Altered NMDA Glutamate Receptor Antagonist Response in Individuals With a Family Vulnerability to Alcoholism
Abstract
OBJECTIVE: A family history of alcoholism is a risk factor for the development of ethanol dependence. Ethanol is an antagonist of the N-methyl-d-aspartate (NMDA) glutamate receptor, and alterations in NMDA receptor function are thought to be involved in ethanol abuse and dependence. The purpose of this study was to determine in healthy individuals with no ethanol dependence whether response to the NMDA receptor antagonist ketamine would differentiate those with a family history of ethanol dependence from those without such a family history. METHOD: Healthy subjects between the ages of 21 and 30 received 40-minute intravenous infusions of saline, low-dose ketamine (0.1 mg/kg), and high-dose ketamine (0.5 mg/kg) on three separate test days in a randomized order under double-blind conditions. The healthy individuals with at least one first-degree relative and another first- or second-degree relative with ethanol dependence (N=16) were compared with those who had no family history of ethanol dependence in any first- or second-degree relative (N=29). Outcome measures included the Brief Psychiatric Rating Scale, Clinician-Administered Dissociative States Scale, verbal fluency, Hopkins Verbal Learning Test, a biphasic alcohol effects scale, visual analog scales of mood states, and ketamine levels. RESULTS: During ketamine infusion, individuals with a family history of ethanol dependence showed an attenuated response in terms of perceptual alterations and dysphoric mood relative to those without such a family history. CONCLUSIONS: These data suggest that alterations in NMDA receptor function may contribute to subjective response to ethanol and therefore also to the risk of developing alcoholism.
There is extensive evidence that a family history of alcoholism is a risk factor for the development of ethanol problems (1). A substantial part of the tendency for ethanol dependence to run in families is attributable to genetic factors (2). Healthy individuals with a family history of ethanol dependence exhibit a relatively lower intensity of subjective intoxication, ataxia, body sway, or flushing to moderate doses of ethanol in a laboratory setting than do healthy individuals with no family history of alcoholism. These findings have clinical importance, since follow-up studies have shown that a reduced sensitivity to the dysphoric or adverse effects of ethanol is the single strongest predictor of the subsequent development of alcoholism (3).
Antagonists of the N-methyl-d-aspartate (NMDA) receptor have provided an important mechanism for evaluating the pathophysiology of neuropsychiatric disorders, such as Alzheimer’s disease, anxiety, depression, and schizophrenia. For example, administration of the NMDA antagonist ketamine to healthy subjects and the subsequent cognitive deficits (4, 5) and perceptual changes (6–9) have led to further understanding of the pathophysiology of schizophrenia (6–9).
NMDA glutamate receptors are among the highest affinity ethanol targets in the brain (10). NMDA receptor antagonists substitute for ethanol in drug discrimination studies conducted in animals, particularly for higher ethanol doses (11–13). Postmortem studies of brain tissue suggest that certain subunits of NMDA receptors are increased in cortical structures of ethanol-dependent individuals (14), and in vivo ethanol withdrawal increases cerebrospinal fluid glutamate levels. The NMDA antagonist ketamine produced dose-related ethanol-like effects in recently detoxified ethanol-dependent patients, and the intensity and the degree of similarity of the ethanol-like effects of ketamine were greater at higher doses (7). Patients also showed reductions in their sensitivity to some of the negative effects (i.e., perceptual, mood, and cognitive effects) of ketamine (15). These group differences in ketamine response could reflect a response to prolonged ethanol exposure. However, ketamine response could also be a heritable trait marker for a family history of ethanol dependence.
The purpose of the current study was to examine whether healthy individuals at increased familial risk to develop ethanol dependence exhibit alterations in NMDA receptor function. This objective was evaluated by comparing among healthy individuals the dose-related effects of the NMDA receptor antagonist ketamine in those with a strong family history of ethanol dependence versus those with no such family history.
Method
Subjects
In this institutional review board-approved study, healthy individuals (N=45) were recruited by advertisements and compensated for their participation. The subjects included in the study 1) were between the ages of 21–30, 2) had no lifetime axis I psychiatric or substance use disorder, and 3) had biological mothers with no history of alcoholism, in order to ensure that any effects were not the result of direct toxicity of alcohol during pregnancy. Individuals with a history of consultation for an emotional difficulty, those with a psychiatric illness in a first- or second-degree relative, and those who were alcohol naive were excluded from participation.
