Reduction of Cancer-Specific Thought Intrusions and Anxiety Symptoms With a Stress Management Intervention Among Women Undergoing Treatment for Breast Cancer
Abstract
Objective: After surgery for breast cancer, many women experience anxiety relating to the cancer that can adversely affect quality of life and emotional functioning during the year postsurgery. Symptoms such as intrusive thoughts may be ameliorated during this period with a structured, group-based cognitive behavior intervention. Method: A 10-week group cognitive behavior stress management intervention that included anxiety reduction (relaxation training), cognitive restructuring, and coping skills training was tested among 199 women newly treated for stage 0-III breast cancer. They were then followed for 1 year after recruitment. Results: The intervention reduced reports of thought intrusion, interviewer ratings of anxiety, and emotional distress across 1 year significantly more than was seen with the control condition. The beneficial effects were maintained well past the completion of adjuvant therapy. Conclusions: Structured, group-based cognitive behavior stress management may ameliorate cancer-related anxiety during active medical treatment for breast cancer and for 1 year following treatment. Group-based cognitive behavior stress management is a clinically useful adjunct to offer to women treated for breast cancer.
Approximately 216,000 new cases of breast cancer were diagnosed in the United States in 2004 (1) . Diagnosis and treatment of breast cancer are clearly stressful events (2) involving invasive medical procedures with aversive side effects of treatment such as pain, nausea, vomiting, and fatigue (3) . Recurring thoughts about the diagnosis and treatment are common (4) . Although diagnostic levels of posttraumatic stress disorder (PTSD) are relatively rare (5) , PTSD-like symptoms and subthreshold levels of PTSD are more frequent (6 , 7) . After treatment, many survivors experience residual psychological strain from the diagnosis, shifts in social support, fear of recurrence, and fear of death (8) .
Factors that influence distress after breast cancer treatment include coping style and social support (6 , 9 , 10) . Prospective studies reveal that optimism (11) , coping strategies such as positive reframing and acceptance (11 , 12) , and social support (13) yield less distress in the months after treatment. Similarly, psychosocial interventions that foster optimistic appraisals, build coping strategies, and bolster social support should benefit women treated for breast cancer (14) .
Psychosocial interventions for persons with cancer and other chronic illnesses typically use cognitive behavior techniques, often incorporating skills training and relaxation training. The interventions typically focus on reducing general distress, and they appear effective (15 – 17) . However, most studies test interventions in heterogeneous patient groups, such as subjects with different types and stages of cancer at very different points in their treatment experience. It seems desirable to target more specifically the concerns of a particular form of cancer at a particular stage of treatment, such as breast cancer patients who recently had surgery and are now receiving adjuvant therapy. Concerns especially salient during this period would include recurrence, abandonment by friends, and damage from toxic adjuvant therapy (8) .
A structured cognitive behavior intervention reduced general distress among stage II and stage III breast cancer patients in one study (18) , but only among women who entered the study with elevated symptoms of cancer-related intrusive thoughts, as measured by the Impact of Event Scale. That study also did not evaluate effects beyond postintervention (4 months), and it did not test whether the intervention reduced those intrusive thoughts. In another study, a psycho-educational intervention improved quality of life among stage I–III breast cancer patients (19) , although effects were restricted to general measures of functioning. Yet another study (20) found that supportive-expressive group psychotherapy reduced cancer-related distress among metastatic breast cancer patients during the year after diagnosis, but the study design did not include random assignment of patients to a treatment condition.
We have developed a cognitive behavior stress management intervention for breast cancer patients designed to facilitate adjustment during and after treatment (14) . It provides training in anxiety-reduction skills (muscle relaxation, guided imagery), cognitive restructuring, coping skills, and interpersonal skills with a supportive group of breast cancer patients at similar points in medical treatment. In one trial, this intervention increased perceptions of benefit from the cancer experience after surgery for stage I and stage II breast cancer, and the effects persisted a year after treatment (21) .
This article reports the findings of a new randomized trial. It focused specifically on reducing anxiety and distress during the period of medical treatment for nonmetastatic breast cancer. All participants had undergone surgery just before enrollment, and most were about to begin adjuvant therapy. We expected the intervention to decrease intrusive thoughts over the short-term and well after the end of medical treatment. Secondary measures were clinician-rated anxiety symptoms and general emotional distress.
