Neural Circuitry Engaged During Unsuccessful Motor Inhibition in Pediatric Bipolar Disorder
Abstract
Objective: Deficits in motor inhibition may contribute to impulsivity and irritability in children with bipolar disorder. Studies of the neural circuitry engaged during failed motor inhibition in pediatric bipolar disorder may increase our understanding of the pathophysiology of the illness. The authors tested the hypothesis that children with bipolar disorder and comparison subjects would differ in ventral prefrontal cortex, striatal, and anterior cingulate activation during unsuccessful motor inhibition. They also compared activation in medicated versus unmedicated children with bipolar disorder and in children with bipolar disorder and attention deficit hyperactivity disorder (ADHD) versus those with bipolar disorder without ADHD. Method: The authors conducted an event-related functional magnetic resonance imaging study comparing neural activation in children with bipolar disorder and healthy comparison subjects while they performed a motor inhibition task. The study group included 26 children with bipolar disorder (13 unmedicated and 15 with ADHD) and 17 comparison subjects matched by age, gender, and IQ. Results: On failed inhibitory trials, comparison subjects showed greater bilateral striatal and right ventral prefrontal cortex activation than did patients. These deficits were present in unmedicated patients, but the role of ADHD in mediating them was unclear. Conclusions: In relation to comparison subjects, children with bipolar disorder may have deficits in their ability to engage striatal structures and the right ventral prefrontal cortex during unsuccessful inhibition. Further research should ascertain the contribution of ADHD to these deficits and the role that such deficits may play in the emotional and behavioral dysregulation characteristic of bipolar disorder.
Considerable data suggest the importance of studying the neural circuitry mediating motor inhibition in pediatric bipolar disorder. Behavioral data in pediatric bipolar disorder suggest deficits in motor inhibition (1) , whereas behavioral data in adults with bipolar disorder show similar deficits and relate them to the impulsivity characteristic of both mania and depression (2) . In addition, functional magnetic resonance imaging (fMRI) studies in both adolescents and adults with bipolar disorder indicate differences in striatal activation between patients and comparison subjects while they are performing motor inhibition tasks (3 , 4) . Taken together, this research suggests that pediatric bipolar disorder is associated with perturbed functioning in a striatally based circuit, a hypothesis that could be tested by an imaging study of motor inhibition in pediatric bipolar disorder. Finally, in comparison youth, deficits in motor inhibition have been linked to irritability (5) . Irritability is a common and disabling symptom in pediatric bipolar disorder, and the presence of irritability and motor disinhibition in both pediatric bipolar disorder and attention deficit hyperactivity disorder (ADHD) has been one cause of the diagnostic confusion between these two illnesses. Thus, a study of motor inhibition in pediatric bipolar disorder would set the stage for future neuroimaging studies of pathophysiological commonalities and distinctions between bipolar disorder and ADHD.
Indeed, studies have indicated that motor inhibition is mediated by several regions of interest that are also implicated in the pathophysiology of bipolar disorder (3 , 6–15) , including the ventral prefrontal cortex, striatum, and anterior cingulate. One study found that in relation to comparison subjects, children with bipolar disorder and a family history of bipolar disorder had increased ventral prefrontal cortex activation while viewing emotional pictures or performing a spatial working memory task (12) . In the same study, the patients had greater anterior cingulate activation than the comparison subjects during the spatial working memory task, consistent with studies in bipolar adults (7 , 13) . Finally, five studies in bipolar disorder (three adult and two pediatric) reported patient-comparison differences in striatal activation on paradigms involving emotional stimuli, an interference task, and a spatial working memory task (3 , 4 , 9 , 12 , 16) .
When we study motor inhibition in youth with bipolar disorder, two potential confounds deserve attention: medication and comorbid ADHD. We recruited enough children with bipolar disorder to compare neural activation in medicated versus unmedicated patients and in those with ADHD versus those without ADHD. Such comparisons are rare in the literature. Four fMRI studies included enough unmedicated bipolar patients to analyze their data separately from those of medicated patients (4 , 17–19) . All other published fMRI studies in bipolar disorder (child or adult) have included predominantly medicated subjects (3 , 6 , 13–16) . Similarly, although studies have found comorbid ADHD in at least 60% of the children with bipolar disorder (20) , only one previous fMRI study, to our knowledge, compared activation in bipolar children with and without comorbid ADHD (19) .
