Prediction of Neuropsychological Performance by Neurological Signs in Schizophrenia
Abstract
OBJECTIVE: The major purposes of this study were 1) to examine whether neurological signs predict cognitive performance in both schizophrenic patients and healthy subjects and 2) to determine the ability of neurological signs and neuropsychological tests to discriminate schizophrenic patients from healthy subjects. METHOD: Eighty-five patients with a DSM-III-R diagnosis of schizophrenia and 36 normal comparison subjects were included in the study. All subjects were administered a comprehensive neuropsychological test battery, and neurological signs were assessed with the Neurological Evaluation Scale. Stepwise regression analyses were used to predict neuropsychological test performance from the subscale scores on the Neurological Evaluation Scale. Forward stepwise linear discriminant function analyses were used to examine the discriminative ability of neurological subscale scores, neuropsychological test scores, and the two combined. RESULTS: Scores on the Neurological Evaluation Scale predicted the neuropsychological test performance of both patients and comparison subjects. The sensory integration subscale score was the most frequent predictor of neuropsychological test performance. In contrast, the “others” subscale, which includes frontal release signs, abnormalities in eye movements, and short-term memory, was the most highly discriminating subscale, correctly classifying 78.5% of the total study group. The best predictors from the neuropsychological battery (category fluency and Trail Making Test, part A, time test) correctly classified 81.8%. When both sets of variables were used, the Neurological Evaluation Scale “others” subscale entered the discriminant function first. CONCLUSIONS: Neurological signs are reliably related to measures of neuropsychological performance and also reliably discriminate between patients and healthy subjects. However, some neurological signs may be more sensitive to the presence of schizophrenia, while others may be more predictive of neuropsychological performance.
Patients with schizophrenia are characterized by the presence of neurological abnormalities that are not specific to the disease but are more prevalent in such patients than in persons with other mental illnesses and normal control subjects (1). These neurological abnormalities classically have been divided into “hard” signs and “soft” signs. The former are signs that are localizable to a specific brain area, while the latter are considered nonspecific and nonlocalizing. Schizophrenic patients have been shown to have a greater prevalence of both soft and hard signs when compared with normal control subjects (1). These differences do not appear to be attributable to either medication status or the presence of medication-induced neurological side effects (e.g., tardive dyskinesia, extrapyramidal symptoms).
The significance of neurological abnormalities in schizophrenia is unclear. It has been argued that they represent a trait characteristic of the disorder, and they have been observed to be associated with positive family history (1). Genetic (2), environmental (3), or intrauterine or perinatal (4) etiologies have been proposed for these signs. It is possible, then, that they are indicators of prior brain insults/injuries undetected in earlier life that predispose to schizophrenia or are modifying factors for the outcome of the disease. An approach for examining their significance is to study the correlations of neurological signs with other illness manifestations. In previous studies, these signs have been correlated with negative or deficit symptoms (5–7), the disorganized subtype (8), poorer outcome (9), lower psychosocial performance (10), earlier age at onset (11), and repeated episodes of violence (12).
The relation between neurological signs and another important disease manifestation, cognitive impairment, has not received much attention in the past. Most but not all previous studies have found a correlation between general cognitive dysfunction and neurological signs (13–16). Neurological signs have been correlated with markers of organicity such as lower IQ (17, 18) and impaired performance on the Mini-Mental State examination (11, 17, 19). Cuesta et al. (20) reported that seven “frontal” neurological signs correlated with poor cognitive testing performance.
Two recent studies, however, have pointed to a more selective correlation between neurological signs and neuropsychological impairment. Mohr et al. (21), using the Neurological Evaluation Scale, studied neurological signs in a group of 93 patients with schizophrenia. Neurological Evaluation Scale total score and subscale scores correlated with scores on the Wisconsin Card Sorting Test and the Weigl Sorting Test (measures thought to represent frontal lobe function) after control for performance on the Raven Standard Progressive Matrices. Correlations were highest for the sequencing of complex motor acts subscale of the Neurological Evaluation Scale. The authors suggested that this relationship was suggestive of prefrontal cortical dysfunction.