Method
Participants
Participants were 199 nonmetastatic breast cancer patients. Some received letters from their physician, others from the American Cancer Society. The study was described as an opportunity for women undergoing treatment for breast cancer to learn stress management. Interested women called and spoke with a female assistant who screened for eligibility. Participants were required to have been diagnosed with breast cancer at stage III or below and to have had surgery within the past 8 weeks. Potential participants with prior cancer (N=35), prior psychiatric treatment for a serious disorder (hospitalization or a formal diagnosis of psychosis, major depressive episode, panic attacks, suicidality, or substance dependence [N=17]), or a lack of fluency in English (N=3) were excluded. Of women contacted by letter, approximately 70% called for information; of those who called and met inclusion criteria, 57.2% participated in the first assessment ( Figure 1 ).
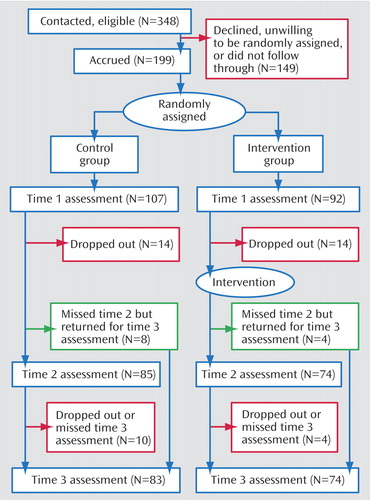
The outcome variables were collected at study entry (time 1), and 6 and 12 months after entry (times 2 and 3). Attrition is described in Figure 1 . Attrition did not differ significantly by condition at time 2 (χ 2 =0.40, df=1, p>0.54) or time 3 (χ 2 =1.21, df=1, p>0.38). We used an intent-to-treat analysis, estimating missing data using full information maximum likelihood (subsequently described); thus the entire sample was represented in all analyses.
At each time point, those who dropped out were compared on key variables with those retained. Those stopping before time 2 were more likely to be Hispanic (χ 2 =16.89, df=2, p<0.001) and younger (F=8.06, df= 1, 197, p<0.005). There were no significant differences in terms of cancer stage (χ 2 =6.60, df=3, p>0.08), number of positive nodes (F=0.33, df=1, 197, p>0.55), marital status (χ 2 =0.82, df=1, p>0.40), presence versus absence of chemotherapy (χ 2 =0.00, df=1, p>0.99) or radiation (χ 2 =1.76, df=1, p>0.20), or any outcome variable assessed at time 1. Those who stopped between times 2 and 3 did not differ from completers on any outcome assessed at time 2 or on any medical or demographic variable.
Procedure
Participants completed initial assessment upon recruitment, 4–8 weeks postsurgery. They then were randomly assigned to the intervention or control condition. The intervention occurred over a 10-week period, beginning 10–12 weeks after surgery. Women in the control condition were invited to attend a 1-day educational seminar during this period (80 attended; attendance did not relate to any outcome variable). A second assessment occurred 3 months after the intervention ended (6 months after the initial assessment). A third assessment occurred 6 months later. Thus the period of follow-up spanned approximately 1 year after random assignment.
Participants in both conditions met in groups of up to eight in a room equipped with flat couches for muscle relaxation exercises, and a table and chairs for group discussions. Both the intervention and the control seminar were co-led by female postdoctoral fellows and advanced predoctoral trainees in clinical psychology. Leaders rotated between intervention and control cohorts. Assessments were handled by persons not conducting the intervention with that cohort.
Intervention condition
The closed, structured group intervention met weekly for 10 2-hour sessions (14) . It interwove cognitive behavior stress management techniques with didactics, including in-session experiential exercises and out-of-session assignments (e.g., practicing relaxation). Women received recordings of their group leader reciting relaxation exercises, which they were urged to use daily. The intervention aimed at coping better with daily stressors and optimizing use of social resources, focusing on cancer- and treatment-related issues. The intervention used group members and leaders as role models (for positive social comparisons and social support); encouraged emotional expression; replaced doubt appraisals with confidence (22) ; honed skills in anxiety reduction (by muscle relaxation and relaxing imagery [23] ) and skills in conflict resolution and emotional expression (via assertion training [24] ). On average, participants attended 7.08 sessions (SD=2.58, median=8, range=1–10).