Here we used the stop signal task, a motor inhibition paradigm used to study ADHD (21) . We were interested in the circuitry engaged during unsuccessful motor inhibition because the failure to inhibit inappropriate motor responses is an important symptom of pediatric bipolar disorder. Two features of the stop signal paradigm facilitate such study. First, the paradigm adjusts difficulty on inhibitory trials based on subject performance, ensuring a relatively large number of unsuccessful inhibitory (stop incorrect) trials to contrast with successful inhibitory (stop correct) trials and with go trials. Second, all trials begin with a go signal, followed in approximately 25% of trials by a stop signal. Thus, stop correct and stop incorrect trials involve identical stimuli but differ in the presence or absence of a motor response, whereas stop incorrect and go trials involve the same motor response but differ in the signal preceding the response. Contrasts using these event types can thus control for both stimulus properties and motor response, isolating the circuitry involved in successful and unsuccessful inhibition.
In sum, we used rapid event-related fMRI and the stop signal task to study motor inhibition in pediatric bipolar disorder, focusing on circuitry engaged during unsuccessful inhibition. Because the ventral prefrontal cortex, anterior cingulate, and striatum have been implicated in both motor inhibition and bipolar disorder, we hypothesized that patients and comparison subjects would differ in activation in these regions. In addition, we compared activation in medicated versus unmedicated patients and in bipolar patients with ADHD versus bipolar patients without ADHD.
Method
Subjects
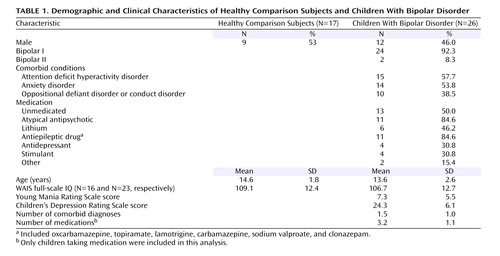
The patient group consisted of 39 youth recruited through advertisements to patient advocacy groups. All patients met DSM-IV diagnostic criteria for bipolar disorder and exhibited the narrow phenotype of bipolar disorder, i.e., each had had at least one hypomanic or manic episode meeting full duration criteria, including an expansive elevated mood (22) . Exclusions were severe pervasive developmental disorder, substance use within the past 3 months, chronic medical illness, or IQ <70. A best-estimate diagnostic approach was used, integrating data from the Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (K-SADS-PL) (23) (parents and children were interviewed separately by clinicians with master’s-level degrees or greater; kappa >0.9), treating clinicians, and medical records. Comorbid diagnoses were assigned only if patients met criteria for the comorbid diagnosis while they were euthymic. Thus, the diagnosis of comorbid ADHD could not be due to overlap between symptoms of ADHD and mania, hypomania, or depression.
We excluded data from subjects with inadequate task performance (i.e., <65% correct go trials) or excessive movement (>2.5 mm in any plane) or artifact, or who were unable to complete the procedure. Data were not usable for 13 patients, and the clinical data that follows includes only the 26 patients with usable data.
Fifteen of the patients (57.7%) had comorbid ADHD ( Table 1 ). Clinicians completed mood ratings (the Young Mania Rating Scale) (24) and the Children’s Depression Rating Scale (25) within 24 hours of scanning ( Table 1 ). Five patients were hypomanic (Young Mania Rating Scale score >12 but <20), and the remaining 21 were euthymic. Thirteen patients (50%) were unmedicated ( Table 1 ). Stimulants were not withheld for scanning. Unmedicated patients were not medication naive but had been withdrawn from psychotropic medications and were free from such medications for a minimum of four drug half-lives.
Unmedicated comparison subjects (N=26) had no diagnoses on the K-SADS, no DSM-IV diagnoses in first-degree relatives (family history ascertained by parent interview), no chronic medical illnesses, and IQ >70. Of the 26 scanned comparison subjects, data from 17 were usable.
The groups did not differ significantly on age, gender, or IQ ( Table 1 ). The study was approved by the NIMH institutional review board; participants and a legal guardian provided written informed assent or consent.
Behavioral Task
The task was based on previous published work (26) . On all trials, a white fixation cross appeared for 500 msec; it was replaced by an “X” or an “O” go signal for 1000 msec. On a button box, the subjects pressed “1” for “X” and “2” for “O.” The subjects were told to respond within 1000 msec unless the stop signal appeared (i.e., the background changed to red). This occurred on 25% of the trials (i.e., stop trials). In that circumstance, they were instructed not to press either button.