After signing informed consent, subjects underwent a screening that included the Structured Clinical Interview for DSM-III-R (SCID) (16) to rule out any axis I disorders and a physical screening to ensure they were medically healthy. Subjects were asked to report their alcohol consumption for the 30 days preceding the intake assessment. Family history was formally evaluated by interviewing the subject to obtain the psychiatric status (including substance abuse/dependence, mood disorder, etc.) of all first- and second-degree biological relatives (including parents) using a questionnaire based on the SCID (H.R. Kranzler, personal communication, 2002). Subjects with a family history of ethanol dependence (N=16) had a biological father and another first- or second-degree biological relative with history of alcoholism. The remaining subjects (N=29) had no family history of alcoholism in any first or second-degree relatives. Eighteen of these 29 subjects included in the data analysis were individuals who met entry criteria for this study but who participated in a previous near-identical ketamine study, described in another report (6). There were no significant differences between groups in age, weight, or years of education. Similarly, there was no significant difference in the amount of self-reported alcohol use between groups.
Procedures
Subjects completed 3 test days at least 3 days apart in a randomized order under double-blind conditions. Prior to the administration of any study medication, subjects were warned that ketamine had addictive potential and that its effects resembled the effects of ethanol. The individuals with a family history of ethanol dependence in particular were encouraged not to participate if they were concerned about their increased risk for the development of a substance use disorder. Prior to each test session, participants fasted overnight and remained in a fasting state during the test session. They were tested at approximately 8:30 a.m., and an intravenous line was placed at that time. On each test day, patients received a 40-minute intravenous infusion containing saline solution; ketamine hydrochloride, 0.1 mg/kg; or ketamine hydrochloride, 0.5 mg/kg (Ketalar, Parke-Davis, Kalamazoo, Mich.). This method of administration was similar to that reported previously in healthy subjects and ethanol-dependent patients (6, 7). Another intravenous catheter was placed and used for sampling plasma for hormonal analyses and to determine plasma ketamine levels. Blood was drawn to determine ketamine levels 10 and 80 minutes after the initiation of ketamine infusion. The order of multiple assessments was standardized.
Psychosis, dysphoria, negative symptoms, and behavior changes were assessed by using the Brief Psychiatric Rating Scale (BPRS) (17) as in prior studies of ketamine effects (6, 7, 18). Four key BPRS items for the positive symptoms of psychosis were used: conceptual disorganization, hallucinatory behavior, suspiciousness, and unusual thought content. Dysphoria was derived from the BPRS anxious-depression factor, comprising the items anxiety, guilt feelings, depressive mood, somatic concern, tension, and motor retardation (19). Three BPRS items for negative symptoms were used: blunted affect, emotional withdrawal, and motor retardation. Behavior changes were derived from the BPRS activation subscale, which comprises the items of tension, mannerisms, and posturing and excitement (19). The BPRS was administered at baseline and 10, 40, 80, 110, and 230 minutes after the start of the infusion. The Clinician-Administered Dissociative States Scale (20), which has 19 self-reported items and eight observer-rated items scored from 0 (not at all) to 4 (extremely), was administered at baseline and after 10, 40, 80, 110, and 230 minutes.
Mood ratings were assessed at 10, 20, 40, 80, 110, 170, and 230 minutes using visual analog scales. These scales are 100-mm visual analog scales marked proportionately to the perceived intensity of the subjective experience (0=not at all, 100=extremely) for the following mood states: depressed, high, anxious, drowsy, nauseous, and hungry. These mood rating scales have shown convergent validity with other measures of mood states during similar challenge studies (7, 21, 22). Subjective intoxication ratings were also assessed at 10, 20, 40, 80, 110, 170, and 230 minutes using visual analog scales. Ketamine-induced effects, which are similar to the effects of alcohol, were measured by a biphasic alcohol effects scale in which the ascending scale measured activating alcohol-like symptoms such as euphoria while the descending scale measured sedation.
