annual congress of the European College of Neuropsychopharmacology, Paris, Oct. 31–Nov. 4, 1998. Received Nov. 16, 1998; revisions received April 14 and June 17, 1999; accepted July 29, 1999. From Lilly Research Laboratories; the Kinsey Institute, Indiana University, Bloomington; and Clinical Studies, Ltd., Philadelphia. Address reprint requests to Dr. Michelson, Lilly Research Laboratories, Lilly Corporate Center D.C. 2423, Indianapolis, IN 46285; [email protected] (e-mail). Supported by a grant from Lilly Research Laboratories.
 |
 |
1. Rosen RC, Lane RM, Menza M: Effects of SSRIs on sexual function: a critical review. J Clin Psychopharmacol 1999; 19:67–85Crossref, Medline, Google Scholar
2. Reimherr FW, Amsterdam JD, Quitkin FM, Rosenbaum JF, Fava M, Zajecka J, Beasley CM Jr, Michelson D, Roback P, Sundell K: Optimal length of continuation therapy in depression: a prospective assessment during long-term fluoxetine treatment. Am J Psychiatry 1998; 155:1247–1253Google Scholar
3. Frank E, Kupfer DJ, Perel JM, Cornes C, Jarrett DB, Mallinger AG, Thase ME, McEachran AB, Grochocinski VJ: Three-year outcomes for maintenance therapies in recurrent depression. Arch Gen Psychiatry 1990; 47:1093–1099Google Scholar
4. Balogh S, Hendricks SE, Kang J: Treatment of fluoxetine-induced anorgasmia with amantadine. J Clin Psychiatry 1992; 53:212–213Medline, Google Scholar
5. Balon R: Intermittent amantadine for fluoxetine-induced anorgasmia. J Sex Marital Ther 1996; 22:290–292Crossref, Medline, Google Scholar
6. Gitlin MJ: Treatment of sexual side effects with dopaminergic agents (letter). J Clin Psychiatry 1995; 56:124Medline, Google Scholar
7. Shrivastava RK, Shrivastava S, Overweg N, Schmitt M: Amantadine in the treatment of sexual dysfunction associated with selective serotonin reuptake inhibitors. J Clin Psychopharmacol 1995; 15:83–84Crossref, Medline, Google Scholar
8. Norden MJ: Buspirone treatment of sexual dysfunction associated with selective serotonin re-uptake inhibitors. Depression 1994; 2:109–112Crossref, Google Scholar
9. Tyrer G, Steel JM, Ewing DJ, Bancroft J, Warner P, Clarke BF: Sexual responsiveness in diabetic women. Diabetologia 1983; 24:166–171Crossref, Medline, Google Scholar
10. Bancroft J, Tyrer G, Warner P: The classification of sexual problems in women. Br J Sex Med 1982; 30–37Google Scholar
11. Graham CA, Ramos R, Bancroft J, Maglaya C, Farley TM: The effects of steroidal contraceptives on the well-being and sexuality of women: a double-blind, placebo-controlled, two-centre study of combined and progestogen-only methods. Contraception 1995; 52:363–369Crossref, Medline, Google Scholar
12. Hamilton M: Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 1967; 6:278–296Crossref, Medline, Google Scholar
13. Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J: An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571Crossref, Medline, Google Scholar
14. Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA: Manual for the State-Trait Anxiety Inventory. Palo Alto, Calif, Consulting Psychologists Press, 1983Google Scholar
15. Cawood EH, Bancroft J: Steroid hormones, the menopause, sexuality and well-being of women. Psychol Med 1996; 26:925–936Crossref, Medline, Google Scholar
16. The Canadian MS Research Group: A randomized controlled trial of amantadine in fatigue associated with multiple sclerosis. Can J Neurol Sci 1987; 14:273–278Crossref, Medline, Google Scholar
17. Moryl E, Danysz W, Quack G: Potential antidepressive properties of amantadine, memantine and bifemelane. Pharmacol Toxicol 1993; 72:394–397Crossref, Medline, Google Scholar
18. Krupp LB, Coyle PK, Doscher C, Miller A, Cross AH, Jandorf L, Halper J, Johnson B, Morgante L, Grimson R: Fatigue therapy in multiple sclerosis: results of a double-blind, randomized, parallel trial of amantadine, pemoline, and placebo. Neurology 1995; 45:1956–1961Google Scholar
19. Nierenberg AA, Dougherty D, Rosenbaum JF: Dopaminergic agents and stimulants as antidepressant augmentation strategies. J Clin Psychiatry 1998; 59(suppl 5):60–64Google Scholar