On the first stop trial, the stop signal appeared 250 msec after the go signal. Subsequent stop-signal timing was based on subject performance. If the subject successfully inhibited, the next stop signal appeared 50 msec later than on the last stop trial, making inhibition more difficult; if the subject failed to inhibit, the signal appeared 50 msec earlier than on the last stop trial, making inhibition easier. The trials were separated by 750 msec.
Before scanning, the subjects were trained to achieve a mean reaction time of less than 1000 msec on go trials. The subjects received feedback after each block during scanning; they were told to speed up if the mean reaction time exceeded 1000 msec.
Scanning Acquisition
Scanning occurred in a General Electric Signa 3T magnet (Milwaukee). Images were presented by means of Avotec Silent Vision Glasses (Stuart, Fla.) mounted on the head coil above the subjects’ eyes. Gradient echo planar images were acquired after sagittal localization and a manual shim procedure. Echo planar images were 23 contiguous 5-mm axial slices that were parallel to the anterior commissure posterior commissure line, with echo planar images in single-shot gradient echo T 2 * weighting (matrix 64×64, TR=2000 msec, TE=40 msec, field of view=240 mm, voxels=3.75×3.75×5 mm).
The subjects completed four runs, each with 32 go, 16 stop, and 16 blank trials distributed randomly throughout each block. Blank null trials were included based on the “rapid event-related” paradigm of Friston et al. (27) to allow deconvolution of unique events occurring close in time and to provide a baseline for comparison.
A high-resolution T 1 -weighted anatomical image with a standardized magnetization-prepared gradient echo sequence (180 1-mm sagittal slices, field of view=256, number of excitations=1, TR=11.4 msec, TE=4.4 msec, matrix=256×256, TI=300 msec, bandwidth=130 Hz/pixel, 33kHz/256 pixels) was acquired for spatial normalization.
Data Analysis
Behavioral Data
The following were recorded during scanning: reaction time and accuracy on go trials, accuracy on stop trials, and inhibit delay (i.e., the interval between the onsets of the go and stop signals). The stop signal reaction time, or the speed of inhibition, was calculated. When a subject inhibited successfully on 50% of stop trials, the stop signal reaction time was the mean go reaction time minus the mean inhibit delay (26) . Because the subjects’ accuracy on stop trials may have deviated from 50%, an interpolation algorithm was used to calculate stop signal reaction time: the mean stop signal delay was subtracted from the go reaction time at the Xth percentile of go reaction times, where X is the subject’s percent accuracy on stop trials.
Primary Analyses of Imaging Data
Analyses were conducted with SPM99 (Wellcome Department of Imaging Neuroscience, University College, London). Preprocessing included slice time correction, motion correction, and spatial normalization. At the subject level, event-related response amplitudes were estimated by using the general linear model. Event types included unsuccessful stop (stop incorrect), correct go (go; N.B. hereafter, go refers only to correct go trials) and successful stop (stop correct) trials. A rectangular pulse (2250 msec, the event length) was used to model each event, convolved with the hemodynamic response function provided by statistical parametric mapping. A high-pass filter of 0.024 Hz was applied.
Contrast images were created for each subject by using pairwise comparisons of event-related response amplitudes. The primary analyses compared patients and comparison subjects on activation on stop incorrect versus go trials and on stop correct versus stop incorrect trials. For both contrasts, subsequent analyses compared medicated patients to comparison subjects, unmedicated patients to comparison subjects, and unmedicated to medicated patients. We also compared patients with bipolar disorder with ADHD to comparison subjects, patients with bipolar disorder without ADHD to comparison subjects, and patients with bipolar disorder and ADHD to patients with bipolar disorder without ADHD on both contrasts.
Before group analysis, each contrast image was intensity normalized. These normalized contrast images were smoothed by using an isotropic Gaussian kernel (full width at half maximum=11.4 mm). A group-level random-effects model was employed to provide population-level inference. A small volume-corrected region-of-interest analysis was used on the bilateral ventral prefrontal cortex, striatum (accumbens, caudate, putamen), and anterior cingulate. Significance was set at p<0.05, corrected for the number of voxels within each region of interest.
Region-of-interest templates were defined with MedEx software, drawn by hand in the coronal plane, on the canonical single subject’s structural MRI images supplied by SPM99. Coordinates were in Montreal Neurological Institute space.
