Correlation Between Dopamine D2 Receptors in the Ventral Striatum and Central Processing of Alcohol Cues and Craving
Abstract
OBJECTIVE: Alcohol and other drugs of abuse stimulate dopamine release in the ventral striatum, which includes the nucleus accumbens, a core region of the brain reward system, and reinforce substance intake. Chronic alcohol intake is associated with down-regulation of central dopamine D2 receptors, and delayed recovery of D2 receptor sensitivity after detoxification is positively correlated with high risk for relapse. Prolonged D2 receptor dysfunction in the ventral striatum may interfere with a dopamine-dependent error detection signal and bias the brain reward system toward excessive attribution of incentive salience to alcohol-associated stimuli. METHOD: Multimodal imaging, with the radioligand [18F]desmethoxyfallypride and positron emission tomography as well as functional magnetic resonance imaging (fMRI), was used to compare 11 detoxified male alcoholics with 13 healthy men. The authors measured the association of D2-like dopamine receptors in the ventral striatum with alcohol craving and central processing of alcohol cues. RESULTS: Activation of the medial prefrontal cortex and striatum by alcohol-associated stimuli, relative to activation by neutral visual stimuli, was greater in the detoxified alcoholics than in the healthy men. The alcoholics displayed less availability of D2-like receptors in the ventral striatum, which was associated with alcohol craving severity and with greater cue-induced activation of the medial prefrontal cortex and anterior cingulate as assessed with fMRI. DISCUSSION: In alcoholics, dopaminergic dysfunction in the ventral striatum may attribute incentive salience to alcohol-associated stimuli, so that alcohol cues elicit craving and excessive activation of neural networks associated with attention and behavior control.
Dopamine release in the ventral striatum, which includes the nucleus accumbens, follows drug and alcohol intake (1, 2). It has been suggested that dopamine release in this central area of the brain reward system is strongly rewarding and that drug intake is reinforced by the hedonic affect elicited by drugs and drug-associated stimuli (3). It has also been postulated that dopamine release in the nucleus accumbens attributes “incentive salience” to drug-associated stimuli, thus increasing the motivational value and attentional processing of drug cues, and it has been suggested that cue-induced dopamine release mediates drug “wanting” rather than drug “liking” (4). This hypothesis was based on the observation of Schultz et al. (5) that unexpected rewards and conditioned reward-indicating stimuli elicited dopamine signaling in the nucleus accumbens. When, however, primary rewards were fully expected because of the association with preceding reward-indicating cues, there was no increase in dopamine discharge. Schultz et al. (5) suggested that dopamine discharge represents an error-detection signal that is increased upon confrontation with unexpected rewards or reward-indicating stimuli and decreased if conditioned cues are not followed by the reward.
Sustained cue-induced changes in dopamine discharge may code reward uncertainty and elicit a nonselective form of attention or arousal (6). The ventral striatum is closely connected with the anterior cingulate (7) and the dorsal striatum (8). Cue-induced activation of the anterior cingulate and adjacent medial prefrontal cortex may mediate an attentional response to drug cues (9, 10), while cue-induced dopamine release in the dorsal striatum can trigger relapse into drug-taking behavior (11).
Alcohol, like other drugs of abuse, stimulates dopamine release and induces a down-regulation of striatal dopamine D2 receptors (12). Longitudinal studies have shown that D2 receptor down-regulation is most prominent directly after detoxification and recovers during abstinence (13, 14). Delayed recovery of central dopaminergic neurotransmission predicts a higher risk for relapse among detoxified alcoholics (13–15).
To further elucidate the association between individual differences in D2 receptor dysfunction in the ventral striatum (particularly the nucleus accumbens), central processing of alcohol-associated cues, and alcohol craving, we used functional magnetic resonance imaging (fMRI) in combination with positron emission tomography (PET) to study detoxified male alcoholics and age-matched comparison subjects. We tested the hypothesis of Robinson and Berridge (4) that low availability of D2 receptors in the ventral striatum/nucleus accumbens mediates excessive attribution of incentive salience to alcohol-associated cues, causing a pathological “wanting” to consume alcohol. We also tested the hypothesis that in alcoholics a dysfunction of ventral striatum D2 receptors interferes with a dopamine-dependent error detection signal (5), so that alcohol-associated cues elicit excessive activation of the medial prefrontal cortex/anterior cingulate and dorsal striatum.
