Independent Domains of Inhibitory Gating in Schizophrenia and the Effect of Stimulus Interval
Abstract
Objective: Patients with schizophrenia are known to have inhibitory gating deficits in the suppression of evoked potential P50 response to repeated stimuli and the prepulse inhibition of the startle response. In the current study, the authors aimed to determine whether these two inhibitory gating measures are related in schizophrenia patients or whether abnormal P50 suppression and abnormal prepulse inhibition are independent neurophysiological characteristics of schizophrenia. The authors hypothesized that the relationship of the two measures may vary as a function of interstimulus intervals of stimulus presentations. Method: Fifty-nine schizophrenia patients and 17 healthy comparison subjects were tested on both P50 suppression and prepulse inhibition. P50 suppression was measured using paired clicks with 500-msec interstimulus intervals. Prepulse inhibition was measured by using a series of prepulse-pulse pairs with interstimulus intervals ranging from 30 to 500 msec. Results: Patients showed reduced P50 suppression and prepulse inhibition in relation to healthy comparison subjects. Concordance analysis showed that abnormal P50 suppression and abnormal prepulse inhibition do not necessarily occur together. Prepulse inhibition was most prominent at the 120-msec interstimulus interval, which was not correlated to P50 suppression. At the 500-msec interstimulus interval, prepulse inhibition was significantly but negatively correlated to P50 suppression. Prepulse inhibition at the other interstimulus intervals was not correlated with P50 suppression. Conclusions: These neurophysiological measures lack robust and direct relationships and likely mark independent aspects of abnormal brain inhibitory functions in schizophrenia.
Problems in sensory and sensorimotor gating, as measured by suppression of the P50 evoked potential and prepulse inhibition of the startle response, are observed in schizophrenia and thought to mark the disease’s liability (1 – 3) . The P50 suppression and prepulse inhibition measures share the underlying conceptual framework of inhibitory “gating” to physiological responses. In addition, the two measures are similar on several other features: auditory stimuli are the most commonly used stimulation method in both measures; the first stimulus in both of these paradigms uses a nonstartling sound, and a subsequent stimulus is presented as part of paired stimuli with a certain interstimulus interval in both tasks.
Given these similarities, it is puzzling that previous reports showed a general lack of or only indirect correlation between these two measures in healthy subjects (4 – 7) , in individuals with schizotypal personality disorders (8) , and in animal studies (9) . However, previous studies did not examine the correlations between the two measures in schizophrenia patients, in which P50 suppression and prepulse inhibition abnormalities are frequent. In addition, it is important to determine whether a lack of correlation also means a lack of concordance of abnormal P50 suppression and abnormal prepulse inhibition in patients. Knowledge of the latter issue is critical in assigning the phenotypic status of these measures in genetic studies.
In previous experiments, the interstimulus interval for P50 suppression was always 500 msec, whereas the interstimulus intervals for prepulse inhibition were 30 msec (8) , 60 msec (6) , or 120 msec (4 , 5 , 7) . Timing is a basic determinant for signal processing in the neural circuitry. The use of different intrapair intervals between P50 and prepulse inhibition tasks may critically contribute to how performance on the two tasks is related. We hypothesized that these two measures may indeed be similar, but the strength of the correlations is a function of the interstimulus interval. Thus, our study systematically varied the interstimulus intervals for prepulse inhibition (from 30 msec to 500 msec) and examined how variations in the interstimulus interval may affect the P50-prepulse inhibition correlations. Of particular interest was examining the correlation when the interstimulus interval becomes similar and then overlaps (at 500 msec). Although one can make an argument for examining P50 suppression at the 60–120-msec interstimulus interval, an interval when prepulse inhibition is most robust, this was not done in our study because most patients with schizophrenia show normal P50 suppression at the 100-msec interstimulus interval (10) .