Cognitive functions were assessed as follows. Verbal fluency was assessed by using a task that required subjects to generate as many words as possible during a 1-minute interval that began with a specified letter. Equivalent versions of this task were administered on the 3 test days at 20 minutes after the initiation of infusion by using letters equated for frequency in English (23). The Hopkins Verbal Learning Test is a word list learning test of verbal memory (24). This test is sensitive to the amnesic effects of NMDA antagonists and has the advantage of six different versions that permit multiple episodes of testing. The procedures associated with the test allow some degree of distinction between immediate recall, delayed recall (after 30 minutes), and recognition. This test was administered 20 minutes after infusion initiation.
Samples for blood ketamine levels were drawn during the laboratory session by a research nurse 10 and 80 minutes after infusion initation. Samples were spun immediately and then frozen in airtight vials. Plasma ketamine concentrations were measured by reversed-phase high-performance liquid chromatography with ion pairing. Variation between days over a range (400 ng/ml to 20 ng/ml) varied from 2.1% to 7.8%.
Data Analysis
All analyses were performed by using SAS Version 8.2. Cumulative logit generalized estimating equation models for ordinal data were fitted in PROC GENMOD for the analysis of the BPRS, visual analog scale, and Clinician-Administered Dissociative States Scale data. Group (family history, no family history), dose (high, low, placebo), and time and all possible interactions of these variables were considered as predictors. Sex was included as a covariate. Independence working correlation matrix was assumed. The analysis was restricted only to dose levels and time points for which there was variability in the data. Alcohol consumption was considered as a covariate but was nonsignificant and dropped from the final model for parsimony. Model-based post hoc between-group comparisons of high-dose ketamine response were performed for the time points considered in the model. Between-group comparisons were also performed by using Fisher’s exact test for all dose and time combinations, even the ones at which there was not enough variability to include the data in the general model.
The verbal fluency data were analyzed by using a mixed model with fixed group, dose, and group-by-dose effects and a random subject effect in SAS PROC MIXED. The group-by-dose interaction was tested first and was followed by Bonferroni-adjusted individual comparisons. The ascending and descending scales of the biphasic alcohol effects scale were assessed log transformed, and each scale was analyzed with a mixed model with fixed effects of dose, group, and time and all possible interactions, a random subject effect, and heterogeneous autoregressive structure over time. Baseline was included as a covariate.
The immediate recall data from the Hopkins Verbal Learning Test were analyzed by using a mixed model with fixed effects of group, dose, and time and a random subject effect. Perseverations and sex were used as covariates. Recognition and delay variables were tested with both 1) a mixed model with fixed effects of group and dose and a random subject effect, with immediate recall data as a covariate; and 2) a nonparametric model to test effects of treatment and family history.
Ketamine levels were also assessed log transformed and analyzed with a mixed model with fixed effects of dose, group, and time and all possible interactions. Correlations between blood values and the main outcome variables were also analyzed by using a logistic model.
Results
Behavioral and Subjective Effects
Psychotic response
Individuals with a family history of ethanol dependence had a blunted psychotic response to ketamine relative to those with no such family history (Figure 1). In the analysis of the four key positive symptom scores, scores were first clustered into low (≤4) or high (≥5) scores for analysis in a logistic regression generalized estimating equation model. However, for the placebo and low-dose ketamine (0.1 mg/kg) infusions, subjects in both groups exhibited low scores at almost all the time points. As a result, it was necessary to limit the analysis to the higher ketamine (0.5 mg/kg) dose. Within the ketamine 0.5 mg/kg test day, high responses were limited to the assessment made 10 minutes following the initiation of ketamine infusion. The effect of group was significant, with subjects who had no family history of alcoholism exhibiting greater response than those with a family history of ethanol dependence (Table 1).
Dysphoria
Ketamine produced a more pronounced dysphoric mood response in individuals with no family history of ethanol dependence, as measured by the BPRS anxious-depression factor. In the analysis of this factor, scores were first clustered into low (≤6), medium (7, 8), or high (≥9) scores. The analysis was restricted to the 10- and 80-minute time points, which revealed a significant group-by-dose-by-time interaction (Table 1).
Negative symptoms
Subjects with no family history of ethanol dependence also exhibited more pronounced ketamine-induced negative symptoms (blunted affect, emotional withdrawal, and psychomotor retardation). In the analysis of these three key negative symptom scores, scores were first clustered into low (≤3) or high (≥4) scores. The model could only be fit to the high-dose ketamine comparisons. There was a significant group-by-time effect (Table 1). Two post hoc between-group comparisons at the 10- and 80-minute time points were performed; only the comparison at 80 minutes was significant (χ2=4.15, df=1, p<0.05), with the post hoc results confirmed by Fisher’s exact test (p=0.055).