Control condition
Participants in the control condition received a condensed educational version of the information from the intervention, lasting 5–6 hours. However, it lacked the therapeutic group environment and emotional support, the opportunity to hear group members’ weekly frustrations and triumphs, opportunities to role play the techniques and receive group feedback, the weekly home practice, and the opportunity to observe other members model new appraisals, relaxation techniques, and coping strategies.
This procedure has at least two benefits over a no-treatment control condition. By providing information relevant to breast cancer experiences, it diminishes differential attrition in the control condition—a major pitfall of no-treatment control. Providing information related to adjustment also creates a stronger test of the intervention’s impact. The main drawback is that this control provides a dose of most ingredients of the intervention, thus working against predictions. This procedure does not control for attention time, with less than one-third the contact hours of the cognitive behavior stress management groups (20 versus 6 hours). However, our aim was to evaluate whether the intervention would have specific and durable effects on cancer-related anxiety symptoms in women undergoing active medical treatment.
Measures
Thought intrusion and avoidance
Our primary measure assessed intrusive thoughts about breast cancer and attempts to suppress such thoughts, both important indicators of event-related distress. The Impact of Event Scale is a 15-item self-report instrument assessing degree of thought intrusion and avoidance about particular life situations (here the diagnosis of and treatment for breast cancer), with response options coded 0, 1, 3, and 5. The Impact of Event Scale has two subscales. The intrusion scale measures the extent of unwanted thoughts and images related to the stressor (e.g., “I had trouble falling asleep or staying awake because pictures or thoughts about it came into my mind”). Our average alpha reliability was 0.86. The avoidance scale assesses conscious attempts to prevent oneself from thinking about the situation (e.g., “I tried not to think about it”). Average alpha was 0.80. For each scale, item responses were averaged.
Interviewer-rated anxiety
A secondary measure was anxiety symptoms measured by the Hamilton Rating Scale for Anxiety. Procedures for scoring these clinical ratings followed the format of the structured interview guide. High interrater reliability, internal consistency, and discriminant validity have been reported for this scale (25) . Assessors making the ratings were trained by a clinical psychologist with extensive training in the use of this measure.
Emotional distress
Another secondary measure was emotional distress, measured with the Affects Balance Scale (26) . This measure, used in prior breast cancer research (27) , has scales assessing negative affect, depression, hostility, guilt, and anxiety across the past week. A negative total can also be computed (28) . Items are emotion-descriptive adjectives; respondents indicate the extent to which they have been feeling each emotion on a scale ranging from 1 (never) to 5 (always). Item responses were averaged. Average alpha was 0.86.
Data Analysis
Intervention effects were tested by latent growth-curve modeling (29 – 31) , a form of structural equation modeling. In latent growth-curve modeling, a trajectory of change over measurements is computed for each participant. Differences among persons in the properties of these curves then can be predicted from other variables (32) . The properties of interest are the intercept (the trajectory’s starting value) and the slope of change over repeated measurements. These properties were modeled as latent variables from the data collected at times 1, 2, and 3. The key predictor was intervention versus control condition (coded as 1 versus 0). For the slope, loadings represent the time variable tied to each assessment point: 0 represents the initial assessment, 6 represents the 6 months elapsed until the second assessment, and 12 the time elapsed until the third assessment. The structure of this model is shown in Figure 2 .