The cingulate was defined as the gyrus that abutted the corpus callosum and extended laterally to the white matter. The anterior and posterior division was based on the approximate midpoint of the corpus callosum (28) . The putamen was defined by the gray matter between the internal capsule and the insula, while the caudate was defined by the gray matter between the lateral ventricle and the internal capsule (29) . The rostral inferior boundary of the caudate was the nucleus accumbens, which was delimited superiorly by a line connecting the inferior corner of the lateral ventricle and the inferior point of the internal capsule abutting the accumbens and laterally by a vertical line passing from the internal capsule to the base of the brain (30) . The rostral extent of the nucleus accumbens coincided with the emergence of the striatum; caudally, the accumbens extended to the anterior commissure (30) . Ventral prefrontal cortex boundaries were defined as the last slice with the anterior horizontal ramus and the last slice containing the olfactory sulcus, the anterior horizontal ramus, and the olfactory sulcus (31 , 32) .
Results
Behavioral Data
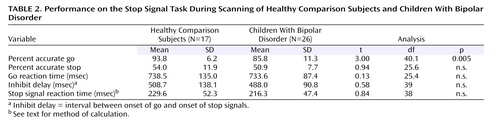
There were no between-group differences in mean inhibit delay (indicating that the groups did not differ in the amount of task difficulty adjustment), go reaction time, stop accuracy, or stop signal reaction time, but the patients had significantly lower go accuracy than the comparison subjects ( Table 2 ). In the fMRI analysis, to control for between-group differences in stop and go accuracy, only correct go trials were used; stop correct and stop incorrect trials were analyzed separately. There were no significant behavioral differences between unmedicated and medicated patients or between patients with bipolar disorder with ADHD and patients with bipolar disorder without ADHD.
Imaging Data
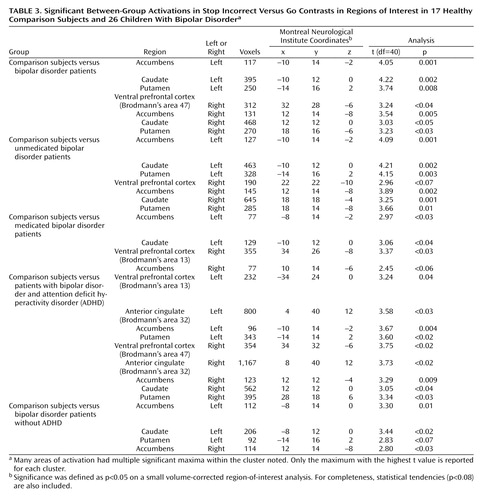
Stop Incorrect Versus Go ( Table 3 )
All Bipolar Patients Versus Comparison Subjects
The patients with bipolar disorder did not show increased activation relative to the comparison subjects in any region of interest. However, the comparison subjects had greater activation than the patients in the bilateral caudate, putamen, accumbens, and right ventral prefrontal cortex ( Figure 1 , upper image).
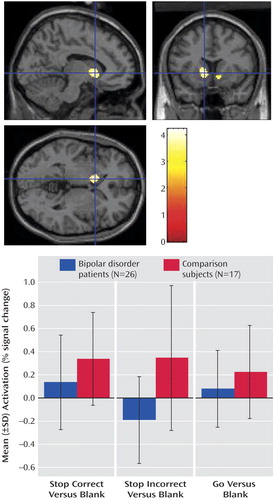
a Data shown at p<0.005, whole-brain uncorrected for the purposes of presentation in upper image (peak activation in left striatum: x=–10, y=12, z=0; t=4.22, k E =395, p=0.002). Activation in the left striatum at x=–10, y=12, z=0 in comparison subjects and patients with bipolar disorder in the lower image.
Effect of Medication
The two patient groups (medicated and unmedicated) did not differ from each other in any region of interest. However, each patient group did differ from the comparison subjects. Specifically, the comparison subjects had greater activation than the unmedicated patients in the bilateral accumbens, caudate, and putamen, with a tendency toward increased activation in the ventral prefrontal cortex. Similarly, the comparison subjects had greater activation than the medicated patients in the left accumbens, left caudate, and right ventral prefrontal cortex, with a tendency toward greater activation in the right accumbens.
Effect of Comorbid ADHD
The patients with bipolar disorder and ADHD and the patients with bipolar disorder without ADHD did not differ in any region of interest. The comparison subjects had greater activation than the patients with bipolar disorder with ADHD in the striatum (bilateral accumbens and putamen and right caudate), bilateral anterior cingulate, and bilateral ventral prefrontal cortex. Differences between comparison subjects and patients with bipolar disorder without ADHD were limited to the striatum, with comparison subjects having greater activation in the bilateral accumbens and left caudate and a tendency toward greater activation in the left putamen.