Method
Subjects and Instruments
The local ethics committee approved the study, and written informed consent was obtained from all participants after the procedures had been fully explained. Eleven male alcoholics (mean age=44.5 years, SD=6.5, range=35–57) and 13 age-matched healthy comparison subjects (mean age=43.2, SD=9.5, range=32–60) were included. The patients suffered from alcohol dependence according to ICD-10 and DSM-IV criteria and had no other psychiatric axis I disorder, no past history of drug dependence, and no current drug abuse according to random urine drug testing and the Structured Clinical Interview for DSM-IV-TR (SCID) (16). The patients had abstained from alcohol in a supervised inpatient treatment program for 2 to 4 weeks (subject to random alcohol breath tests and urine drug tests). The comparison subjects had no psychiatric axis I or II disorder according to the SCID (16) and the Structured Clinical Interview for DSM-IV Personality Disorders (17). The severity of current alcohol craving was measured in the morning before fMRI with the Alcohol Craving Questionnaire (18), an internationally widely used scale with good test-retest reliability (r=0.85, p<0.001), when tested in our laboratory in 46 alcoholics on two separate days, and high internal consistency, when tested in 243 alcoholics (Cronbach’s alpha=0.96, p<0.001).
PET
Measurement of D2 receptor availability
We used PET and the benzamide radioligand [18F]desmethoxyfallypride ([18F]DMFP), which binds with high affinity to D2-like dopamine receptors. A detailed evaluation of this tracer has been published previously (19). PET data were acquired dynamically (28 time frames: four lasting 1 minute apiece, three for 2 minutes, three for 3 minutes, 15 for 5 minutes, and three for 10 minutes) with an ECAT EXACT PET scanner (Siemens Medical Solutions, Erlangen, Germany) after administration of a mean of 194 MBq of [18F]DMFP (SD=27) to image D2 receptor binding. Specific activity at the time of injection was a mean of 267 GBq/μmol (SD=283), resulting in injected tracer masses of <1 μmol. The subjects rested in the scanner with their eyes closed. A pixelwise simplified reference tissue model using the cerebellum as a control region, i.e., free of D2 receptors, was applied to the dynamic PET data, yielding parametric images of the binding potential (20). The binding potential is a composite function of parameters determined by the ratio of Bmax (the total concentration of specific binding sites) to Kd (the equilibrium dissociation constant). The images were coregistered with individual MRI scans and stereotactically normalized into Talairach space (21); with respect to the automatic normalization procedure, great care was taken to manually ensure the correct coregistration in each case.
Correlation with alcohol craving
A categorical comparison of the two groups (alcoholics and comparison subjects) was performed with statistical parametric mapping (SPM 99) (22). We tested the hypothesis that alcoholics display fewer D2 receptors in the ventral striatum/nucleus accumbens and that alcohol craving is inversely related to the availability of D2 receptors in the nucleus accumbens. A linear regression analysis was performed with SPM 99 to detect brain areas in which alcohol craving was significantly associated with D2 receptor availability. The input matrices in this analysis were the PET parametric images containing the D2 receptor availability for each voxel. Unlike brain imaging analysis based on a priori defined regions of interest, correlations with SPM 99 offer an observer-independent method to detect the brain areas in which D2 receptor availability is directly associated with alcohol craving. Since the comparison subjects reported hardly any alcohol craving, correlations between craving and D2 receptor availability were computed separately for the alcoholics and comparison subjects. The significance level for the group contrast and the correlation with alcohol craving was p<0.05 (corrected for the striatal volume of interest defined in Talairach space).