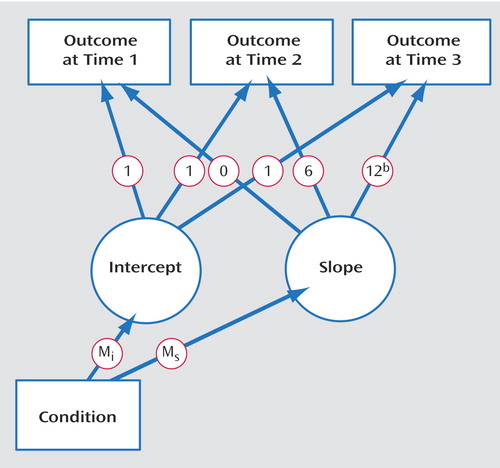
a Outcome variables at three assessments (at 6-month intervals) are used to define two latent variables (intercept and slope), with experimental condition (intervention versus control) used to predict those latent variables. M i is the differential effect of the intervention on the intercept of the growth curves; M s is the differential effect of the intervention on change over time.
b In some models tested, this loading was estimated rather than specified as 12.
We focus on paths from experimental condition to the intercept (M i ) and to the slope (M s ). The path from condition to intercept reflects the group difference in initial values. This path should be nonsignificant, reflecting no difference between groups at time 1. The path from condition to slope reflects the extent to which change in the dependent variable over time relates to experimental condition. We expect this effect to be significant, indicating a difference in mean trajectories between groups. This effect is analogous to a group-by-time interaction in repeated measures ANOVA.
An important advantage of latent growth-curve modeling over repeated measures ANOVA is its ability to use all available data (which is also true of random regression modeling). In ANOVA, participants who are missing any data are deleted. This reduces sample size (and power) and yields biased estimates, therefore compromising efforts to use an intent-to-treat approach (33) . This problem is particularly acute in clinical trials, which often have considerable missing data. Latent growth-curve modeling uses a process called full information maximum likelihood (FIML). FIML uses all available data for each person, estimating missing information from relations among variables in the full sample. These procedures have been shown to be quite robust even when there is a great deal of missing data (34) . Our analyses used FIML, implemented in the program Mplus (35) . Thus all participants are represented in all main analyses.
Another advantage of latent growth-curve modeling is its flexibility in addressing nonlinear change. For example, the benefit of an intervention may plateau rather than continue to grow. Latent growth-curve modeling can address such nonlinearities by estimating the later time point instead of specifying it. In effect, this draws a line from time 1 to 2, and estimates how many months would pass (at the current rate of change) by the time the line reached the level of the time-3 data point. If an outcome stopped changing entirely at time 2, time 3 would be estimated at 6 months; if it continued to change at a constant rate, time 3 would be estimated at 12 months. Random regression modeling does not incorporate this particular flexibility (although it has others) because it treats time as a variable rather than a loading. In the analyses reported here, we examined models in which time 3 was specified as 12 months after time 1 as well as models in which time 3 was freely estimated.
We report several standard indices of model fit, including the chi-square statistic, testing the null hypothesis that the specified model fits the pattern of associations in the data (the ideal is a nonsignificant chi-square). We also report comparative fitness index, for which values above 0.95 indicate good fit; the root mean square error of approximation, for which values below 0.05 indicate good fit; and the standardized root mean square residual, for which values below 0.10 indicate good fit (36) . Specific effects were tested with the z statistic, using a 0.05 two-tailed significance level throughout. Effect sizes are reported as Cohen’s d, for which values of 0.20 are regarded as relatively small, 0.50 as medium, and 0.80 as large (37) .
Results
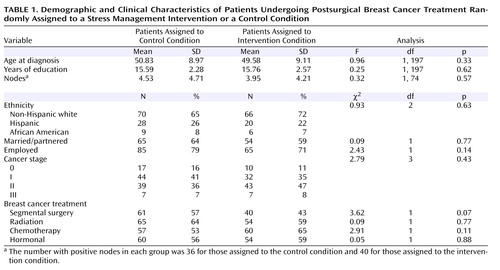
Characteristics of the participants, by assigned condition, are presented in Table 1 . Comparisons revealed no significant difference between conditions. All variables in Table 1 were examined as potential control variables. Most were tested as direct influences on the latent variables (direct projections to intercept and slope in Figure 2 ). However, life stress unrelated to breast cancer varied greatly across assessments; thus it was examined for separate effects on the outcomes at separate time points. We included control variables only if they contributed significantly or improved overall model fit. Only noncancer stress had such an effect (only on general distress) and thus was included in that model.