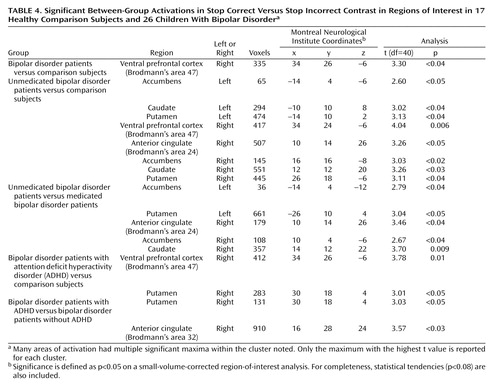
Stop Correct Versus Stop Incorrect ( Table 4 )
All Bipolar Patients Versus Comparison Subjects
The patients had increased activation in relation to the comparison subjects in the right ventral prefrontal cortex but did not differ in any other region of interest.
Effect of Medication
The medicated patients and the comparison subjects did not differ in any region of interest. The unmedicated patients had significantly greater activation than the comparison subjects in the bilateral accumbens, caudate, and putamen; in the right ventral prefrontal cortext; and in the right anterior cingulate. Similarly, the unmedicated patients had significantly greater activation than the medicated patients in the bilateral accumbens, left putamen, right anterior cingulate, and right caudate.
Effect of Comorbid ADHD
The patients with bipolar disorder without ADHD and the comparison subjects did not differ in any region of interest. The patients with bipolar disorder with ADHD had greater activation than the comparison subjects in the right putamen and the right ventral prefrontal cortex. The patients with bipolar disorder and ADHD also had greater activation than the patients with bipolar disorder without ADHD in the right putamen and right anterior cingulate.
Across-Condition Comparison at Peak Voxels
These results indicate greater striatal and right ventral prefrontal cortex activation in the comparison subjects than in the patients in the stop incorrect versus go contrast, and greater striatal and right ventral prefrontal cortex activation in the patients than in the comparison subjects in the stop correct versus stop incorrect contrast. These findings could both be due to increased striatal and ventral prefrontal cortex activation in comparison subjects versus patients on incorrect stop trials. To explore this possibility, for the right ventral prefrontal cortex and each striatal region of interest, we identified the voxel showing the greatest between-group difference in the stop incorrect versus go contrast. For that voxel, we plotted the mean contrast value on the stop correct versus blank, stop incorrect versus blank, and go versus blank contrasts. Blank null trials were used in these contrasts so that the responses to the other conditions could be estimated efficiently (27) . This yielded a series of histograms, one for each striatal area and one for the right ventral prefrontal cortex. Each histogram showed a similar pattern: i.e., the comparison subjects showed significantly greater activation than the patients on stop incorrect trials in all regions (see Figure 1 for a representative plot of activation in the left caudate).
Discussion
Given the importance of failed inhibition in the clinical presentation of pediatric bipolar disorder, we used rapid event-related fMRI to study the neural circuitry engaged during unsuccessful inhibitory trials (i.e., on the stop incorrect versus go and stop correct versus stop incorrect contrasts). Between-group differences on both contrasts were due to greater bilateral striatal and right ventral prefrontal cortex activation in the comparison subjects than in the patients with bipolar disorder in the stop incorrect condition. Although the data indicated that these deficits occur in both unmedicated and medicated patients, the role of comorbid ADHD is unclear.
Although stop signal reaction time did not differ between these small study groups, data from a larger sample indicated a tendency suggesting that patients with bipolar disorder may be slower than comparison subjects to inhibit prepotent responses (1) . Whether or not there are behavioral differences between patients with bipolar disorder and comparison subjects on the stop signal task, the question of whether the two groups engage similar regions while performing motor inhibition is of interest. Moreover, children with bipolar disorder have deficits on other motor regulation tasks (1 , 33) . Neuroimaging and basic research implicate the striatum in the learning and execution of motor programs (34) . Research in adults indicates striatal engagement with increasing neurocognitive load (35) , and animal research suggests that striatal dopaminergic neurons produce error signals (36) . Data presented here and elsewhere (1) suggest that motor inhibition deficits in pediatric bipolar disorder may reflect a failure to engage the striatum appropriately during failed inhibition.