fMRI
Alcohol-associated stimuli
For fMRI, 15 alcohol-associated stimuli that were previously shown to evoke alcohol craving in alcoholics (23, 24), 15 abstract pictures with the same type of color and complexity, and 15 affectively neutral pictures from the International Affective Picture System (25) were presented to all subjects. fMRI data were acquired on a separate day at least 24 hours after the PET scan. Because of motion artifacts, fMRI data were available for nine alcoholics and 12 comparison subjects. The subjects were instructed to passively view the stimuli, because even simple cognitive rating tasks can reduce limbic activation elicited by affective stimuli (26); after each scanning session, arousal and the valence of each picture were rated with the Self-Assessment Manikin (27). The alcohol-associated and control stimuli were presented in a block design (three pictures per category, 19.8 seconds per block) in a standard clinical 1.5 T MRI scanner (Siemens Vision, Siemens Medical Solutions, Erlangen, Germany). A standard echo planar imaging sequence (TR=1.8 msec, TE=66 msec, α=90°) was used with an in-plane resolution of 64×64 pixels (number of slices=24, thickness=4 mm, gap=1 mm, field of view=220×220 mm, repetition time=3.3 seconds). A T1-weighted image data set (voxel size=1×1×1 mm) was acquired for anatomical reference.
Data analysis
The fMRI data were analyzed with SPM 99. The first five volumes of each functional time series were discarded to eliminate T1 effects. All volumes were realigned to the remaining first volume to correct for between-scan movements. The structural three-dimensional data set was coregistered to the first T2* image. The structural image was spatially normalized to a standard template by using a 12-parameter affine transformation with additional nonlinear components. The nonlinear transformation was subsequently applied to the T2* data. The functional data were smoothed by using an isotropic Gaussian kernel for individual analysis (6 mm full width at half maximum [FWHM]) and for group analysis (12 mm FWHM).
Statistical analysis was performed by modeling alcohol versus control conditions (alcohol-associated stimuli versus affectively neutral and abstract stimuli; boxcar functions convolved with the hemodynamic response function) as explanatory variables within the context of the general linear model on a voxel-by-voxel basis with SPM 99. Data were analyzed for each subject individually (threshold p<0.001, uncorrected). To detect between-group differences, we included the individual contrast images, i.e., the differences in blood-oxygen-level-dependent (BOLD) response between conditions, of all subjects in each group (patients and comparison subjects) in a second-level analysis. On the basis of previous studies, we hypothesized that the alcohol-associated stimuli would activate the anterior cingulate, adjacent medial prefrontal cortex, and striatum more than would the control stimuli (10, 28–34). The significance level was p<0.05, corrected for the volume of interest in the medial prefrontal cortex/anterior cingulate and striatum defined in Talairach space.
Correlations Between PET and fMRI Data
We tested the hypothesis that D2 receptor availability in the ventral striatum/nucleus accumbens is negatively correlated with cue-induced functional activation in brain areas identified in the group contrast (alcoholics versus comparison subjects). Therefore, we correlated the individual fMRI BOLD contrast (difference in signal change between the alcohol-associated and affectively neutral or abstract stimuli) in the local maxima of brain areas identified in the group contrast (in this study, the striatum and medial prefrontal cortex; see Results section) with D2 receptor availability in the nucleus accumbens/ventral striatum; the Bonferroni correction was applied for multiple testing. Changes in the BOLD response can be assessed by linear combinations of the estimated general linear model parameters (beta values) and are contained in the individual contrast images (percentage signal change). D2 receptor availability in the nucleus accumbens/ventral striatum as defined in Talairach space was bilaterally measured and averaged. In all subjects, individually coregistered MRI scans were used to confirm that the selected voxels were located in the nucleus accumbens/ventral striatum (35) within the limits defined by Mawlawi et al. (36).
We also performed linear regression analyses with SPM 99 to confirm that significant associations between D2 receptor availability in the nucleus accumbens/ventral striatum and cue-induced functional brain activation were located in brain areas identified in the group-by-condition interaction (alcoholics versus comparison subjects and alcohol-associated versus control cues; p<0.05 corrected for the volume of interest). Correlations were carried out separately for the alcoholics and comparison subjects to avoid floor effects, because the comparison subjects showed no alcohol craving and hardly any alcohol-associated brain activation in the anterior cingulate/medial prefrontal cortex or striatum in the current study or previous studies (33, 34).