Thought Intrusion and Avoidance
In the analysis of thought intrusion (per the Impact of Event Scale), the model specifying time 3 as 12 months after time 1 did not fit the data (this analysis had no control variable). When time 3 was allowed to be freely estimated, however, model fit was very good (χ 2 =0.15, df=1, p=0.69; comparative fit index=1.00; root mean square error of approximation=0.00; standardized root mean square residual=0.006). The value estimated for time 3 was 7.02 months, indicating only slight changes from time 2 to time 3. Experimental condition did not predict intercept (z=0.83), indicating success of randomization.
Condition did significantly predict slope (z=3.64, p<0. 001; Cohen’s d=1.22). Estimated values for the two conditions at the three assessments are in Figure 3 . The mean difference between groups at time 2 was tested by centering the intercept at time 2 and recomputing the model. Condition now had a significant relation to intercept (z=2.38, p<0. 03; Cohen’s d=0.43), indicating a significant difference at 6 months. A similar test of the difference at time 3 also indicated a significant difference (z=2.86, p<0.005; Cohen’s d=0.55).
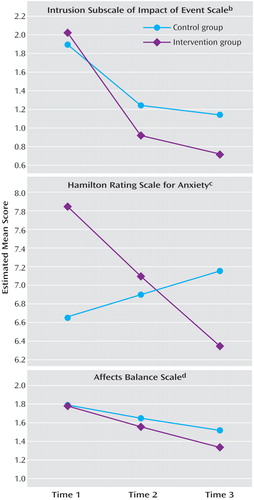
a For thought intrusions, time 3 was estimated as 7.02 months (to yield the best linear relation) rather than 12 months. Means incorporate all observed data and all data estimated using full information maximum likelihood procedures for missing assessments.
b Group effect on slope: z=3.64, p<0.001; Cohen’s d=1.22. Groups differ at time 2 (z=2.38, p<0.03; Cohen’s d=0.43) and time 3 (z=2.86, p<0.005; Cohen’s d=0.29).
c Group effect on slope: z=2.71, p<0.003; Cohen’s d=0.74. No significant between-group differences at any time.
d Group effect on slope: z=2.48, p<0.02; Cohen’s d=0.33. Groups differ at time 3 (z=2.63, p<0.01; Cohen’s d=0.43).
In contrast, the intervention did not affect avoidance of cancer-related thoughts. Avoidance fell significantly over time, but not varying by condition.
Anxiety
The next dependent variable was the Hamilton anxiety symptom score (again no control variable). The model fit the data well when time was specified as 0, 6, and 12 months (χ 2 =0.32, df=2, p=0.85; comparative fit index=1.00; root mean square error of approximation=0.00; standardized root mean square residual=0.008). Condition related significantly to slope (z=2.71, p<0.004; Cohen’s d=0.74), indicating differential change across time. Condition’s effect on intercept was not significant (z=1.47), despite the tendency of the experimental group toward higher anxiety ratings at baseline than the control group ( Figure 3 ); nor was condition’s effect on intercept significant at time 3 (z=1.36), despite a tendency in the opposite direction.
Emotional Distress
The final outcome was the Affects Balance Scale index of negative emotions, similar to outcomes used in many psychosocial intervention studies with cancer patients. Concurrent stress unrelated to cancer was included as a control variable at each assessment, since doing so improved model fit significantly. The overall model fit the data well when time was specified as 0, 6, and 12 months (χ 2 =12.86, df=8, p=0.12). However, modification indices suggested inclusion of two additional paths, from noncancer stress at time 1 to both the intercept and the slope of the latent variable. This indicates that initial noncancer stress had a residual effect on negative emotions throughout the subsequent year. Including those paths improved overall model fit significantly, resulting in a very good fit (χ 2 =2.26, df=6, p=0.89; comparative fit index=1.00; root mean square error of approximation=0.00; standardized root mean square residual=0.019). Condition did not predict variation in intercept (z=–0.092) but had a significant relation to slope (z=2.48, p<0.02; Cohen’s d=0.33) ( Figure 3 ). The effect of condition on distress was most evident at the 12-month follow-up assessment, where the difference was significant (z=2.63, p<0.01; Cohen’s d=0.43).