Although another study found decreased striatal activation in adult patients with bipolar disorder versus comparison subjects (4) , our findings contrast with four studies showing increased—rather than decreased—striatal activation in patients with bipolar disorder versus comparison subjects (3 , 9 , 12 , 16) . We examined activation on a trial-by-trial basis in relation to subject behavior, unlike prior studies in which behavioral data were not obtained (3 , 16) , block designs were used (4 , 12) , or ceiling effects occurred (9) . Most important, these studies used different behavioral paradigms. We observed increased striatal activation in the patients versus the comparison subjects (i.e., a pattern opposite to the one here) when the subjects processed emotional stimuli (37) , suggesting that patterns of striatal activation may vary as a function of the behavioral paradigm. A similar phenomenon has been reported with regard to engagement of the dorsolateral prefrontal cortex in patients with schizophrenia (38) .
These results may elucidate how striatal dysfunction contributes to disinhibition and other impairments in pediatric bipolar disorder. We speculate that in patients with bipolar disorder, the lack of a striatal error signal during failed inhibition contributes to failures in motor learning and thus to motor regulatory deficits. Such deficits in motor regulation may in turn be related to the emotional dysregulation that is the hallmark of bipolar disorder because data in comparison children suggest that irritability and decreased motor inhibition may be related mechanistically. That is, in comparison children, impaired motor inhibition (i.e., increased stop signal reaction time) is associated with increased intensity of experienced anger (5) . If the impulsivity seen in patients with bipolar disorder across the developmental spectrum is associated with failure to engage the striatum in situations requiring motor inhibition, then therapeutic interventions that facilitate striatal engagement in such contexts might be explored.
We found between-group differences in the right ventral prefrontal cortex that, although somewhat less robust and consistent than those in the striatum, followed a similar pattern; i.e., the comparison subjects showed greater ventral prefrontal cortex activation than patients on failed inhibitory trials. The congruence of our ventral prefrontal cortex and striatal findings is consistent with that of nonhuman primate research indicating a functional circuit including these two regions (39) .
With three exceptions (4 , 17 , 18) , fMRI studies have not examined the impact of either medication or comorbid illnesses on neural activation in bipolar disorder. On a simple motor task, Caligiuri et al. (17) found that cortical and subcortical activation differed more markedly between unmedicated bipolar adults and comparison subjects than between medicated bipolar adults and comparison subjects. Similarly, two other studies (4 , 18) and ours found more marked differences in activation between unmedicated bipolar patients and comparison subjects than between medicated bipolar patients and comparison subjects, suggesting that studies of medicated patients might be prone to type II, rather than type I, errors.
To our knowledge, only one previous study (19) has considered the impact of comorbid ADHD on fMRI results. We found that children with bipolar disorder with or without ADHD differed from comparison subjects in striatal activation during failed inhibition (i.e., on the stop incorrect versus go contrast). However, on the stop correct versus stop incorrect contrast, the patients with bipolar disorder and ADHD but not the patients with bipolar disorder without ADHD differed from the comparison subjects, and the patients with bipolar disorder and ADHD differed from the patients with bipolar disorder without ADHD in activation on several regions of interest. Thus, the impact of comorbidity on fMRI results in pediatric bipolar disorder may depend on the specifics of the contrast and the behavioral paradigm. Ultimately, our results cannot dissociate abnormalities due to bipolar disorder from those due to ADHD. It is also unclear whether the pathophysiology of ADHD symptoms is the same in children with, versus without, bipolar disorder or whether ADHD in children with bipolar disorder is a phenocopy of more common forms of ADHD. Indeed, although we found increased right ventral prefrontal cortex activation in patients with bipolar disorder and ADHD versus comparison subjects on the stop correct versus stop incorrect contrast, some (21) , but not other (40) , studies have found that children with ADHD in relation to comparison subjects have reduced activation in the right inferior frontal prefrontal cortex during successful inhibition (21) . Follow-up research might compare fMRI data from children with ADHD only to children with bipolar disorder. Also, further study is needed to ascertain the impact of mood state on our results. Because 21 of 26 patients included were euthymic, the deficits we identified may be trait related, but more definitive work is needed to dissociate the effects of both mood state and comorbid ADHD.
In sum, our data indicate that in relation to comparison subjects, children with bipolar disorder may have deficits in their ability to engage striatal structures and the right ventral prefrontal cortex during unsuccessful inhibition. These results may give clues to the pathophysiology of disinhibition and impulsivity in bipolar disorder.