In the exploratory part of the study, we assessed the effects of potentially confounding variables: smoking, age at onset of alcohol dependence, severity of alcoholism (37), lifetime alcohol intake (38), number of previous detoxifications, and range of time from last drink to scanning on the severity of alcohol craving, D2 receptor binding in the ventral striatum, and cue-induced brain activation. For this exploratory part of the study, all p values are given for descriptive reasons.
Results
The alcoholics had significantly lower D2 receptor availability than the healthy comparison subjects in the bilateral putamen and adjacent nucleus accumbens/ventral striatum (for Talairach coordinates –28, 10, –8, t=2.21; for coordinates 20, 12, –6, t=2.39; df=22, p<0.05, corrected for the striatal volume of interest). In the alcoholics, greater severity of alcohol craving was significantly and exclusively associated with low bilateral D2 receptor availability in the nucleus accumbens/ventral striatum (Figure 1).
The correlation was remarkably symmetrical for the nucleus accumbens/ventral striatum (for right-side Talairach coordinates 16, 14, –6, r=–0.91; for left-side coordinates –15, 14, –6, r=–0.90; N=11, p<0.05, corrected for striatal volume of interest; the finding remained statistically significant at p<0.05 when multiple comparisons in the whole brain were corrected). The radioligand [18F]DMFP also binds to D2 receptors outside of the striatum to a low but significant extent (19). However, alcohol craving was not associated with D2 receptor availability outside of the ventral striatum. Among the healthy comparison subjects, no significant correlation was observed between alcohol craving and D2-like receptor availability.
The fMRI images showed that activation by visual alcohol-associated stimuli, beyond that produced by the control stimuli, was significantly greater for the alcoholics than for the healthy volunteers in the left medial prefrontal cortex (Brodmann’s area 10, coordinates –18, 52, –8, t=2.05) and left caudate (coordinates –6, 9, 8, t=2.54) (df=19, all p<0.05 corrected for volume of interest). In the alcoholics, D2 receptor availability in the ventral striatum was significantly and negatively correlated with cue-induced fMRI activation in the left medial prefrontal cortex (Brodmann’s area 10, coordinates –18, 52, –8: r=–0.82, N=9, p<0.05 after Bonferroni correction) but not in the left caudate (coordinates –6, 9, 8: r=0.16, N=9, p=0.66). No significant associations were observed in the comparison subjects in these regions of interest.
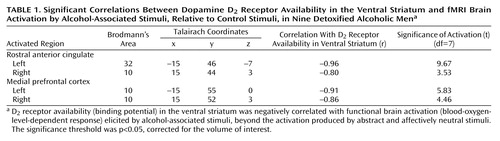
Using SPM 99, we also assessed the association between the availability of D2 receptors in the nucleus accumbens/ventral striatum and brain activation elicited by alcohol versus control cues. We observed that among the alcoholics, low striatal D2 receptor availability was associated with greater cue-induced activation of the bilateral rostral anterior cingulate (Brodmann’s area 32/10) and medial prefrontal cortex (Brodmann’s area 10) (Table 1 and Figure 2).
Among the healthy comparison subjects, D2 receptor availability was not associated with cue-induced brain activation in the cingulum or medial prefrontal cortex.
We did not observe significant correlations between the main outcome variables of the study (severity of alcohol craving, mean D2 receptor availability in the ventral striatum, cue-induced activation of the medial prefrontal cortex) and potentially confounding variables: smoking, age at onset of alcohol dependence, severity of alcoholism, lifetime alcohol intake, number of previous detoxifications, or range of time between last drink and scanning (r=0.46–0.01, p>0.10 in all cases, N=11 for alcohol craving and mean D2 receptor availabilitiy, N=9 for cue-induced brain activation).