Discussion
Perhaps the most common psychological challenge of cancer patients is anxiety from diagnosis, effects of adjuvant therapy, and fear of recurrence (8 , 18 , 38) . Thought intrusions about these adversities are commonly experienced symptoms among breast cancer patients—symptoms that can compromise quality of life beyond the physical demands of these medical treatments (4) .
We tested whether a group-based cognitive behavior stress management intervention would reduce such symptoms among women emerging from surgery and moving through adjuvant therapy. The intervention blended skill learning with exposure to group members’ weekly experiences, opportunities to role play, and opportunities to observe other members modeling new appraisals, relaxation techniques, and coping strategies, all within a supportive group environment. Women receiving cognitive behavior stress management showed a significant reduction in cancer-specific thought intrusions relative to those in the control condition. This effect persisted at 9 months postintervention. Since most women were completing adjuvant therapy by the second assessment, the third assessment reflects the durability of this effect.
Our prior trial (21) did not find this effect on intrusive thoughts. One potentially important difference between studies is that the current group had higher intrusion scores on the Impact of Event Scale than our prior patient group. In fact, scores in this present group exceeded those of several other recent studies, including one with metastatic disease (6 , 39 , 40) . It is likely that beneficial effects of cognitive behavior stress management depend partly on there being enough thought intrusion that there is room for change to occur.
Cognitive behavior stress management had a smaller but significant effect on interviewer-rated assessments of general anxiety symptoms. To our knowledge, this is the first study to demonstrate psychosocial intervention effects on interviewer-rated anxiety among women undergoing treatment for breast cancer. Cognitive behavior stress management caused a downward trajectory in anxiety ratings, whereas subjects in the control condition had no comparable decline over time. Unfortunately, a tendency toward higher Hamilton scores in the cognitive behavior stress management group at baseline led to a crossover effect, such that the groups did not differ significantly at any time point.
On general distress—a typical measure in psychosocial interventions with cancer patients—cognitive behavior stress management also caused significant reduction compared with the control condition. It is of interest that general distress, although not cancer-related thought intrusion or rated anxiety, responded both to the intervention and to stressors outside the cancer experience. This suggests the desirability of distinguishing measures specific to the experience of cancer from measures of overall distress. The latter reflect an amalgam of experiences, both cancer-related and otherwise. This distinction is rarely made in the psycho-oncology literature. It seems important, however, for researchers to include measures that are sensitive and specific to the breast cancer experience, particularly in testing effects of psychosocial interventions.
We should note some study limitations. The patient group was self-selected, middle-class, educated, and mostly white. There remains a need for tests of culturally appropriate interventions among Hispanic and African American women, whose concerns may or may not be the same as those of the women studied here (41) . Further, although the intervention was effective on some measures, it did not influence active avoidance of cancer-related thoughts. Finally, not all variables that might be relevant to these outcomes were examined (e.g., days since completion of adjuvant therapy, medications such as antiemetics for treatment side effects).
Nonetheless, the effects were moderately large in size (37) , meeting or exceeding those in other group interventions (42) , even some with far shorter follow-up periods (18) . Thus, it appears that a group-based stress management intervention can significantly decrease cancer-specific intrusive thoughts, general anxiety symptoms, and overall negative mood in women who are moving through their medical treatment for breast cancer. These beneficial effects are more pronounced than those from a more limited psychoeducational experience (43) . Further, they persisted at least 9 months after the completion of the intervention, well past completion of adjuvant therapy. Cognitive behavior stress management thus may be a clinically useful adjunct for women treated for breast cancer.