Behavior changes
In the analysis of the BPRS activation subscale, scores were first clustered into low (≤3) or high (≥4) scores. The model could only fit to the high dose of ketamine and to the 10-minute time point. Although there were no significant group differences, there is some indication that the ketamine effect was sustained in the healthy subjects who had no family history of alcoholism relative to those with a family history of ethanol dependence. Post hoc analysis with Fisher’s exact tests showed a significant between-group comparison at the high-dose 80-minute time point (p<0.02).
Dissociation
The subjects with a family history of ethanol dependence had a blunted dissociative response to ketamine relative to those without such a family history, as measured by the Clinician-Administered Dissociative States Scale (Figure 2). In the analysis of the Clinician-Administered Dissociative States Scale scores, scores were first clustered into low (0–2), medium (3–10), high (11–25), or very high (≥26) scores. The analysis was restricted to the 10- and 80-minute time points. There was a significant group-by-time effect (Table 1) with significant between-group differences occurring at the 80-minute time point only (χ2=11.50, df=1, p=0.0007), which was confirmed by Fisher’s exact test (p≤0.01).
Intensity of mood states
Subject ratings from the visual analog scale were categorized into five 20-mm increments (0–20, 21–40, etc.). On the basis of visual analog scale ratings for “high” and “drowsy,” ketamine produced significant euphoria (dose-by-time effect: (χ2=54.12, df=6, p<0.01). No group-related differences were significant in the analysis. There were no other significant effects for the other mood states assessed with the visual analog scale.
Subjective intoxication
There were significant ketamine-induced effects, which are similar to the effects of alcohol, as measured by a biphasic alcohol effects scale. For both the ascending and descending scales, ketamine showed significant effects of dose (F=4.05, df=2, 56.9, p=0.02 and F=19.1, df=2, 31.1, p<0.01, respectively), time (F=3.33, df=6, 202, p<0.01; F=23.4, df=6, 159, p<0.01), and dose-by-time (F=3.02, df=12, 203, p<0.01; F=9.92, df=12, 130, p<0.01), but no group-related differences were significant in this analysis.
Cognitive Effects
On the Hopkins Verbal Learning Test, there was a significant ketamine effect on immediate recall (F=16.84, df=2, 234, p<0.001), with lower scores during the high dose relative to both the low dose and placebo (p<0.001, p<0.001). Although there was a significant group effect on immediate recall in which those individuals with a family history of ethanol dependence had lower scores than those without such a family history (F=8.78, df=1, 234, p<0.004), there was no significant dose-by-group effect. There was no significant group effect or dose effect for either delayed recall or recognition.
On the verbal fluency test, there was not a significant group effect or a group-by-dose effect (F=1.67, df=2, 76.4, p=0.20). However, post hoc tests of the within-group dose effect showed a significant effect for individuals with no family history of ethanol dependence (F=5.72, df=2, 76.6, p=0.005) in which the higher the dose, the lower the verbal fluency scores; this was not seen in subjects with a family history of ethanol dependence (F=0.08, df=2, 76.2, p=0.92).
Blood Level Effects
As expected, there were significant effects on ketamine plasma levels for dose (F=8.01, df=1, 90, p<0.001) and time (F=10.24, df=1, 90, p<0.001). However, there was no statistically significant difference in ketamine levels between individuals with a family history of ethanol dependence and those with no such family history. Ketamine blood levels were not significantly correlated with the intensity of the psychotic or dissociative responses.
Discussion
The principal finding from this study is that in healthy individuals with no ethanol dependence, response to the NMDA receptor antagonist ketamine was altered in those with a family history of ethanol dependence relative to those with no such family history. Specifically, these data show that individuals with a family history of ethanol dependence exhibited blunted psychosis, dysphoric mood, negative symptom, and perceptual responses but sustained activation to ketamine relative to individuals with no such family history. The impact of possible confounding factors was considered. However, the groups were matched by age, and there were no significant differences in demographic characteristics except for sex, and this was added as a covariate to the models. Similarly, since there were no significant differences in ketamine plasma levels between the groups, nor was there a significant effect of plasma ketamine level on the intensity of the main outcome variables, our results are not likely to have arisen from group differences in drug metabolism. A second finding from this study is, as expected, that subjects as a group perceived ketamine to be similar to alcohol and experienced it as euphoric and sedating. However, there were no group differences in perceptions of similarity to alcohol or in experiences of sedation or being “high.”