In the second study, Flashman et al. (22) assessed 22 neurological signs, including six developmental reflexes. Patients with neurological signs (N=68) and those without (N=108) were compared. The only neuropsychological tests that discriminated between the two groups after control for lifetime medication exposure, tardive dyskinesia, and extrapyramidal symptoms were those involved with motor speed and motor coordination (e.g., finger tapping, the Purdue pegboard task, and the Trail Making Test, part B). Tests of general cognitive function, attention, frontal lobe function, and memory failed to distinguish between the two groups.
Neurological signs have therefore been related to both global and selective cognitive impairments. These discrepant results could be attributed to variability in the assessment of neurological signs and neuropsychological tests, not using validated standardized scales, differential attention to potential confounding variables, and/or population differences among studies. The current study was an attempt to clarify previous findings by using a validated neurological scale and a comprehensive neuropsychological test battery. A further goal was to assess the ability of the Neurological Evaluation Scale to discriminate between normal comparison subjects and patients with schizophrenia.
METHOD
All patients attending the Maryland Psychiatric Research Center Outpatient Research Program who met the DSM-III-R criteria for chronic schizophrenia and had both a neurological and a neuropsychological assessment performed within 3 months of each other were included in the study. Eighty-five patients were selected. All available sources of information were used for making the diagnosis, including the Structured Clinical Interview for DSM-III-R—Patient Version (23), direct assessment, family informants, and past medical records. Patients with an organic brain disorder, mental retardation, or a history of drug abuse/dependence were excluded from the study.
Thirty-six normal comparison subjects were recruited from the general population through advertisements. The comparison subjects did not have past or current DSM-III-R axis I or axis II disorders, as determined by the Structured Clinical Interview for DSM-III-R, or a history of organic brain disorder, mental retardation, or severe head trauma.
Patients and comparison subjects gave written informed consent before examination, and the comparison subjects were reimbursed for participation in the study.
The patients were interviewed while clinically stable. The majority of the patients were receiving conventional antipsychotics (either fluphenazine or haloperidol) at the time of testing; two patients were drug free, and one patient was taking clozapine. The Brief Psychiatric Rating Scale (BPRS) (24) was used to measure symptoms. Dyskinetic and parkinsonian movements were assessed with the Maryland Psychiatric Research Center Involuntary Movement Scale (25). Mean neuroleptic doses for each group were determined with the use of the conversion system developed by Schooler (26). Anticholinergic medication was rated as present or not.
Neurological signs were evaluated with the Neurological Evaluation Scale (27). The scale comprises 26 items designed to assess three functional areas of interest: sensory integration, motor coordination, and sequencing of complex motor acts. In addition, short-term memory, frontal release signs, and eye movement abnormalities are included in an “others” category. The items are presented in appendix 1. The total score and scores for each of the four subscales were used in the study to measure severity of neurological impairment. The interrater reliability (intraclass correlation coefficient) for the subscale scores and total score ranged from 0.63 (for motor coordination) to 0.99 (for sensory integration). Total time for the administration of the Neurological Evaluation Scale was approximately 45 minutes.
The neuropsychological test battery (28–37) is presented in appendix 2. Neuropsychological tests were administered according to standardized testing procedures. The Mini-Mental State examination (38) was administered before the Neurological Evaluation Scale. All other tests were administered in a fixed rather than a random order to ensure that more difficult tasks did not occur consecutively. Frequent breaks were allowed, and if necessary, tests were administered in two test sessions. Not all patients and comparison subjects completed all measures in the battery, so the number of patients and comparison subjects varies from test to test.
In the statistical analysis, the distribution of each variable was examined with the Kolmogorov-Smirnov test to determine whether it was normally distributed. Scores on the Trail Making Test, parts A and B (measured as time to complete the test in seconds), which were not normally distributed, were log transformed to normalize them. Derived scores were used for the Trail Making Test, part B, and the Stroop Color and Word Test so that measures focused on the executive function construct (37). For the Trail Making Test, we measured the difference between each subject’s actual part B score and the part B score estimated by the regression of the log-transformed part A time on the log-transformed part B time (39). The regression equation was computed using the data from the comparison subjects. A similar approach was used to estimate the residual score for the Stroop color-word measure. The residual score was the difference between the observed color-word score and the score predicted by the color-word score with the color score regression in the comparison group. Raw scores were used for the individual subscales of the Wechsler Memory Scale—Revised. Demographic variables of patients and comparison subjects were compared by t tests and chi-square analyses. Differences in scores on the Neurological Evaluation Scale were also compared by t tests.