Discussion
This study revealed two major findings. First, it lends direct support to the hypothesis of Robinson and Berridge (4) that dopamine dysfunction in the ventral striatum, which includes the nucleus accumbens, is associated with alcohol craving. In previous studies, individual differences in the speed of recovery of central dopaminergic neurotransmission predicted the relapse risk of alcoholics during early abstinence (13–15). In accordance with these observations, we found considerable variation in D2 receptor availability in the ventral striatum/nucleus accumbens of alcoholics who had abstained from alcohol for 3 weeks. It is currently not known whether the low D2 receptor availability observed represents a counteradaptive down-regulation triggered by chronic alcohol-associated dopamine release or whether it is due to alcoholism-related neurotoxicity. In any case, our study suggests a potential mechanism by which persistently low levels of D2 receptors in the ventral striatum may contribute to excessive alcohol urges and the motivation for relapse. We presented alcohol-associated pictures in a scanner while the alcohol-dependent patients were participating in a detoxification program. These patients were well informed that no alcoholic beverages were available during or after the brain imaging study. Therefore, the pictures of alcoholic beverages did not indicate the availability of an alcohol or drug reward. In accordance with this setting, the healthy comparison subjects showed no activation of the anterior cingulate or frontal cortex. In contrast, alcohol-associated stimuli elicited increased activation of the medial prefrontal cortex and caudate among the alcohol-dependent patients. The strength of the activation of the medial prefrontal cortex (Brodmann’s area 10) and the adjacent anterior cingulate was inversely correlated with the availability of dopamine receptors in the ventral striatum. A lack of D2 receptors in the ventral striatum, particularly the nucleus accumbens, may interfere with the normal role of dopaminergic neurotransmission as an error-detection signal that indicates the availability of reward (5). Patients may thus lack a dopamine-dependent striatal gating function (39) that filters out stimuli that are not followed by reward (40). As a consequence, alcohol-associated stimuli that were not followed by alcohol intake in this study elicited excessive activation of the medial prefrontal cortex and anterior cingulate. The anterior cingulate is a part of the medial prefrontal cortex, which is thought to be important in attention (41, pp. 175–176), conditioned drug seeking (10, 42), and craving (29, 30, 33). Cue-induced stimulation of the medial prefrontal cortex and anterior cingulate may thus reflect enhanced attentional processing of alcohol-related stimuli (41, p. 175), which become ”wanted” and elicit drug craving (4).
Some potential limitations of the study should be addressed. The patients rested in the PET scanner with their eyes closed. While they were told to relax, some of them may have experienced various degrees of alcohol craving, which might affect acute dopamine release in the ventral striatum and interact with radioligand binding to striatal D2 receptors. Correlations do not imply causation, and the exact nature of the interaction between D2 receptor availability in the ventral striatum and alcohol craving remains to be elucidated in further studies.
This study showed that alcohol craving is associated with a low availability of D2 receptors in the bilateral ventral striatum, including the nucleus accumbens, a central area of the brain reward system. Among alcoholics but not healthy comparison subjects, D2 receptor availability in the bilateral ventral striatum was negatively correlated with cue-induced activation of the frontal cortex and cingulum. Reinstatement of alcohol abuse may depend on dopaminergic dysfunction (12, 14) in the ventral striatum that promotes cue-induced activation of a neuronal network associated with attention, drug reward, and craving (9, 28, 29, 33). These observations indicate that neuroreceptor PET and fMRI can successfully be combined to reveal the in vivo interaction between neurotransmission and the neural networks associated with emotion and attention. They point to a neurobiological correlate of alcohol urges and support the hypothesis that dopaminergic dysfunction in the brain reward system plays a key role in alcohol craving among humans.
 |
Received Aug. 21, 2003; revision received Dec. 16, 2003; accepted Dec. 23, 2003. From the Department of Psychiatry and Department of Medical Psychology of the Charité, Campus Mitte, Berlin; the Department of Nuclear Medicine, Department of Psychiatry, and Institute of Nuclear Chemistry, University of Mainz, Mainz, Germany; the Central Institute of Mental Health; and the Department of Psychiatry, University of Hamburg, Hamburg, Germany. Address reprint requests to Dr. Mann, Department of Addictive Behavior and Addiction Medicine, Central Institute of Mental Health, University of Heidelberg J5, 68159 Mannheim, Germany; [email protected] (e-mail). Supported by the Deutsche Forschungsgemeinschaft (grants HE 2597/4–1 and BA1101/2-1). The authors thank Mary J. Breiner for providing some of the pictures of alcoholic and neutral beverages.