The data suggest that differences in ketamine response between the two groups are reflected in their distinct subjective responses to ethanol. The evidence supporting this interpretation is that both groups reported an equivalently high degree of similarity between the subjective effects of ketamine and their recollection of the subjective effects of ethanol. The similarity of the subjective effects of ketamine to the subjective effects of ethanol was reported by both groups despite the fact that ketamine had a markedly different array of effects in these two groups. If alterations in NMDA receptor antagonist response did not extend to ethanol, one would have expected to find significant differences in the extent of ethanol similarity of ketamine effects in groups distinguished by their ketamine responses. Thus, the current data are also consistent with the extensive literature that suggests that subjects with versus those without a family history of ethanol dependence are distinguished by alterations in ethanol response that resemble their differences in ketamine response in the current study. However, an important limitation of the current study design was the absence of an ethanol test day that would have allowed for a direct comparison of ketamine and ethanol effects in these groups.
A third finding is that ketamine produced some expected cognitive impairments, particularly in memory, but again there were no group differences in cognitive measures in the response to ketamine administration. Of note, however, was the group difference at baseline for one of the cognitive measures: individuals with a family history of ethanol dependence scored significantly lower on a test of verbal memory. Cognitive impairments and other “soft” neurologic signs in individuals with a family history of ethanol dependence relative to those with no such family history have long been of interest as potentially relevant factors that could contribute to the development of ethanol dependence (25). While many studies have found similarities between these groups in learning, memory, and verbal and total intelligence scores, differences in abstract reasoning and logic performance suggest the potential for underlying neurobiological abnormalities in neural circuitry (25). Further, an attenuation of ethanol-induced cognitive deficits has been found in individuals with a family history of ethanol dependence relative to those with no such family history (26). In this study, baseline differences in cognitive function did not influence ketamine response, suggesting that baseline learning and memory could not account for group differences in subjective ketamine responses. Additionally, individuals with a family history of ethanol dependence did not exhibit a reduced sensitivity to the ketamine-induced effects on cognitive function.
Results from this study suggest that altered NMDA receptor function may contribute to the altered ethanol response in individuals with a family history of ethanol dependence relative to those with no such family history. These NMDA receptor functional changes include a shift in the reward valence of the actions of NMDA receptor antagonists, presumably including ethanol, toward the experience of a drug of predominately reduced dysphoric effects. The present results are also consistent with data from recently detoxified individuals (27) showing that when ethanol-dependent patients are subtyped, those with a family history of ethanol dependence show reduced ketamine induction of affect blunting, dysphoric mood, and impaired performance on the Wisconsin Card Sorting Test. However, other ketamine responses that discriminated healthy subjects with a family history of ethanol dependence from those without such a history did not differentiate ethanol-dependent patients with versus without a family history of ethanol dependence, including sustained or enhanced euphoria. These data suggest that family history of ethanol dependence and ethanol dependence convey convergent, but not overlapping, alterations in NMDA receptor function.
Changes in the response to ketamine could reflect alterations in NMDA receptor function related to voltage-gated ion channels, NMDA receptor-related signal processing cascades, modulation of the phosphorylation state of NMDA receptor subunits, and shifts in the levels or posttranscriptional processing of NMDA receptor subunit mRNAs or proteins (28). Alternatively, these responses could reflect network consequences of alterations in NMDA receptor function, such as the stimulation of the release of glutamate and dopamine (29). Clinical evidence has shown that drugs that attenuate glutamate release or glutamatergic excitability via blockade of voltage-gated cation channels reduce the dysphoric effects of ketamine and promote the euphoric effects of ketamine in both healthy subjects (30) and recovering ethanol-dependent patients (31). In fact, pretreatment with calcium channel blockers in individuals with no family history of ethanol dependence leads to a ketamine response that is more consistent with that of individuals who have a family history of ethanol dependence (30), suggesting that the alteration reflects dysfunction related to voltage-gated cation channels. Nevertheless, other mechanisms have not been definitively ruled out.