Stepwise regression analysis was used to predict neuropsychological test performance from scores on the neurological signs subtests. Possible confounding factors were based on previous studies showing that different variables relate to neurological signs (1, 14, 40–42). Separate regression models were constructed for the patient and comparison groups. For comparison subjects the covariates were age, sex, race, and parental socioeconomic status. To control for the potentially confounding effects of ancillary variables, these variables, common to comparison subjects and patients, were entered as a block at step 1 of the regression analyses. For patients the additional covariates were BPRS total score, parkinsonism rating from the Involuntary Movement Scale, tardive dyskinesia rating from the Involuntary Movement Scale, medication status, and anticholinergic medication. To facilitate direct comparison between comparison subjects and patients, covariates for the patients were entered in two blocks, the first including the same covariates used for the normal comparison subjects and the second block including the additional patient covariates. Because the Neurological Evaluation Scale “others” subscale includes two memory items (appendix 1), the regression analysis for neuropsychological tests related to memory function were performed excluding those two items.
Finally, three forward stepwise linear discriminant function analyses, with jackknifed classification matrices, were performed to examine the discriminant ability of neurological signs and neuropsychological tests to categorize the subjects as patients or comparison subjects. This linear discriminant function model is developed using all of the subjects minus one (N–1). The resulting linear discriminant function is used to predict the group membership of the omitted subject. The process is repeated for all subjects. This method provides for internal replication, minimizes bias, is conservative, and ensures maximal interpretability (43). Separate discriminant functions were derived for the Neurological Evaluation Scale subscales and the neuropsychological tests. In the third discriminant analysis, scores on both the Neurological Evaluation Scale subscales and the neuropsychological tests were entered to find the variables that maximized correct classification. All neuropsychological tests included in appendix 2 were available for entering in the discriminant analyses, with the exception of three (Continuous Performance Test, Span of Apprehension, and Mini-Mental State examination), which were not available for some patients, and the WAIS-R, which was not available for the comparison subjects.
RESULTS
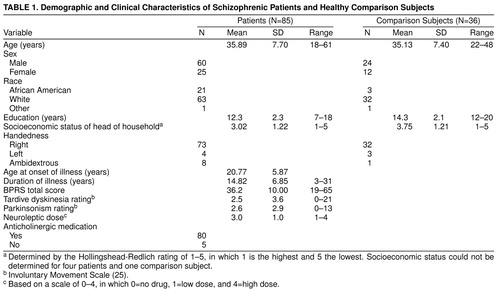
The demographic and clinical variables of the patients and normal comparison subjects are presented in table 1. There were no significant differences between the two groups in age, sex, race, or socioeconomic status of the head of household. The comparison subjects had significantly more years of education than the patients (t=4.46, df=119, p<0.001). Neurological Evaluation Scale scores and neuropsychological test scores of the patients and comparison subjects are reported in table 2 and table 3, respectively. Patients had significantly higher Neurological Evaluation Scale total scores and scores on the sensory integration, motor coordination, sequencing of complex motor acts, and “others” subscales. Because education significantly differed between the groups, an analysis of covariance controlling for education was performed. All differences remained significant.
Regression analyses for patients and comparison subjects are reported in table 4 and table 5. For patients, neurological signs subscale scores significantly increased the variance explained for 15 of 19 test results after control for covariates. For comparison subjects, one-half of the neuropsychological test results were also predicted by neurological subtest scores. Sensory integration was the Neurological Evaluation Scale subscale score most frequently found to increase the variance explaining the neuropsychological test results. As shown in Table 4, sensory integration significantly predicted the results of 12 of the 19 neuropsychological tests for the patients. For the comparison subjects (table 5) fewer neuropsychological test results were predicted by neurological subscale scores: sensory integration scores significantly predicted the results of three of the 17 neuropsychological tests, and scores on sequencing of complex motor acts predicted the results of four of the 17 tests. Although some neuropsychological test results were predicted by more than one neurological subscale score, in most cases a neuropsychological test result was predicted by performance on only one of the neurological subtests.