Method
Subjects
Fifty-nine outpatients with schizophrenia (ages 18–65) were tested on both tasks. The Structured Clinical Interview for DSM-IV (SCID) was administered to all subjects to obtain DSM-IV diagnoses. The patients were individuals with DSM-IV schizophrenia (N=55) or Research Diagnostic Criteria schizoaffective disorder (mainly schizophrenia, N=4) who were medicated and clinically stable. Five patients were taking first-generation antipsychotic medications (chlorpromazine-equivalent dose: mean=690.0 mg/day, SD=424.8), and 54 patients were taking second-generation antipsychotic medications, including 18 taking clozapine (dosage: mean=465.8 mg/day, SD=131.6); 19 taking olanzapine (mean=21.4 mg/day, SD=10.1), nine taking risperidone (mean=5.2 mg/day, SD=2.3), four taking quetiapine (mean=500.0 mg/day, SD=383.0); two taking aripiprazole (10 mg/day), and one taking ziprasidone (120 mg/day). One patient was taking both clozapine and olanzapine; four patients were taking medications of both generations. Symptoms in patients were measured with the 20-item Brief Psychiatric Rating Scale (BPRS) using 1–7 scores for each item. Seventeen healthy comparison subjects (no axis I or II diagnosis or family history of psychosis based on family history Research Diagnostic Criteria) were recruited using local community newspaper advertisements. The University of Maryland’s institutional review board approved written informed consent obtained from each subject.
Laboratory Procedures
P50 Suppression
A hearing screen was carried out. All subjects had a hearing threshold below 35 dB at 500 Hz. P50 methods were similar to those previously reported (11) . EEGs were recorded (skin impedance <5 Kohms) by the 28 electrode Quick-Cap (Neuromedical Supplies, El Paso, Tex.) and a Neuroscan SynAmp sampling (Neuromedical Supplies, El Paso, Tex.) at 1000 Hz using bandpass 0.1 to 100 Hz to yield 500-msec epochs, including a 100-msec prestimulus window. The subjects were presented with 150 pairs of 1-msec, 72-dB click stimuli through a pair of loudspeakers. Clicks within pairs were presented 500 msec apart, while pairs of clicks occurred at a 10-second interval. The sound intensity was measured by placing the Sound Level Meter (model 2700, Quest Technologies, Oconomowoc, Wis.) (on the A scale, sound pressure level mode, peak level at 60–120-dB range) next to the ear. Trials containing artifacts (75 μV) were rejected by a threshold filter. Epochs were then visually examined to reject additional apparent artifact. The vertex channel CZ was used for P50 data analysis (10) . Time-locked evoked potentials were obtained by averaging all artifact-free epochs, filtered at 10–100 Hz, 24- dB slopes. For S1, P50 was defined as the most positive deflection 35–75 msec after stimulus presentation. Amplitudes were measured by the absolute differences between the positive peak and the preceding negative trough. For S2, P50 was selected within a window of the S1 latency 10 ± msec for each subject. P50 suppression was defined by percent suppression in P50 amplitudes (% suppression = [S1 response – S2 response]/S1 response × 100). Percent suppressions instead of ratios were used for both P50 and prepulse inhibition so that they were directly comparable.
Prepulse Inhibition
The orbicularis oculi electromyographic activity was recorded from the right eye, filtered (1-1000 Hz, 60-Hz notch filter), and digitized at a 1-kHz rate. The acoustic stimuli were generated by a Psylab Stand Alone monitor and a tone generator (both from Contact Precision Instruments, Cambridge, Mass.) and delivered with headphones. Electromyographic activity was directed through a Grass A.C. Amplifier (model 1CP511, Astro-Med., Inc., West Warwick, R.I.) and was acquired by using commercially available hardware and software (BioPac, Gloeta, Calif.). The sound intensity was measured under the same settings as above by using a headphone coupler (Model EC-9A, Quest Technologies, Oconomowoc, Wis.) with a standard 1-lb weight strapped on the headphone during measurement. The electromyographic activity recording was processed offline with a 100-Hz high-pass filter and baseline correction by using a 100-msec prestimulus baseline. Response onset was defined by the first crossing from baseline within a 20–120-msec window after stimulus onset. Peak response amplitude was calculated by the difference of the most positive peak and most negative trough in a 20- to 150- msec window after pulse onset. Nonresponders to startle stimuli were defined as subjects who responded to less than 50% of the first eight pulse-alone trials. A session started with a 3-minute acclimation period with 70-dB white noise. Startling pulse-alone trials contained 116-dB white noise lasting 40 msec, and the prepulse-pulse trials contained a 20-msec, 80-dB white noise prepulse or 10 dB above the background noise. The following prepulse-pulse interstimulus intervals, or lead conditions, were tested: 30, 45, 60, 75, 90, 120, 180, 240, and 500 msec. The first three startle responses were discarded. After the three trials, each interstimulus interval condition was presented six times, while the pulse-alone trial was presented 12 times, for a total of 66 trials per session in pseudorandom order. Intertrial intervals varied from 12 to 20 seconds. A session lasted for about 20 minutes. Prepulse inhibition was calculated for each interstimulus interval condition as percent suppression in response amplitudes (% prepulse inhibition = [startle alone – lead condition]/startle alone × 100). During both measurements, the subjects were told to relax and keep their eyes open.