1. American Cancer Society: Cancer Facts and Figures, 2004. Atlanta, American Cancer Society, 2004Google Scholar
2. van’t Spijker A, Trijsberg R, Duivenvoorden H: Psychological sequelae of cancer diagnosis: a meta-analytic review of 58 studies after 1980. Psychosom Med 1997; 59:280–293Google Scholar
3. Jacobsen PB, Bovbjerg DH, Schwartz MD, Hudis CA, Gilewski TA, Norton L: Conditioned emotional distress in women receiving chemotherapy for breast cancer. J Consult Clin Psychol 1995; 63:108–114Google Scholar
4. Jacobsen PB, Widows M, Hann D: Posttraumatic stress disorder symptoms after bone marrow transplantation for breast cancer. Psychosom Med 1998; 60:366–371Google Scholar
5. Kangas M, Henry JL, Bryant RA: Posttraumatic stress disorder following cancer: a conceptual and empirical review. Clin Psychol Rev 2002; 22:499–524Google Scholar
6. Cordova MJ, Andrykowski MA, Kenady DE, McGrath PC, Sloan DA, Redd WH: Frequency and correlates of posttraumatic-stress-disorder-like symptoms after treatment for breast cancer. J Consult Clin Psychol 1995; 63:981–986Google Scholar
7. Green BL, Rowland JH, Krupnick JL, Epstein SA, Stockton P, Stern NM, Spertus IL, Steakley C: Prevalence of posttraumatic stress disorder in women with breast cancer. Psychosomatics 1998; 39:102–111Google Scholar
8. Spencer SM, Lehman JM, Wynings C, Arena P, Carver CS, Antoni MH, Derhagopian RP, Ironson G, Love N: Concerns about breast cancer and relations to psychosocial well-being in a multi-ethnic sample of early stage patients. Health Psychol 1999; 18:159–169Google Scholar
9. Glanz K, Lerman C: Psychosocial impact of breast cancer: a critical review. Ann Behav Med 1992; 14:204–212Google Scholar
10. Helgeson VS, Synder P, Seltman H: Psychological and physical adjustment to breast cancer over 4 years: identifying distinct trajectories of change. Health Psychol 2004; 23:3–15Google Scholar
11. Carver CS, Pozo C, Harris SD, Noriega V, Scheier MF, Robinson DS, Ketcham, AS, Moffat FL, Clark KC: How coping mediates the effect of optimism on distress: a study of women with early stage breast cancer. J Personal Soc Psychol 1993; 65:375–390Google Scholar
12. Stanton AL, Snider PR: Coping with a breast cancer diagnosis: a prospective study. Health Psychol 1993; 12:16–23Google Scholar
13. Alferi SM, Carver CS, Antoni MH, Weiss S, Durán RE: An exploratory study of social support, distress, and life disruption among low-income Hispanic women under treatment for early stage breast cancer. Health Psychol 2001; 20:41–46Google Scholar
14. Antoni MH: Stress Management Intervention for Women With Breast Cancer. Washington, DC, American Psychological Association, 2003Google Scholar
15. Andersen BL: Psychological interventions for cancer patients to enhance the quality of life. J Consult Clin Psychol 1992; 60:552–568Google Scholar
16. Trijsburg RW, van Knippenberg FCE, Rijpma SE: Effects of psychological treatment on cancer patients: a critical review. Psychosom Med 1992; 54:489–517Google Scholar
17. Andersen BL: Biobehavioral outcomes following psychological interventions for cancer patients. J Consult Clin Psychol 2002; 70:590–610Google Scholar
18. Andersen BL, Farrar W, Golden-Kreutz D, Glaser R, Emery C, Crespin T, Shapiro C, Carson W: Psychological, behavioral and immune changes after a psychological intervention: a clinical trial. J Clin Oncol 2004; 22:3570–3580Google Scholar
19. Helgeson VS, Cohen S, Schulz R, Yasko J: Education and peer discussion group interventions and adjustment to breast cancer. Arch Gen Psychiatry 1999; 56:340–347Google Scholar
20. Classen C, Butler LD, Koopman C, Miller E, DiMiceli S, Giese-Davis J, Fobair P, Carlson RW, Kraemer HC, Spiegel D: Supportive-expressive group therapy and distress in patients with metastatic breast cancer. Arch Gen Psychiatry 2001; 58:494–501Google Scholar