Three forward stepwise linear discriminant function analyses with jackknifed classification matrices were run to examine the discriminant ability of neurological signs, the neuropsychological test battery, and both assessments together. In the first analysis, with neurological signs, the scores on the four Neurological Evaluation Scale subscales were available for entering. Only one variable, the Neurological Evaluation Scale “others” subscale score, met the inclusion criterion (F=4.0 to enter) (F=56.5, df=1, 120, p<0.00001). This variable accounted for an overall 78.5% correct jackknife classification. Individually, 75.3% of the schizophrenic patients were correctly classified (empirical sensitivity), and 86.1% of the comparison subjects were correctly classified (empirical specificity). In the second analysis, with the neuropsychological tests, at step 1, category fluency met the entry condition (F=53.3, df=1, 199, p<0.00001). This variable accounted for an overall 78.5% correct jackknife classification. At step 2, the Trail Making Test, part A (time), entered (F=13.3, df=1, 119, p<0.0001). These two variables accounted for an overall 81.8% correct jackknife classification. Individually, 80.0% of the schizophrenic patients were correctly classified, and 86.1% of the comparison subjects were correctly classified. None of the remaining neuropsychological variables met the entry criteria for the linear discriminant function. In the third analysis, when both neurological and neuropsychological variables were available to enter, the Neurological Evaluation Scale “others” subscale score entered first (F=56.5, df=1, 199, p<0.00001). At step 2 category fluency entered, at step 3 the logical memory subscale (Wechsler Memory Scale—Revised) entered, and at step 4 the Trail Making Test, part A, entered. Together these last three variables added only 3.3% to the classification matrix. Overall, the four variables accounted for an 81.8% correct jackknife classification matrix. Individually, 91.5% of the schizophrenic patients were correctly classified, and 78.0% of the comparison subjects were correctly classified.
DISCUSSION
Patients with schizophrenia exhibit more neurological signs than normal comparison subjects; in the present study the difference was significant for the total score and the scores on each of the subscales derived from the standardized scale used for this study (27). This replicates our previous finding (27). In the examination of the relation between neurological signs and neuropsychological testing, the major findings were that 1) for both comparison subjects and patients, Neurological Evaluation Scale scores significantly predicted most neuropsychological test results; 2) sensory integration predicted most of the neuropsychological test results; and 3) neurological items grouped in the Neurological Evaluation Scale “others” subscale discriminated patients from comparison subjects better than any other neurological subscale. When neurological scores were entered in the linear discriminant function with all of the neuropsychological test results, the “others” subscale scores entered into the analysis first.
Previous studies have shown that global neurological impairment relates to global neuropsychological function or that global neurological impairment relates to specific neuropsychological function (frontal function, motor speed, and motor coordination). In the present study, while the first case seems to be true, a third possibility arises: specific neurological impairments are related to a broad array of neuropsychological test results. In the regression analyses, and after control for all theoretical confounding factors, neurological subscores increased the variance explained for most neuropsychological test results among the patients and for eight of the 17 neuropsychological tests performed by the comparison subjects. The lesser association of neuropsychological test results and neurological signs among the normal comparison subjects could be due to a reduced variance in the comparison subjects or to a lesser degree of association between those variables in persons not affected by schizophrenia. The correlation between neurological signs and neuropsychological test performance suggests that the two types of measures share common variance. However, most of the variance of the latter is not explained by neurological signs, suggesting that both measures also capture unique variance. The finding that neurological impairment correlates with general cognitive impairment and not with frontal function or motor speed differs from the findings of studies by Flashman et al. (22) and Mohr et al. (21) but is in agreement with those of most previous studies (13, 15, 40). Unfortunately, our battery did not include neuropsychological tests that assess motor coordination. The differences among studies could be attributable to the neurological signs and neuropsychological tests assessed, including the use of structured neurological sign assessments, different statistical approaches, differences in samples (patients in the Flashman et al. and Mohr et al. studies were inpatients), or the influence of possible confounding factors. In the study by Flashman et al., the neurological signs used, although similar to those included in the Neurological Evaluation Scale, did not include items of complex motor act sequencing. The disparity in the prevalence of neurological signs between the present study and the Flashman et al. study suggests that a different threshold was used to rate neurological signs as present.