The order of testing was determined by scheduling: 32% of the patients were tested on P50 first and 68% on prepulse inhibition first. The time interval between P50 and prepulse inhibition testing was mean=53, SD=69, range=0–275, median-15 days. There was a minimum of 2 hours of separation between P50 and prepulse inhibition testing if the subjects were tested on the same day. Subjects with any change of medication during the study were not included in this report. We examined P50 suppression and prepulse inhibition as trait measures for schizophrenia; therefore, simultaneous data collection was not sought. All data preprocessing and scoring were performed blind to subject identities and group membership. P50 and prepulse inhibition records were separately blinded.
Analysis
Data distributions were examined by the Kolmogorov-Smirnov goodness of fit test. Analysis of variance (ANOVA) was used to assess differences between patients and comparison subjects in percent suppressions. Pearson’s correlation coefficients were performed separately among the two groups. We used kappa statistics to test the concordance of abnormal P50 suppression and abnormal prepulse inhibition, with abnormality defined as one standard deviation below the mean of the measure in healthy comparison subjects (12) . All tests were two-tailed.
Results
General Characteristics of the Participants
The patients and comparison subjects were not significantly different in age (mean=40.8 years, SD=10.3, and mean=39.8, SD=12.4, respectively; F=1.40, df=1, 75, p=0.71). There was a greater proportion of men in the patient group (13 women and 46 men compared with nine women and eight men in the comparison group; χ 2 =6.13, df=1, p=0.03). However, there were no significant gender differences in P50 suppression (F=1.89, df=1, 174, p=0.17) or prepulse inhibition (at a 120-msec interstimulus interval, F=0.80, df=1, 52, p=0.38). There was also no significant gender-by-group interaction for P50 suppression (F=0.48, df=1, 74, p=0.43) or prepulse inhibition (F=0.21, df=1, 52, p=0.94). Kolmogorov-Smirnov tests showed no significant deviation from normal distributions for P50 suppression and prepulse inhibition variables in either comparison subjects or patients (all p>0.33). In patients, the BPRS total score was mean=37.6, SD=8.4. No significant correlations between BPRS total score and P50 suppression or prepulse inhibition were found.
P50 Suppression
The patients had a reduced percent P50 suppression in relation to the healthy comparison subjects (mean=30.1%, SD=24.1%, versus mean=43.6%, SD=17.3%, respectively; F=4.37, df=1, 74, p=0.04). The patients did not significantly differ from the comparison subjects in S1 latency (mean=57.1 msec, SD=10.3, versus mean=59.1, SD=7.2; F=0.53, df=1, 74, p=0.47), S1 amplitude (mean=3.6 μV, SD=3.1, versus mean=3.5 μV, SD=1.3; F=0.03, df=1, 74, p=0.88), or S2 amplitude (mean=2.5 μV, SD=2.5, versus mean=2.0 μV, SD=0.9; F=0.64, df=1, 74, p=0.43).