21. Antoni MH, Lehman JM, Kilbourn KM, Boyers AE, Culver JL, Alferi SM, Yount SE, McGregor BA, Arena PL, Harris SD, Price AA, Carver CS: Cognitive-behavioral stress management intervention decreases the prevalence of depression and enhances benefit finding among women under treatment for early-stage breast cancer. Health Psychol 2001; 20:20–32Google Scholar
22. Beck AT, Emery G: Anxiety Disorders and Phobias: A Cognitive Perspective. New York, Basic Books, 1985Google Scholar
23. Bernstein B, Borkovec T: Progressive Muscle Relaxation Training: A Manual for the Helping Professions. Champaign, Ill, Research Press, 1973Google Scholar
24. Fensterheim H, Baer J: Don’t Say Yes When You Want to Say No. New York, David McKay, 1975Google Scholar
25. Schwab JJ, Bialow MR, Clemmons RS, Holzer CE: Hamilton rating scale for depression with medical in-patients. Br J Psychiatry 1967; 113:83–88Google Scholar
26. Derogatis LR: The Affects Balance Scale. Baltimore, Clinical Psychometric Research, 1975Google Scholar
27. Northouse LL: A longitudinal study of the adjustment of patients and husbands to breast cancer. Onc Nursing Forum 1990; 17:39–43Google Scholar
28. Derogatis LR, Abeloff MD, Melisaratos N: Psychological coping mechanisms and survival time in metastatic breast cancer. JAMA 1979; 242:1504–1508Google Scholar
29. Duncan TE, Duncan SC, Strycker LA, Li F, Alpert A: An Introduction to Latent Variable Growth Curve Modeling: Concepts, Issues, and Applications. Mahwah, NJ, Erlbaum, 1999Google Scholar
30. Llabre MM, Spitzer SB, Saab PG, Schneiderman N: Piecewise latent growth curve modeling of systolic blood pressure reactivity and recovery from the cold pressor test. Psychophysiol 2001; 38:951–960Google Scholar
31. Muthen B: Latent variable modeling with longitudinal and multilevel data, in Sociological Methodology. Edited by Raferty A. Boston, Blackwell Scientific, 1997, pp 453–580Google Scholar
32. Llabre MM, Spitzer S, Siegel S, Saab PG, Schneiderman N: Applying latent growth curve modeling to the investigation of individual differences in cardiovascular recovery from stress. Psychosom Med 2004; 66:29–41Google Scholar
33. Enders CK: A primer on maximum likelihood algorithms available for use with missing data. Structural Equation Modeling 2001; 8:128–141Google Scholar
34. Schafer JL, Graham JW: Missing data: our view of the state of the art. Psychol Methods 2002; 7:147–177Google Scholar
35. Muthen LK, Muthen B: Mplus User’s Guide. Los Angeles, Muthen & Muthen, 1998Google Scholar
36. Kline R: Principles and Practice of Structural Equation Modeling, 2nd ed. New York, Guilford, 2005Google Scholar
37. Cohen J: Statistical Power Analysis for the Behavioral Sciences, 2nd ed. Hillsdale, NJ, Erlbaum, 1988Google Scholar
38. van der Pompe G, Antoni MH, Heijnen C: The effects of surgical stress and psychological stress on the immune function of operative cancer patients. Psychology and Health 1998; 13:1015–1026Google Scholar
39. Butler LD, Koopman C, Classen C, Spiegel D: Traumatic stress, life events, and emotional support in women with metastatic breast cancer: cancer-related traumatic stress symptoms associated with past and current stressors. Health Psychology 1999; 18:555–560Google Scholar
40. Epping-Jordan JE, Compas BE, Osowiecki DM, Oppedisano G, Gerhardt C, Primo K, Krag DN: Psychological adjustment in breast cancer: processes of emotional distress. Health Psychology 1999; 18:315–326Google Scholar
41. Chamberlain Wilmoth M, Sanders LD: Accept me for myself: African American women’s issues after breast cancer. Onc Nurs Forum 2001; 28:875–879Google Scholar
42. Kissane DW, Boloch S, Smith GC, et al: Cognitive-existential group psychotherapy for women with primary breast cancer: a randomised controlled trial. Psycho-Oncology 2003; 12:532–546Google Scholar
43. Fawzy FI, Fawzy NW, Wheeler JG: A post hoc comparison of the efficiency of a psychoeducational intervention for melanoma patients delivered in group versus individual formats: an analysis of data from two studies. Psycho-Oncology 1996; 5:81–89Google Scholar