Neurological signs were related to a broad range of cognitive processes, including attention, executive function, memory, motor tasks, general cognitive impairment (Mini-Mental State examination), and intelligence (WAIS-R). Thus, there was little evidence for any relation between neurological signs and specific cognitive impairments. However, the Neurological Evaluation Scale sensory integration items were associated with more neuropsychological measures than any other subset of neurological signs, with the largest increment in variance explained, in patients with schizophrenia, associated with the WAIS-R performance. WAIS-R data were not available for the normal comparison subjects, so it is not possible to determine whether the notable variance explained by neurological signs for the WAIS-R in patients can be extrapolated to normal subjects. The Neurological Evaluation Scale sequencing of complex motor acts and “others” subscale scores were related to performance on only two neuropsychological tests, and the Neurological Evaluation Scale motor coordination signs predicted performance on only one of the neuropsychological tests. These results suggest that Neurological Evaluation Scale sensory integration signs, which are associated with parietal lobe injury (44), may be a more sensitive measure of general cognitive integrity than the remaining neurological signs, and that if patients (or normal comparison subjects) exhibit impairments on these signs, then they are more likely to exhibit impaired neuropsychological test performance.
There are several possible explanations for this apparently broad relation between sensory integration and neuropsychological test performance. First, this subscale of the Neurological Evaluation Scale has the highest reliability of any of the subscales. Therefore, the evidence of covariance with neuropsychological performance may simply reflect the superior psychometric properties of this scale. Although we cannot rule out this “artifactual” explanation, it appears highly unlikely. If the Neurological Evaluation Scale sensory integration subscale has the greatest proportion of true score variance, it might also be expected to be the test best able to discriminate patients from control subjects. However, that is not what was observed, since it was the “others” subscale score that was the best discriminator and, in fact, the only Neurological Evaluation Scale variable that entered into the linear discriminant function equation. Two other explanatory frameworks can be considered. First, although abnormalities of the parietal cortex have not been highlighted in the schizophrenia literature on postmortem findings or those from structural and functional imaging studies, a number of recent studies have suggested that schizophrenia involves a diffuse gray matter abnormality or an abnormality of the heteromodal association cortex, including the inferior parietal cortex (45). It is plausible that evidence of functional compromise of the parietal cortex provides an index of the “extent” of cortical pathology. Such a marker of compromised cortical function would be expected to be related to multiple cognitive functions, including those not primarily dependent on the parietal lobe. In essence, that is what we observed in our patient group. This argument, however, does not provide any explanation for the fact that the sensory integration subscale score of the Neurological Evaluation Scale also contributed to the regressions in the normal comparison group. An alternative explanation is that the correlations reflect the fact that the parietal lobe plays a critical role in the support of more complex, widely distributed cognitive functions as the principal site of polysensory, polymodal integration. Thus, an impairment (or, possibly, normal individual differences) in these functions might be expected to be related to fairly broad cognitive consequences.
These three explanations (psychometric artifact, disease-specific implications of parietal function, and cognitive implications of parietal function) are not necessarily mutually exclusive, and it is difficult to evaluate their relative merits in this data set with confidence. However, the present data suggest that further studies of parietal lobe structure and function in schizophrenia may prove to be fruitful. If a high correlation between Neurological Evaluation Scale sensory integration items and neuropsychological impairment is confirmed in further studies, this simple, inexpensive, and easy-to-administer subscale could be used to screen patients for neuropsychological impairment.