Prepulse Inhibition
Nonresponder criteria were met in four comparison subjects (23.5%) and 19 patients (32.2%) (χ 2 =0.47, df=1, 76, p=0.49) who were not included in calculations of prepulse inhibition. In both groups, the largest prepulse inhibition effect was obtained at the 120-msec interstimulus interval ( Figure 1 ), supporting the use of this interval as the primary lead condition for prepulse inhibition (3) . Repeated-measures ANOVA showed a nonsignificant interstimulus interval-by-diagnosis interaction (F=0.25, df=1, 36, p=0.62) but a significant diagnosis-by-main effect interaction (F=4.00, df=1, 36, p=0.05). Post hoc tests showed that at the 120-msec interstimulus interval, there was a significant difference in prepulse inhibition between patients and comparison subjects (mean=35.4%, SD=26.0%, versus mean=55.3%, SD=14.1%, respectively; F=6.89, df=1, 52, p=0.01). Prepulse inhibition also differed between groups at a 90-msec interstimulus interval (F=4.58, df=1, 49, p=0.04) and a 30-msec interstimulus interval (F=4.54, df=1, 44, p=0.04). The patients had less startle inhibition across all interstimulus intervals. The patients and comparison subjects showed no significant differences in startle response amplitudes to the pulse-alone trials (mean=45.4 μV, SD=31.9, versus mean=55.8 μV, SD=35.6, respectively; F=0.99, df=1, 52, p=0.32), the prepulse-pulse trials at a 120-msec interstimulus interval (mean=25.1 μV, SD=14.3, versus mean=23.7 μV, SD=15.6, respectively; F=0.09, df=1, 52, p=0.76), or the prepulse-pulse trials at any other interstimulus intervals (all p>0.25).
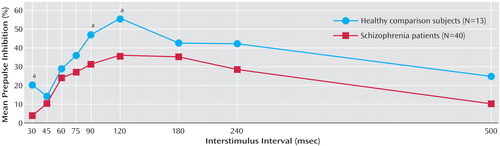
a Significant difference between patients and comparison subjects (p<0.05).
Correlation of P50 Suppression and Prepulse Inhibition
In schizophrenia patients, P50 suppression and prepulse inhibition at the primary lead of 120-msec interstimulus intervals were not correlated (N=40; r=0.02, p=0.89). At the 500-msec interstimulus interval (identical interstimulus interval), prepulse inhibition was significantly but negatively correlated to P50 suppression (r=–0.34, p=0.03), i.e., less P50 suppression was paradoxically associated with more prepulse inhibition. There were no significant correlations between P50 and other prepulse inhibition interstimulus intervals (all p>0.31) for patients. There were also no significant correlations between P50 and any prepulse inhibition interstimulus intervals among comparison subjects (N=13; all p>0.13) ( Figure 2 ).

a The arrow indicates the most commonly used interstimulus interval for prepulse inhibition.
b r=–0.34, p=0.03.
It is unclear why only prepulse inhibition at the 500-msec interstimulus interval showed significant correlations with P50 and why it was unexpectedly an inverse relationship. An examination of the data suggested that about 20% of the patients had prepulse facilitation, i.e., prepulse induced a stronger response compared to response to startle pulse alone, which was known to occur in longer prepulse-pulse intervals (13) . One possibility was that prepulse facilitation might be associated with more P50 suppression. To test the a posteriori hypothesis, the patients were divided into prepulse facilitation (prepulse inhibition <0%) or no prepulse facilitation (prepulse inhibition >0%). The patients showing prepulse facilitation had more P50 suppression (N=12; mean=42.4%, SD=24.4%) compared to the rest of the patients (mean=24.3%, SD=28.4%; F=4.13, df=1, 58, p=0.05).
Effects of Antipsychotic Medications
There was an insufficient number of patients taking first-generation antipsychotic medications (N=5) to compare the effects of first- versus new-generation antipsychotic medications. Patients taking clozapine did not significantly differ from patients not taking clozapine in prepulse inhibition at the 120-msec interstimulus interval (mean=39.6%, SD=21.8%, versus mean=33.3%, SD=28.3%, respectively; F=0.48, df=1, 38, p=0.49). The two patient groups were also not significantly different in P50 suppression (patients taking clozapine: mean=37.5%, SD=25.3%, versus patients not taking clozapine: mean=25.4%, SD=21.5%; F=2.98, df=1, 57, p=0.09). However, this could be a type I error because several previous studies have suggested that clozapine may increase P50 suppression and prepulse inhibition (14 – 18) .
Concordance of P50 Suppression and Prepulse Inhibition Abnormalities
The hypothesis that P50 suppression and prepulse inhibition abnormalities co-occur was not supported (four groups: 12 patients with abnormal P50 suppression only, 10 with abnormal prepulse inhibition only, 12 who were normal on both measures, and six who were abnormal on both; kappa=–0.12, p=0.52). Concordant cases (N=18) did not occur more frequently than discordant cases (N=22). The hypothesis of co-occurrence of P50 and prepulse inhibition abnormalities at any other interstimulus interval was also not supported (all kappa >0.22 in absolute values; all p>0.15), with the exception of prepulse inhibition at a 240-msec interstimulus interval (kappa=–0.29, p<0.03, not significant after correction for multiple comparisons).