1. McClure EB, Treland JE, Snow J, Schmajuk M, Dickstein DP, Towbin KE, Charney DS, Pine DS, Leibenluft E: Deficits in social cognition and response flexibility in pediatric bipolar disorder. Am J Psychiatry 2005; 162:1644–1651Google Scholar
2. Swann AC, Dougherty DM, Pazzaglia PJ, Pham M, Moeller FG: Impulsivity: a link between bipolar disorder and substance abuse. Bipolar Disord 2004; 6:204–212Google Scholar
3. Blumberg HP, Martin A, Kaufman J, Leung HC, Skudlarski P, Lacadie C, Fulbright RK, Gore JC, Charney DS, Krystal JH, Peterson BS: Frontostriatal abnormalities in adolescents with bipolar disorder: preliminary observations from functional MRI. Am J Psychiatry 2003; 160:1345–1347Google Scholar
4. Strakowski SM, Adler CM, Holland SK, Mills NP, Delbello MP, Eliassen JC: Abnormal fMRI brain activation in euthymic bipolar disorder patients during a counting Stroop interference task. Am J Psychiatry 2005; 162:1697–1705Google Scholar
5. Hoeksma JB, Oosterlaan J, Schipper EM: Emotion regulation and the dynamics of feelings: a conceptual and methodological framework. Child Dev 2004; 75:354–360Google Scholar
6. Blumberg HP, Leung HC, Skudlarski P, Lacadie CM, Fredericks CA, Harris BC, Charney DS, Gore JC, Krystal JH, Peterson BS: A functional magnetic resonance imaging study of bipolar disorder: state- and trait-related dysfunction in ventral prefrontal cortices. Arch Gen Psychiatry 2003; 60:601–609Google Scholar
7. Elliott R, Ogilvie A, Rubinsztein JS, Calderon G, Dolan RJ, Sahakian BJ: Abnormal ventral frontal response during performance of an affective go/no go task in patients with mania. Biol Psychiatry 2004; 55:1163–1170Google Scholar
8. Strakowski SM, Adler CM, Holland SK, Mills N, Delbello MP: A preliminary fMRI study of sustained attention in euthymic, unmedicated bipolar disorder. Neuropsychopharmacology 2004; 29:1734–1740Google Scholar
9. Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips ML: Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biol Psychiatry 2004; 55:578–587Google Scholar
10. Blumberg HP, Stern E, Ricketts S, Martinez D, de Asis J, White T, Epstein J, Isenberg N, McBride PA, Kemperman I, Emmerich S, Dhawan V, Eidelberg D, Kocsis JH, Silbersweig DA: Rostral and orbital prefrontal cortex dysfunction in the manic state of bipolar disorder. Am J Psychiatry 1999; 156:1986–1988Google Scholar
11. Rubinsztein JS, Fletcher PC, Rogers RD, Ho LW, Aigbirhio FI, Paykel ES, Robbins TW, Sahakian BJ: Decision-making in mania: a PET study. Brain 2001; 124:2550–2563Google Scholar
12. Chang K, Adleman NE, Dienes K, Simeonova DI, Menon V, Reiss A: Anomalous prefrontal-subcortical activation in familial pediatric bipolar disorder: a functional magnetic resonance imaging investigation. Arch Gen Psychiatry 2004; 61:781–792Google Scholar
13. Lennox BR, Jacob R, Calder AJ, Lupson V, Bullmore ET: Behavioural and neurocognitive responses to sad facial affect are attenuated in patients with mania. Psychol Med 2004; 34:795–802Google Scholar
14. Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW: Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci 2003; 6:115–116Google Scholar
15. Rubia K, Smith AB, Brammer MJ, Taylor E: Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage 2003; 20:351–358Google Scholar
16. Malhi GS, Lagopoulos J, Sachdev P, Mitchell PB, Ivanovski B, Parker GB: Cognitive generation of affect in hypomania: an fMRI study. Bipolar Disord 2004; 6:271–285Google Scholar
17. Caligiuri MP, Brown GG, Meloy MJ, Eberson SC, Kindermann SS, Frank LR, Zorrilla LE, Lohr JB: An fMRI study of affective state and medication on cortical and subcortical brain regions during motor performance in bipolar disorder. Psychiatry Res 2003; 123:171–182Google Scholar
18. Blumberg HP, Donegan NH, Sanislow CA, Collins S, Lacadie C, Skudlarski P, Gueorguieva R, Fulbright RK, McGlashan TH, Gore JC, Krystal JH: Preliminary evidence for medication effects on functional abnormalities in the amygdala and anterior cingulate in bipolar disorder. Psychopharmacology (Berl) 2005; 183:308–313Google Scholar
19. Adler CM, Delbello MP, Mills NP, Schmithorst V, Holland S, Strakowski SM: Comorbid ADHD is associated with altered patterns of neuronal activation in adolescents with bipolar disorder performing a simple attention task. Bipolar Disord 2005; 7:577–588Google Scholar