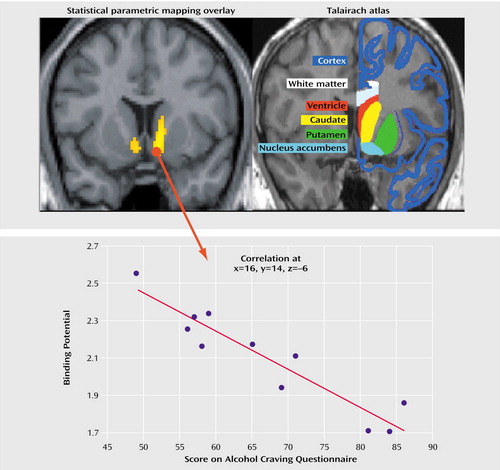
Figure 1. Correlation Between Dopamine D2 Receptor Availability in the Ventral Striatum and Alcohol Craving Among 11 Detoxified Alcoholic Mena
aCraving for alcohol was significantly and negatively correlated with D2 receptor availability in the bilateral nucleus accumbens/ventral striatum in the alcohol-dependent patients after 3 weeks of abstinence but not in healthy comparison subjects. For illustrative purposes a significance threshold of p<0.001 was applied. The image at the upper left is a coronal view of the results of a PET correlation analysis of D2 receptor availability and alcohol craving. The image at the upper right indicates that the areas of significant correlation correspond well to the ventral striatum/nucleus accumbens, as shown by comparison to the standardized anatomic brain atlas of Talairach and Tournoux (21). The scatterplot at the bottom shows the correlation between D2 receptor availability (binding potential) in the right ventral striatum/nucleus accumbens and acute alcohol craving.
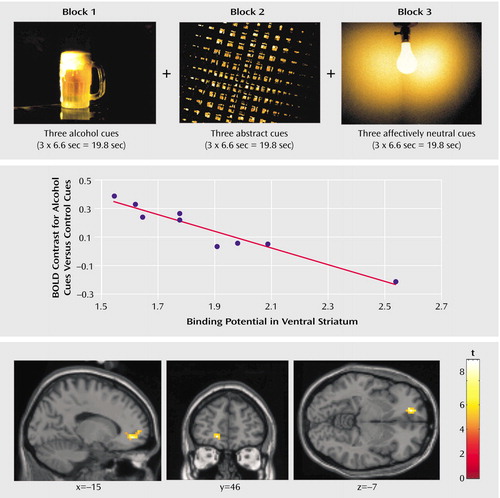
Figure 2. Correlation Between Dopamine D2 Receptor Availability in the Ventral Striatum and fMRI Brain Activation by Alcohol-Associated Stimuli, Relative to Control Stimuli, in Nine Detoxified Alcoholic Mena
aThe design of the fMRI block paradigm is shown at the top. The middle and bottom sections show a scatterplot and the localization, respectively, of the correlation between D2 receptor availability (binding potential) in the ventral striatum and functional brain activation (blood-oxygen-level-dependent [BOLD] response) elicited by the alcohol-associated stimuli, beyond the activation produced by the abstract and neutral control stimuli, in the left medial prefrontal cortex of the alcoholics (for presentation, p<0.001 uncorrected).