Discussion
We found that patients with schizophrenia, as a group, have abnormal evoked potential P50 suppression and prepulse inhibition. However, concordance analysis suggested that deficits in these measures do not co-occur in individual patients by more than chance. Correlation analyses further support the independence of the two gating measures. The lack of substantial correlation was generally true across the range of interstimulus intervals (30–500 msec) that were typically necessary to elicit prepulse inhibition (13) .
The neural circuits for P50 suppression and prepulse inhibition are partially known. For P50 suppression, data implicate the temporal-parietal cortical region, hippocampus, and prefrontal cortex (2 , 19–21) . Prepulse inhibition is robustly regulated by a number of forebrain and other brain structures, including the hippocampus, medial prefrontal region, basolateral amygdala, nucleus accumbens, striatum, and pontine startle circuitry (22) . Based on the current description of the two neural circuits, perhaps the hippocampus and the frontal area are involved in both pathways. However, given the trivial correlations and the lack of robust concordance in abnormalities, deficits in the same locus could not be the primary pathology because a common mechanism would entail substantial interrelation between the two measures. A more plausible explanation is that of multifactorial biochemical or anatomic dysfunctions related to the illness, and the P50 and prepulse inhibition paradigms may have elicited partially nonoverlapping aspects of the multifactorial disease-related processes. In this context, we note that several drugs, including ketamine and a serotonin 5-HT 2a/2c agonist, are shown to have differential effects on the two gating measures (23 – 26) .
This study suggests that these neurophysiological markers associated with schizophrenia could be sorted independently. When applying Mendel’s second law, one would speculate that the genetic bases of the two inhibitory endophenotypes are different. Once the sensitivity/specificity of these measures are better established, genetic studies may use these findings to enhance the homogeneity of patient grouping by, for instance, defining subgroups of patients based on the concordance assignment of these endophenotypes.
1. Venables PH: Input dysfunction in schizophrenia. Prog Exp Pers Res 1964; 1:1–47Google Scholar
2. Freedman R, Adler LE, Myles-Worsley M, Nagamoto HT, Miller C, Kisley M, McRae K, Cawthra E, Waldo M: Inhibitory gating of an evoked response to repeated auditory stimuli in schizophrenic and normal subjects: human recordings, computer simulation, and an animal model. Arch Gen Psychiatry 1996; 53:1114–1121Google Scholar
3. Braff DL, Grillon C, Geyer MA: Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry 1992; 49:206–215Google Scholar
4. Schwarzkopf SB, Lamberti JS, Smith DA: Concurrent assessment of acoustic startle and auditory P50 evoked potential measures of sensory inhibition. Biol Psychiatry 1993; 33:815–828Google Scholar
5. Oranje B, van Berckel BN, Kemner C, van Ree JM, Kahn RS, Verbaten MN: P50 suppression and prepulse inhibition of the startle reflex in humans: a correlational study. Biol Psychiatry 1999; 45:883–890Google Scholar
6. Light GA, Braff DL: Measuring P50 suppression and prepulse inhibition in a single recording session. Am J Psychiatry 2001; 158:2066–2068Google Scholar
7. Brenner CA, Edwards CR, Carroll CA, Kieffaber PD, Hetrick WP: P50 and acoustic startle gating are not related in healthy participants. Psychophysiology 2004; 41:702–708Google Scholar
8. Cadenhead KS, Light GA, Geyer MA, McDowell JE, Braff DL: Neurobiological measures of schizotypal personality disorder: defining an inhibitory endophenotype? Am J Psychiatry 2002; 159:869–871Google Scholar