A strength of this study is its use of a study design that mirrors previous work with ketamine, including similar doses and outcome measures, in ethanol-dependent individuals, so the results can be compared. However, there are several limitations to this study. First, individuals were recruited by advertisement, and family history of alcoholism was assessed by self-report. Interviewing affected relatives of subjects who claimed to have a positive family history in order to confirm the diagnosis of alcoholism was not done for practical reasons. Similarly, the recruitment of offspring of a treatment-seeking population would be more accurate but was ruled out because of practical reasons. Therefore, the diagnosis of alcohol dependence in the family member could not be confirmed. However, we surmise that bias in reporting familial affection is more likely to occur in the other direction, i.e., in failing to attribute deserved diagnoses to relatives, so the healthy sample may have included individuals with a family member with alcohol dependence that was unrecognized. In any event, if such errors occurred in either direction, they would tend to decrease the power of the study rather than lead to a positive finding. Second, although alcohol dependence is moderately heritable, not all individuals with a family history carry the risk of developing alcoholism, although for that reason we included only those with multiple family members with the diagnosis. Arguably, since we sampled only unaffected subjects past the major period of lifetime risk for developing alcohol dependence, the subjects we studied, who have successfully avoided developing alcohol dependence despite a family history, would be those among the at-risk population who have protective factors as well as risk factors. That is, our study design may have preferentially included individuals who were unlikely to become ethanol dependent despite their family history. Human subject considerations are thought to mandate the selection of such a sample. Third, the results could be explained by some confounding factor that was not tested, such as the presence of a personality disorder.
In summary, this study reported alterations in response to the NMDA receptor antagonist ketamine between healthy subjects with a family history of ethanol dependence relative to those without such a family history. The subjects with a family history experienced dysphoric responses to ketamine that were markedly reduced and some mild enhancement in euphoria. An inherited underlying alteration in NMDA receptor antagonist response may have implications for the subsequent response of these individuals to ethanol ingestion. If NMDA antagonists are not perceived as unpleasant, it may shift the reward valence of ingestion of these substances. Clinically, these individuals may lack the “warning signs” to stop heavy drinking when consuming moderate amounts of alcohol. In the right environmental and social context, the loss of a potentially important brake on drinking may promote heavy drinking. The strength of the current findings suggest that future candidate gene investigations might concentrate on genes that influence glutamatergic function. In these studies, the response to ketamine or other NMDA receptor antagonists might prove to be a useful phenotype.
 |
Received Sept. 26, 2002; revisions received May 13 and Dec. 9, 2003; accepted Dec. 15, 2003. From the Department of Veterans Affairs Alcohol Research Center, VA Connecticut Healthcare System; the National Institute on Alcohol and Alcohol Abuse Center for the Translational Neuroscience of Alcoholism, New Haven, Conn.; the Clinical Neuroscience Research Unit, Abraham Ribicoff Research Facilities, New Haven, Conn.; and the Departments of Psychiatry, Laboratory Medicine, and Epidemiology and Public Health (Division of Biostatistics), Yale University School of Medicine, New Haven, Conn. Address reprint requests to Dr. Petrakis, West Haven VA Medical Center (116-A), 950 Campbell Ave., West Haven, CT 06516; [email protected] (e-mail). Supported by grants from the National Institute on Alcohol Abuse and Alcoholism (1R01 AA-10121-01, RO1 AA-12308-01, KO2 AA-00261-01, and P50 AA-12870-01) to Drs. Krystal and Petrakis; and Merit Review grants and funding from the Department of Veterans Affairs VA-Yale Alcohol Research Center to Drs. Krystal and Petrakis. The authors thank Rachel Alpert; William Ford; Angelina Genovese, R.N.C., M.B.A; Elizabeth O’Donnell, R.N.; and Jane Zhang, Ph.D., for their contributions as well as the staffs of the Biostudies Unit and Substance Abuse Treatment Unit of VA Connecticut Healthcare System.