1. Robbins TW, Everitt BJ: Neurobehavioral mechanisms of reward and motivation. Curr Opin Neurobiol 1996; 6:228–236Crossref, Medline, Google Scholar
2. Koob GF, Le Moal M: Drug abuse: hedonic homeostatic dysregulation. Science 1997; 278:52–58Crossref, Medline, Google Scholar
3. Wise RA: Actions of drugs of abuse on the brain reward systems. Pharmacol Biochem Behav 1980; 13:213–223Crossref, Medline, Google Scholar
4. Robinson TE, Berridge KC: The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev 1993; 18:247–291Crossref, Medline, Google Scholar
5. Schultz W, Dayan P, Montague PR: A neural substrate of prediction and reward. Science 1997; 275:1593–1599Crossref, Medline, Google Scholar
6. Fiorillo CD, Tobler PN, Schultz W: Discrete coding of reward probability and uncertainty by dopamine neurons. Science 2003; 299:1898–1902Crossref, Medline, Google Scholar
7. Alexander GE, DeLong MR, Strick PL: Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci 1986; 9:357–381Crossref, Medline, Google Scholar
8. Haber SN, Fudge JL, McFarland NR: Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci 2000; 20:2369–2382Crossref, Medline, Google Scholar
9. Breiter HC, Gollub RL, Weisskoff RM, Kennedy DN, Makris N, Berke JD, Goodman JM, Kantor HL, Gastfried DR, Riorden JP, Mathew RT, Rosen BR, Hyman SE: Acute effects of cocaine on human brain activity and emotion. Neuron 1997; 19:591–611Crossref, Medline, Google Scholar
10. Tzschentke TM: Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol 1998; 56:613–672Crossref, Medline, Google Scholar
11. Ito R, Dalley JW, Robbins TW, Everitt BJ: Dopamine release in the dorsal striatum during cocaine seeking behavior under the control of a drug-associated cue. J Neurosci 2002; 22:6247–6253Crossref, Medline, Google Scholar
12. Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzmann R, Ding YS, Pappas N, Shea C, Piscani K: Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res 1996; 20:1594–1598Crossref, Medline, Google Scholar
13. Dettling M, Heinz A, Dufeu P, Rommelspacher H, Gräf K-J, Schmidt LG: Dopaminergic responsivity in alcoholism: trait, state, or residual marker? Am J Psychiatry 1995; 152:1317–1321Link, Google Scholar
14. Heinz A, Dufeu P, Kuhn S, Dettling M, Graf K, Kurten I, Rommelspacher H, Schmidt LG: Psychopathological and behavioral correlates of dopaminergic sensitivity in alcohol-dependent patients. Arch Gen Psychiatry 1996; 53:1123–1128Crossref, Medline, Google Scholar
15. George DT, Rawlings R, Eckhardt MJ, Phillips MJ, Shoaf S, Linnoila M: Buspirone treatment of alcoholism: age of onset, and cerebrospinal fluid 5-hydroxyindoleacetic acid and homovanillic acid concentrations, but not medication treatment, predict return to drinking. Alcohol Clin Exp Res 1999; 23:272–278Medline, Google Scholar
16. First MB, Spitzer RL, Gibbon M, Williams JB: Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/ PSY SCREEN). New York, New York State Psychiatric Institute, Biometrics Research, 2001Google Scholar
17. First MB, Gibbon M, Spitzer RL, Williams JB, Benjamin L: Structured Clinical Interview for DSM-IV Personality Disorders (SCID-II): Interview and Questionnaire. Washington, DC, American Psychiatric Press, 1997Google Scholar
18. Singleton EG, Henningfield JE, Tiffany ST: Alcohol Craving Questionnaire (ACQ-NOW): Background and Administration Manual. Baltimore, National Institute on Drug Abuse, Addiction Research Center, 1994Google Scholar
19. Gründer G, Siessmeier T, Piel M, Vernaleken I, Buchholz HG, Zhou Y, Hiemke C, Wong D, Roesch F, Bartenstein P: Quantification of D2-like dopamine receptors in the human brain with [18F]desmethoxyfallypride. J Nucl Med 2003; 44:109–116Medline, Google Scholar
20. Gunn RN, Lammertsma AA, Hume SP, Cunningham, VJ: Parametric imaging of ligand-receptor binding in PET using a simplified reference region model. Neuroimage 1997; 6:279–287Crossref, Medline, Google Scholar
21. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain: Three-Dimensional Proportional System. New York, Thieme Medical, 1988Google Scholar