9. Ellenbroek BA, van Luijtelaar G, Frenken M, Cools AR: Sensory gating in rats: lack of correlation between auditory evoked potential gating and prepulse inhibition. Schizophr Bull 1999; 25:777–788Google Scholar
10. Nagamoto HT, Adler LE, Waldo MC, Griffith J, Freedman R: Gating of auditory response in schizophrenics and normal controls: effects of recording site and stimulation interval on the P50 wave. Schizophr Res 1991; 4:31–40Google Scholar
11. Hong LE, Summerfelt A, McMahon RP, Thaker GK, Buchanan RW: Gamma/beta oscillation and sensory gating deficit in schizophrenia. Neuroreport 2004; 15:155–159Google Scholar
12. Louchart-de la Chapelle S, Nkam I, Houy E, Belmont A, Menard JF, Roussignol AC, Siwek O, Mezerai M, Guillermou M, Fouldrin G, Levillain D, Dollfus S, Campion D, Thibaut F: A concordance study of three electrophysiological measures in schizophrenia. Am J Psychiatry 2005; 162:466–474Google Scholar
13. Graham FK, Putnam LE, Leavitt LA: Lead-stimulation effects of human cardiac orienting and blink reflexes. J Exp Psychol Hum Percept Perform 1975; 104:175–182Google Scholar
14. Nagamoto HT, Adler LE, Hea RA, Griffith JM, McRae KA, Freedman R: Gating of auditory P50 in schizophrenics: unique effects of clozapine. Biol Psychiatry 1996; 40:181–188Google Scholar
15. Adler LE, Olincy A, Cawthra EM, McRae KA, Harris JG, Nagamoto HT, Waldo MC, Hall MH, Bowles A, Woodward L, Ross RG, Freedman R: Varied effects of atypical neuroleptics on P50 auditory gating in schizophrenia patients. Am J Psychiatry 2004; 161:1822–1828Google Scholar
16. Hamm AO, Weike AI, Schupp HT: The effect of neuroleptic medication on prepulse inhibition in schizophrenia patients: current status and future issues. Psychopharmacol (Berl) 2001; 156:259–265Google Scholar
17. Braff DL, Geyer MA, Swerdlow NR: Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacol (Berl) 2001; 156:234–258Google Scholar
18. Kumari V, Soni W, Sharma T: Normalization of information processing deficits in schizophrenia with clozapine. Am J Psychiatry 1999; 156:1046–1051Google Scholar
19. Grunwald T, Boutros NN, Pezer N, von Oertzen J, Fernandez G, Schaller C, Elger CE: Neuronal substrates of sensory gating within the human brain. Biol Psychiatry 2003; 53:511–519Google Scholar
20. Boutros NN, Trautner P, Rosburg T, Korzyukov O, Grunwald T, Schaller C, Elger CE, Kurthen M: Sensory gating in the human hippocampal and rhinal regions. Clin Neurophysiol 2005; 116:1967–1974Google Scholar
21. Weisser R, Weisbrod M, Roehrig M, Rupp A, Schroeder J, Scherg M: Is frontal lobe involved in the generation of auditory evoked P50? Neuroreport 2001; 12:3303–3307Google Scholar
22. Swerdlow NR, Geyer MA, Braff DL: Neural circuit regulation of prepulse inhibition of startle in the rat: current knowledge and future challenges. Psychopharmacol (Berl) 2001; 156:194–215Google Scholar
23. de Bruin NM, Ellenbroek BA, Cools AR, Coenen AM, van Luijtelaar EL: Differential effects of ketamine on gating of auditory evoked potentials and prepulse inhibition in rats. Psychopharmacol (Berl) 1999; 142:9–17Google Scholar
24. Riba J, Rodriguez-Fornells A, Barbanoj MJ: Effects of ayahuasca on sensory and sensorimotor gating in humans as measured by P50 suppression and prepulse inhibition of the startle reflex, respectively. Psychopharmacol (Berl) 2002; 165:18–28Google Scholar
25. de Bruin NM, van Luijtelaar EL, Cools AR, Ellenbroek BA: Filtering disturbances in schizophrenic patients: gating of auditory evoked potentials and prepulse inhibition of the acoustic startle response compared: emphasis on the role of dopamine. Curr Neuropharmacol 2003; 142:9–17Google Scholar
26. Swerdlow NR, Geyer MA, Shoemaker JM, Light GA, Braff DL, Stevens KE, Sharp R, Breier M, Neary A, Auerbach PP: Convergence and divergence in the neurochemical regulation of prepulse inhibition of startle and N40 suppression in rats. Neuropsychopharmacology 2006; 31:506–515Google Scholar