Neurological signs included in the “others” subscale of the Neurological Evaluation Scale discriminated between normal subjects and patients as accurately as the comprehensive neuropsychological test battery (78.5% versus 81.8%). In addition, when both assessments were included in the discriminant function analysis, the overall percent of total classification was 81.8%, and the variable that entered first was the Neurological Evaluation Scale “others” subscale score. The “others” subscale includes frontal release signs (primitive reflexes), abnormalities in eye movements, and short-term memory. These findings are in agreement with recent studies, which show that developmental reflexes (e.g., the snout reflex) are more discriminating than soft signs (46). The fact that the Neurological Evaluation Scale was basically as discriminating as a fairly comprehensive neuropsychological battery is somewhat surprising: one might expect that the more refined measurement of memory, attention, and executive function available in the cognitive test battery would more completely separate the diagnostic groups. This was clearly not what we observed. We suggest that the Neurological Evaluation Scale is a good indicator of the presence of an “abnormality”: something that occurs with low frequency among comparison subjects but is present with high frequency in ill patients, including patients who demonstrate generally good intellectual performance. Alternatively, neuropsychological performance is more broadly distributed (in both groups) and is likely influenced by a variety of demographic and educational factors (that may be independent of the illness), which may decrease the sensitivity and specificity of neuropsychological test scores. In the current patient series, we examined the Neurological Evaluation Scale performance of the 15 patients with the highest IQs (the mean of this group across four WAIS-R subtests was 109, slightly above the normal population mean). These patients scored significantly worse than the comparison subjects on the Neurological Evaluation Scale “others” subscale (p=0.005) but did not differ on the other three scales. This finding complements the other results of the study and provides further evidence that the “others” subscale is sensitive to the presence of schizophrenia, even among patients with relatively intact cognitive performance. The high discriminant strength of the “others” subscale supports the inclusion of these items in the study of neurological signs as a phenotypic marker. We suggest that studies designed to address the familial transmission of the disease should include the items from the Neurological Evaluation Scale “others” subscale.
There are some limitations to this study. One is the small number of subjects, especially in the comparison group. Another limitation refers to generalizability; because patients attending our clinic are a group with chronic illness, the present findings may not be generalizable to other settings.
In conclusion, neurological signs seem to be a marker of global cognitive impairment. Although neurological signs have been previously reported as soft signs because of a lack of localizing ability, some subsets of these signs are specifically related to different neuropsychological tests thought to represent function in different brain areas. In addition, a battery of neurological signs, such as the Neurological Evaluation Scale in the present study, has shown the same discriminating power as a comprehensive neuropsychological test battery. Integration of knowledge about the different neurological impairments in schizophrenia should illuminate the pathophysiology of the disease.
Presented in an earlier version at the Winter Workshop on Schizophrenia, Davos, Switzerland, Feb. 7–13, 1998. Received June 12, 1998; revision received Feb. 18, 1999; accepted Feb. 23, 1999. From the Maryland Psychiatric Research Center, University of Maryland School of Medicine. Address reprint requests to Dr. Buchanan, Maryland Psychiatric Research Center, P.O. Box 21247, Baltimore, MD 21228; [email protected] (e-mail). Supported in part by NIMH grants MH-48225 and MH-40279 and grant 97/5091 from Fondo de Investigación Sanitaria, Ministerio de Sanidad y Consumo, Spain.
 |
 |
 |
 |
 |
 |
APPENDIX 1. Items on the Neurological Evaluation ScaleaFor a description of the items, see Buchanan and Heinrichs (27).bAssessed bilaterally.