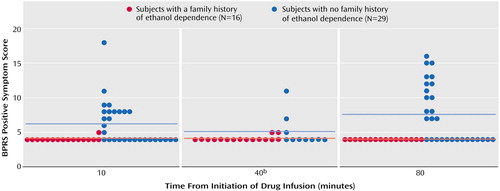
Figure 1. Psychotic Response Among Healthy Subjects During a 40-Minute, High-Dose (0.5 mg/kg) Ketamine Infusion, by Family History of Ethanol Dependencea
aHorizontal lines represent group means.
bData collected from only 10 subjects with no family history of ethanol dependence at this time point.
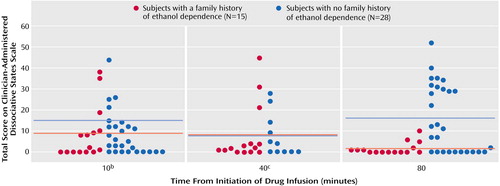
Figure 2. Dissociative Response Among Healthy Subjects During a 40-Minute, High-Dose (0.5 mg/kg) Ketamine Infusion, by Family History of Ethanol Dependencea
aHorizontal lines represent group means.
bData were missing for one subject with no family history of ethanol dependence at this time point.
cAt this time point, data collected from only 11 subjects with no family history of ethanol dependence, and one subject with a family history of ethanol dependence had data missing.
1. Hesselbrock MN, Hesselbrock VM: Relationship of family history, antisocial personality disorder and personality traits in young men at risk for alcoholism. J Stud Alcohol 1992; 53:619–625Crossref, Medline, Google Scholar
2. Kendler KS, Prescott CA, Neale MC, Pedersen NL: Temperance board registration for alcohol abuse in a national sample of Swedish male twins, born 1902 to 1949. Arch Gen Psychiatry 1997; 54:178–184Crossref, Medline, Google Scholar
3. Schuckit MA, Smith TL: An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiatry 1996; 53:202–210Crossref, Medline, Google Scholar
4. Ghoneim MM, Hinrichs JV, Mewaldt SP, Petersen RC: Ketamine: behavioral effects of subanesthetic doses. J Clin Psychopharmacol 1985; 5:70–77Crossref, Medline, Google Scholar
5. Malhotra AK, Pinals DA, Weingartner H, Sirocco K, Missar CD, Pickar D, Breier A: NMDA receptor function and human cognition: the effects of ketamine in healthy volunteers. Neuropsychopharmacology 1996; 14:301–307Crossref, Medline, Google Scholar
6. Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr, Charney DS: Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry 1994; 51:199–214Crossref, Medline, Google Scholar
7. Krystal JH, Petrakis IL, Webb E, Cooney NL, Karper LP, Namanworth S, Trevisan LA, Charney DS: Dose-related ethanol-like effects of the NMDA antagonist, ketamine, in recently detoxified alcoholics. Arch Gen Psychiatry 1998; 55:354–360Medline, Google Scholar
8. Adler CM, Goldberg TE, Malhotra AK, Pickar D, Breier A: Effects of ketamine on thought disorder, working memory, and semantic memory in healthy volunteers. Biol Psychiatry 1998; 43:811–816Crossref, Medline, Google Scholar
9. Adler CM, Malhotra AK, Elman I, Goldberg T, Egan M, Pickar D, Breier A: Comparison of ketamine-induced thought disorder in healthy volunteers and thought disorder in schizophrenia. Am J Psychiatry 1999; 156:1646–1649Link, Google Scholar
10. Grant KA, Lovinger DM: Cellular and behavioral neurobiology of alcohol: receptor-mediated neuronal processes. Clin Neurosci 1995; 3:155–164Medline, Google Scholar
11. Grant K: Strategies for understanding the pharmacological effects of ethanol with drug discrimination procedures. Pharmacol Biochem Behav 1999; 64:261–267Crossref, Medline, Google Scholar
12. Butelman E, Baron S, Wods J: Ethanol effects in pigeons trained to discriminate MK-801, PCP or CGS-19755. Behav Pharmacol 1993; 4:57–60Crossref, Medline, Google Scholar
13. Schechter M, Meehan S, Gordon T, McBurney D: The NMDA receptor antagonist MK-801 produces ethanol-like discrimination in the rat. Alcohol 1993; 10:197–201Crossref, Medline, Google Scholar
14. Michaelis EK, Freed WJ, Galton N, Foye J, Michaelis ML, Phillips I, Kleinman JE: Glutamate receptor changes in brain synaptic membranes from human alcoholics. Neurochem Res 1990; 15:1055–1063Crossref, Medline, Google Scholar