20. Geller B, Luby J: Child and adolescent bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry 1997; 36:1168–1176Google Scholar
21. Rubia K, Smith AB, Brammer MJ, Toone B, Taylor E: Abnormal brain activation during inhibition and error detection in medication-naive adolescents with ADHD. Am J Psychiatry 2005; 162:1067–1075Google Scholar
22. Leibenluft E, Charney DS, Towbin KE, Bhangoo RK, Pine DS: Defining clinical phenotypes of juvenile mania. Am J Psychiatry 2003; 160:430–437Google Scholar
23. Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for Affective Disorders and Schizophrenia for School-Age Children: Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 1997; 36:980–988Google Scholar
24. Fristad MA, Weller EB, Weller RA: The Mania Rating Scale: can it be used in children? a preliminary report. J Am Acad Child Adolesc Psychiatry 1992; 31:252–257Google Scholar
25. Poznanski E, Mokros HB, Grossman J, Freeman LN: Diagnostic criteria in childhood depression. Am J Psychiatry 1985; 142:1168–1173Google Scholar
26. Logan GD, Schachar RJ, Tannock R: Impulsivity and inhibitory control. Psychol Sci 1997; 8:60–64Google Scholar
27. Friston KJ, Zarahn E, Josephs O, Henson RN, Dale AM: Stochastic designs in event-related fMRI. Neuroimage 1999; 10:607–619Google Scholar
28. Szeszko PR, Bilder RM, Lencz T, Pollack S, Alvir JM, Ashtari M, Wu H, Lieberman JA: Investigation of frontal lobe subregions in first-episode schizophrenia. Psychiatry Res 1999; 90:1–15Google Scholar
29. Peterson BS, Thomas P, Kane MJ, Scahill L, Zhang H, Bronen R, King RA, Leckman JF, Staib L: Basal ganglia volumes in patients with Gilles de la Tourette syndrome. Arch Gen Psychiatry 2003; 60:415–424Google Scholar
30. Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS: Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. Neuroimage 2005; 25:1279–1291Google Scholar
31. Monk CS, McClure EB, Nelson EE, Zarahn E, Bilder RM, Leibenluft E, Charney DS, Ernst M, Pine DS: Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage 2003; 20:420–428Google Scholar
32. McClure E, Monk C, Nelson E, Zarahn E, Leibenluft E, Bilder RM, Charney DS, Ernst M, Pine DS: A developmental examination of gender differences in brain engagement during evaluation of threat. Biol Psychiatry 2004; 55:1047–1055Google Scholar
33. Dickstein DP, Garvey M, Pradella A, Greenstein DK, Sharp WS, Castellanos FX, Pine DS, Leibenluft E: Neurological examination abnormalities in children with bipolar disorder or attention deficit hyperactivity disorder. Biol Psychiatry 2005; 58:517–524Google Scholar
34. Rolls ET, Hornak J, Wade D, McGrath J: Emotion-related learning in patients with social and emotional changes associated with frontal lobe damage. J Neurol Neurosurg Psychiatry 1994; 57:1518–1524Google Scholar
35. Bullmore E, Suckling J, Zelaya F, Long C, Honey G, Reed L, Routledge C, Ng V, Fletcher P, Brown J, Williams SC: Practice and difficulty evoke anatomically and pharmacologically dissociable brain activation dynamics. Cereb Cortex 2003; 13:144–154Google Scholar
36. Schultz W: Neural coding of basic reward terms of animal learning theory, game theory, microeconomics and behavioural ecology. Curr Opin Neurobiol 2004; 14:139–147Google Scholar
37. Rich BA, McClure E, Vinton D, Roberson-Nay R, Pine DS, Leibenluft E: Preliminary investigation of misinterpretation of facial expressions in childhood anxiety, bipolar disorder, and their comorbidity. Depress Anxiety 2005; 22:226–227Google Scholar
38. Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR: Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry 2003; 160:2209–2215Google Scholar
39. Ferry AT, Ongur D, An X, Price JL: Prefrontal cortical projections to the striatum in macaque monkeys: evidence for an organization related to prefrontal networks. J Comp Neurol 2000; 425:447–470Google Scholar
40. Schulz KP, Tang CY, Fan J, Marks DJ, Newcorn JH, Cheung AM, Halperin JM: Differential prefrontal cortex activation during inhibitory control in adolescents with and without childhood attention-deficit/hyperactivity disorder. Neuropsychology 2005; 19:390–402Google Scholar