 |
APPENDIX 2. Neuropsychological Test Battery
1. Heinrichs DW, Buchanan RW: Significance and meaning of neurological signs in schizophrenia. Am J Psychiatry 1988; 145:11–18Link, Google Scholar
2. Fish B: Neurobiologic antecedents of schizophrenia in children: evidence for an inherited, congenital neurointegrative defect. Arch Gen Psychiatry 1977; 34:1297–1313Google Scholar
3. Cooper SJ, King DJ: Is schizophrenia a neurodevelopment disorder? (letter). BMJ 1987; 295:1068Crossref, Google Scholar
4. Parnas J, Schulsinger F, Teasdale TW, Schulsinger H, Feldman PM, Mednick SA: Perinatal complications and clinical outcome within the schizophrenia spectrum. Br J Psychiatry 1982; 140:416–420Crossref, Medline, Google Scholar
5. Merriam AE, Kay SR, Opler LA, Kushner SF, van Praag HM: Neurological signs and the positive-negative dimension in schizophrenia. Biol Psychiatry 1990; 28:181–192Crossref, Medline, Google Scholar
6. Buchanan RW, Kirkpatrick B, Heinrichs DW, Carpenter WT Jr: Clinical correlates of the deficit syndrome of schizophrenia. Am J Psychiatry 1990; 147:290–294Link, Google Scholar
7. Smith RC, Kadewari RP: Neurological soft signs and response to risperidone in chronic schizophrenia. Biol Psychiatry 1996; 40:1056–1059Google Scholar
8. Schroder J, Geider FJ, Binkert M, Reitz C, Jauss M, Sauer H: Subsyndromes in chronic schizophrenia: do their psychopathological characteristics correspond to cerebral alterations? Psychiatry Res 1992; 42:209–220Google Scholar
9. Johnstone EC, Macmillan JF, Frith CD, Benn DK, Crow TJ: Further investigation of the predictors of outcome following first schizophrenic episodes. Br J Psychiatry 1990; 157:182–189Crossref, Medline, Google Scholar
10. Wong AH, Voruganti LN, Heslegrave RJ, Awad AG: Neurocognitive deficits and neurological signs in schizophrenia. Schizophr Res 1997; 23:139–146Crossref, Medline, Google Scholar
11. Quitkin F, Rifkin A, Klein DF: Neurologic soft signs in schizophrenia and character disorders: organicity in schizophrenia with premorbid asociality and emotionally unstable character disorders. Arch Gen Psychiatry 1976; 33:845–853Crossref, Medline, Google Scholar
12. Krakowski MI, Convit A, Jaeger J, Lin S, Volavka J: Neurological impairment in violent schizophrenic patients. Am J Psychiatry 1989; 146:849–853Link, Google Scholar
13. Tucker GJ, Campion EW, Silberfarb PM: Sensorimotor functions and cognitive disturbance in psychiatric patients. Am J Psychiatry 1975; 132:17–21Link, Google Scholar
14. Kolakowska T, Williams AO, Jambor K, Ardern M: Schizophrenia with good and poor outcome, III: neurological “soft” signs, cognitive impairment and their clinical significance. Br J Psychiatry 1985; 146:348–357Crossref, Medline, Google Scholar
15. Liddle PF: Schizophrenic syndromes, cognitive performance and neurological dysfunction. Psychol Med 1987; 17:49–57Crossref, Medline, Google Scholar
16. Chen EYH, Lam LCW, Chen RYL, Nguyen DGH: Cognitive correlates of soft signs in schizophrenia (abstract). Schizophr Res 1997; 24:100Crossref, Google Scholar
17. Kennard MA: Value of equivocal signs in neurologic diagnosis. Neurology 1960; 10:753–764Crossref, Medline, Google Scholar
18. Mosher LR, Pollin W, Stabenau JR: Identical twins discordant for schizophrenia: neurologic findings. Arch Gen Psychiatry 1971; 24:422–430Crossref, Medline, Google Scholar
19. Manschreck TC, Ames D: Neurologic features and psychopathology in schizophrenic disorders. Biol Psychiatry 1984; 19:703–719Medline, Google Scholar
20. Cuesta MJ, Peralta V, de Leon J: Neurological frontal signs and neuropsychological deficits in schizophrenic patients. Schizophr Res 1996; 20:15–20Crossref, Medline, Google Scholar
21. Mohr F, Hubmann W, Cohen R, Bender W, Haslacher C, Honicke S, Schlenker R, Wahlheim C, Werther P: Neurological soft signs in schizophrenia: assessment and correlates. Eur Arch Psychiatry Clin Neurosci 1996; 246:240–248Crossref, Medline, Google Scholar
22. Flashman LA, Flaum M, Gupta S, Andreasen NC: Soft signs and neuropsychological performance in schizophrenia. Am J Psychiatry 1996; 153:526–532Link, Google Scholar
23. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R—Patient Version (SCID-P). New York, New York State Psychiatric Institute, Biometrics Research, 1989Google Scholar
24. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812Crossref, Google Scholar
25. Cassady SL, Thaker GK, Summerfelt A, Tamminga CA: The Maryland Psychiatric Research Center Scale and the characterization of involuntary movements. Psychiatry Res 1997; 70:21–37Crossref, Medline, Google Scholar
26. Schooler N: Antipsychotic medications in schizophrenia: effects in acute and maintenance treatment of the illness, in Schizophrenia: Origins, Processes, Treatment, and Outcome. Edited by Cromwell RL, Snyder CR. New York, Oxford University Press, 1993, pp 284–295Google Scholar
27. Buchanan RW, Heinrichs DW: The Neurological Evaluation Scale (NES): a structured instrument for the assessment of neurological signs in schizophrenia. Psychiatry Res 1989; 27:335–350Crossref, Medline, Google Scholar
28. Taylor LB: Psychological assessment of neurosurgical patients, in Functional Neurosurgery. Edited by Rasmussen T, Marino R. New York, Raven Press, 1979, pp 165–180Google Scholar
29. Milner B: Some effects of frontal lobectomy in man, in The Frontal Granular Cortex and Behavior. Edited by Warren JM, Akert K. New York, McGraw-Hill, 1964, pp 313–334Google Scholar
30. Golden CJ: Stroop Color and Word Test: A Manual for Clinical and Experimental Uses. Chicago, Stoelting, 1978Google Scholar
31. Reitan RM, Wolfson D: The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Tucson, Ariz, Neuropsychology Press, 1975Google Scholar
32. Mooney CM: Age in the development of closure ability in children. Can J Psychol 1957; 11:219–226Crossref, Medline, Google Scholar
33. Benton AL, Hannay HJ, Varney NR: Visual perception of line direction in patients with unilateral disease. Neurology 1975; 25:907–910Crossref, Medline, Google Scholar
34. Wechsler D: Wechsler Adult Intelligence Scale—Revised, Manual. Cleveland, Psychological Corp, 1981Google Scholar
35. Wechsler D: A standardized memory scale for clinical use. J Psychol 1945; 19:87–95Crossref, Google Scholar
36. Russell EW: A multiple scoring method for assessment of complex memory functions. J Consult Clin Psychol 1975; 43:800–809Crossref, Google Scholar
37. Lezak MD: Neuropsychological Assessment, 2nd ed. New York, Oxford University Press, 1983Google Scholar
38. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
39. Buchanan RW, Strauss ME, Kirkpatrick B, Holstein C, Breier A, Carpenter WT Jr: Neuropsychological impairments in deficit vs nondeficit forms of schizophrenia. Arch Gen Psychiatry 1994; 51:804–811Crossref, Medline, Google Scholar
40. King DJ, Wilson A, Cooper SJ, Waddington JL: The clinical correlates of neurological soft signs in chronic schizophrenia. Br J Psychiatry 1991; 158:770–775Crossref, Medline, Google Scholar
41. Gupta S, Andreasen NC, Arndt S, Flaum M, Schultz SK, Hubbard WC, Smith M: Neurological soft signs in neuroleptic-naive and neuroleptic-treated schizophrenic patients and in normal comparison subjects. Am J Psychiatry 1995; 152:191–196Link, Google Scholar
42. Barnes TR, Crichton P, Nelson HE, Halstead S: Primitive (developmental) reflexes, tardive dyskinesia and intellectual impairment in schizophrenia. Schizophr Res 1995; 16:47–52Crossref, Medline, Google Scholar
43. Efron B: The Jackknife, the Bootstrap, and Other Resampling Plans: CBMS Monograph 38. Philadelphia, Society for Industrial and Applied Mathematics, 1981Google Scholar
44. Brown JW: Frontal lobe syndromes, in Handbook of Clinical Neurology, vol 45 (revised series 1): Clinical Neuropsychology. Edited by Fredericks JAM. New York, Elsevier, 1985, pp 23–41Google Scholar
45. Pearlson GD, Petty RG, Ross CA, Tien AY: Schizophrenia: a disease of heteromodal association cortex? Neuropsychopharmacology 1996; 14:1–17Google Scholar
46. Ismail B, Cantor-Graae E, McNeil TF: Neurological abnormalities in schizophrenic patients and their siblings. Am J Psychiatry 1998; 155:84–89Link, Google Scholar