15. Krystal J, Karper L, D’Souza DC, Webb E, Bennett A, Abi-Dargham A, Petrakis IL, Brenner L, Morrissey K, Abi-Saab D, Charney DS: Differentiating NMDA dysregulation in schizophrenia and alcoholism using ketamine. Schizophr Res 1995; 15:156Google Scholar
16. Spitzer RL, Williams JBW, Gibbon M, First MB: The Structured Clinical Interview for DSM-III-R (SCID), I: history, rationale, and description. Arch Gen Psychiatry 1992; 49:624–629Crossref, Medline, Google Scholar
17. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812Crossref, Google Scholar
18. Krystal JH, D’Souza DC, Karper LP, Bennett A, Abi-Dargham A, Abi-Saab D, Bowers MB Jr, Jatlow P, Heninger GR, Charney DS: Interactive effects of subanesthetic ketamine and haloperidol. Psychopharmacology (Berl) 1999; 145:193–204Crossref, Medline, Google Scholar
19. Hedlund JL, Vieweg BW: The Brief Psychiatric Rating Scale (BPRS): a comprehensive review. J Operational Psychiatry 1980; 11:48–65Google Scholar
20. Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS, Mazure CM: Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress 1998; 11:125–136Crossref, Medline, Google Scholar
21. Krystal JH, Webb E, Cooney NL, Kranzler HR, Southwick SW, Heninger GR, Charney DS: Serotonergic and noradrenergic dysregulation in alcoholism: m–chlorophenylpiperazine and yohimbine effects in recently detoxified alcoholics and healthy comparison subjects. Am J Psychiatry 1996; 153:83–92Link, Google Scholar
22. Petrakis IL, Trevisan L, Boutros NN, Limoncelli D, Cooney NL, Krystal JH: Effect of tryptophan depletion on alcohol cue-induced craving in abstinent alcoholic patients. Alcohol Clin Exp Res 2001; 25:1151–1155Crossref, Medline, Google Scholar
23. Borkowski JG, Benton AL, Spreen O: Word fluency and brain damage. Neuropsychologia 1967; 5:135–140Crossref, Google Scholar
24. Brandt J: The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. Clin Neuropsychol 1991; 5:125–142Crossref, Google Scholar
25. Schuckit MA: A clinical model of genetic influences in alcohol dependence. J Stud Alcohol 1994; 55:5–17Crossref, Medline, Google Scholar
26. Erblich J, Earleywine M: Children of alcoholics exhibit attenuated cognitive impairment during an ethanol challenge. Alcohol Clin Exp Res 1999; 23:476–482Crossref, Medline, Google Scholar
27. Krystal JH, Petrakis IL, Limoncelli D, Webb E, Gueorgueva R, D’Souza DC, Boutros N, Trevisan L, Charney DS: Altered NMDA glutamate receptor antagonist response in recovering ethanol-dependent patients. Neuropsychopharmacology 2003; 28:2020–2028Crossref, Medline, Google Scholar
28. Krystal JH, Petrakis IL, Mason G, Trevisan L, D’Souza DC: N-methyl-D-aspartate glutamate receptors and alcoholism: reward, dependence, treatment, and vulnerability. Pharmacol Ther 2003; 99:79–94Crossref, Medline, Google Scholar
29. Moghaddam B, Adams B, Verma A, Daly D: Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci 1997; 17:2921–2927Crossref, Medline, Google Scholar
30. Anand A, Charney DS, Oren DA, Berman RM, Hu XS, Cappiello A, Krystal JH: Attenuation of the neuropsychiatric effects of ketamine with lamotrigine: support for hyperglutamatergic effects of N-methyl-D-aspartate receptor antagonists. Arch Gen Psychiatry 2000; 57:270–276Crossref, Medline, Google Scholar
31. Krupitsky EM, Burakov AM, Romanova TN, Grinenko NI, Grinenko AY, Fletcher J, Petrakis IL, Krystal JH: Attenuation of ketamine effects by nimodipine pretreatment in recovering ethanol dependent men: psychopharmacologic implications of the interaction of NMDA and L-type calcium channel antagonists. Neuropsychopharmacology 2001; 25:936–947Crossref, Medline, Google Scholar