22. Friston KJ, Holmes AP, Worsley K, Poline JB, Frith CD, Frackowiak RSJ: Statistical parametric maps in functional brain imaging: a general linear approach. Hum Brain Mapp 1995; 2:189–210Crossref, Google Scholar
23. Grüsser SM, Heinz A, Flor H: Standardized stimuli to assess drug craving and drug memory in addicts. J Neural Transm 2000; 107:715–720Crossref, Medline, Google Scholar
24. Wrase J, Grüsser SM, Klein S, Diener C, Hermann D, Flor H, Mann K, Braus DF, Heinz A: Development of alcohol-associated cues and cue-induced brain activation in alcoholics. Eur Psychiatry 2002; 17:287–291Crossref, Medline, Google Scholar
25. Center for the Study of Emotion and Attention (CSEA-NIMH): The International Affective Picture System (photographic slides). Gainesville, University of Florida, Center for Research in Psychophysiology, 1999Google Scholar
26. Taylor SF, Phan KL, Decker LR, Liberzon I: Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage 2003; 18:650–659Crossref, Medline, Google Scholar
27. Bradley MM, Lang PJ: Measuring emotion: the Self-Assessment Manikin and the Semantic Differential. J Behav Ther Exp Psychiatry 1994; 25:49–59Crossref, Medline, Google Scholar
28. Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF: Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry 1998; 155:124–126Link, Google Scholar
29. Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O’Brien CP: Limbic activation during cue-induced cocaine craving. Am J Psychiatry 1999; 156:11–18Link, Google Scholar
30. Garavan H, Pankiewicz J, Bloom A, Cho J-K, Sperry L, Ross TJ, Salmeron BJ, Risinger R, Kelley D, Stein EA: Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. Am J Psychiatry 2000; 157:1789–1798Link, Google Scholar
31. Kilts CD, Schweitzer JB, Quinn CK, Gross RE, Faber TL, Muhammad F, Ely TD, Hoffman JM, Drexler KP: Neural activity related to drug craving in cocaine addiction. Arch Gen Psychiatry 2001; 58:334–341Crossref, Medline, Google Scholar
32. Wexler BE, Gottschalk CH, Fulbright RK, Prohovnik I, Lacadie CM, Rounsaville BJ, Gore JC: Functional magnetic resonance imaging of cocaine craving. Am J Psychiatry 2001; 158:86–95Link, Google Scholar
33. George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Nahas Z, Vincent DJ: Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Arch Gen Psychiatry 2001; 58:345–352Crossref, Medline, Google Scholar
34. Braus DF, Wrase J, Grüsser SM, Hermann D, Ruf M, Flor H, Mann K, Heinz A: Alcohol-associated stimuli activate the ventral striatum in abstinent alcoholics. J Neural Transm 2001; 108:887–894Crossref, Medline, Google Scholar
35. Haber SN, McFarland NR: The concept of the ventral striatum in nonhuman primates. Ann NY Acad Sci 1999; 877:33–48Crossref, Medline, Google Scholar
36. Mawlawi O, Martinez D, Slifstein M, Broft A, Chatterjee R, Hwang DR, Huang Y, Simpson N, Ngo K, van Heertum R, Laruelle M: Imaging human mesolimbic dopamine transmission with positron emission tomography, I: accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J Cereb Blood Flow Metab 2001; 21:1034–1057Crossref, Medline, Google Scholar
37. Stockwell T, Hodgson R, Edwards G, Taylor C, Rankin H: The development of a questionnaire to measure severity of alcohol dependence. Br J Addict 1979; 74:79–87Crossref, Google Scholar
38. Skinner HA, Sheu WJ: Reliability of alcohol use indices: the Lifetime Drinking History and the MAST. J Stud Alcohol 1982; 43:1157–1170Crossref, Medline, Google Scholar
39. O’Reilly RC, Noelle DC, Braver TS, Cohen JD: Prefrontal cortex and dynamic categorization tasks: representational organization and neuromodulatory control. Cereb Cortex 2002; 12:246–257Crossref, Medline, Google Scholar
40. Contreras-Vidal JL, Schultz W: A predictive reinforcement model of dopamine neurons for learning approach behavior. J Comput Neurosci 1999; 6:191–214Crossref, Medline, Google Scholar
41. Fuster JM: The Prefrontal Cortex: Anatomy, Physiology, and Neuropsychology of the Frontal Lobe. Philadelphia, Lippincott-Raven, 1997Google Scholar
42. Volkow ND, Fowler JS: Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex 2000; 10:318–325Crossref, Medline, Google Scholar



