Longitudinal Study of Cognitive Function in First-Episode and Recent-Onset Schizophrenia
Abstract
OBJECTIVE: Whether cognitive function in schizophrenia deteriorates, improves, or remains stable is a crucial question. Few studies have examined the longitudinal stability of cognitive function and the relationship between cognitive performance and clinical symptoms over time in a cohort of well-treated patients with schizophrenia. METHOD: In the present study, 54 patients with first-episode and recent-onset schizophrenia completed a comprehensive cognitive test battery and were rated on symptom measures at index hospitalization and again after 5 years. RESULTS: Performance IQ and full-scale IQ significantly improved, whereas verbal IQ did not change. Group performance improved on some of the neuropsychological tests, including the Circle A letter-cancellation task, free recall of logical memory test score, and the Wisconsin Card Sorting Test. Mean finger-tapping performance worsened over time, whereas performance on other neuropsychological tests did not change. Negative, psychotic, and disorganized symptoms significantly improved over the time period. Changes in negative symptoms were correlated with performance changes in verbal IQ and full-scale IQ but not performance IQ. Improvement in verbal cognition was observed when negative symptoms improved. Psychotic and disorganized symptom dimensions were not correlated with any IQ measure. CONCLUSIONS: These results indicate that in a cohort of young patients receiving neuroleptic treatment early in their illness, cognitive performance does not deteriorate—and may improve. Only one of the three symptom dimensions—negative—was associated with change in cognitive performance. This study supports the view that negative symptoms are associated with a poor long-term cognitive outcome and may be closely related to the primary cognitive deficit in schizophrenia.
Abnormalities in cognition have been a core feature in the diagnosis of schizophrenia since at least the time of Bleuler (1) and Kraepelin (2). However, despite decades of study, there is no clear understanding of the longitudinal course of cognitive dysfunction. When does cognitive dysfunction begin? Does it predate the onset of psychosis? Is there further decline in function after the onset of psychosis, and, if so, what is the course of the decline? Do factors such as clinical symptoms affect cognitive performance? Many older as well as more recent studies suggest that cognitive dysfunction is present in children who later develop schizophrenia (3–9). The literature documenting the course of deficits after the onset of the illness has been more inconsistent. Many studies have supported the notion of steady decline in cognitive function (10–16). However, other studies have observed improvements in cognitive function over time (11, 17–19). Most recently, prospective longitudinal studies of patients with first-episode schizophrenia have not supported progressive decline in cognitive function but instead have suggested that there may be stability and even mild improvement over time. In a previous study by our center (20), 35 patients with first-episode or recent-onset schizophrenia were followed and administered a comprehensive battery of neuropsychological tests during index hospitalization and at either 1- or 2-year follow-up examinations. Cognitive function remained stable in most domains, with performance on tests of mental set shifting (Trail Making B and Stroop) showing improvement over time. Furthermore, DeLisi et al. (21) evaluated the longitudinal illness course of 20 patients with first-episode schizophrenia at index hospitalization and at 2-, 3-, and 4-year follow-up examinations. The majority of the cognitive test scores did not change over time; improvement occurred in tests of concentration/speed, right hemispheric function, and overall global functioning. These findings are consistent with other longitudinal studies of patients with first-episode and recent-onset schizophrenia (22, 23).
In addition to the examination of the longitudinal course of cognitive deficits, characterization of the specific cognitive deficits has been attempted through cross-sectional studies. While virtually every cognitive domain that has been tested (e.g., attention, memory, and language) has identified deficits (24–31), not all patients with schizophrenia demonstrate the same pattern or degree of impairment. Many studies have explored correlates of cognitive dysfunction in an attempt to develop a parsimonious explanation of the variability in profiles of deficits.
One commonly employed research strategy applied to cross-sectional data has been the dimensional approach (32), in which factor analytic techniques are used to first identify symptom dimensions of schizophrenia. Correlations between the dimensions and cognitive performance are then examined. This allows for each individual to have scores on each dimension. Many studies have demonstrated a relationship between cognitive deficits and negative symptoms, psychotic symptoms, and disorganized symptoms. Results have further highlighted the relevance of sensory modality of the stimuli presented, in addition to the cognitive processes required to perform the task, and seem to be differentially associated with particular symptoms. The negative symptom dimension has the strongest relationship with cognitive performance. As shown in previous studies, it is correlated with deficits on measures of visual and motor processing (27, 33–35), generalized brain dysfunction (36), conceptual thinking, object naming, long-term memory (28, 32), and attentional processes, including visual information processing deficits, a lower signal discrimination (visual continuous performance tasks, forced-choice span of apprehension task), and lower auditory-visual processing capacity and concentration (35, 37–40). In contrast, auditory processing deficits have been related to positive symptoms (33), as well as verbal memory impairments (27, 28) and auditory distractibility (39). The disorganized dimension has been linked to deficits in visual and motor processing (31), concentration, word learning, immediate recall (32), and visual sustained attention (37).
In addition to the relationship between symptoms and cognitive performance, the contributions of external variables have also been studied. For instance, Heaton et al. (41) did not find support for a relationship between age at onset and duration of illness with neuropsychological impairment in schizophrenia. This study further supports the supposition that cognitive decline does not continue throughout the course of the illness. However, Hoff et al. (42) found a relationship between early age at onset and impairment in motor and language tasks. Relationships have been observed between cognitive impairment and medication status, response to treatment (43–47), and diagnostic subtype (33). Cognitive impairments have also been correlated with structural brain abnormalities, including cerebral ventricular enlargement and sulcal widening (48–55) and temporal lobe volume (56). Heaton and Drexler (57) reviewed 10 longitudinal neuropsychological studies of patients with schizophrenia and found that patients tested after the onset of illness and at multiple follow-up intervals did not significantly change or demonstrated modest improvement in cognitive performance (see also reference 22).
Both neurodevelopmental and neurodegenerative models have been used to explain cognitive deficits in schizophrenia (58). A clear consensus has not been established in the literature as to whether cognitive decline occurs as the illness progresses, whether cognitive function remains the same, or whether it improves. Prospective longitudinal study of patients with first-episode and recent-onset schizophrenia can shed light on the degree to which cognitive decline occurs during the long-term course of schizophrenia. The aim of this study was to examine the longitudinal course of cognitive function in a group of patients with first-episode and recent-onset schizophrenia. Patients were observed at index hospitalization and at a 5-year follow-up assessment. Fifty-four patients with schizophrenia spectrum disorders completed a comprehensive cognitive battery of tests and were assessed on multiple clinical measurements at both time periods.
METHOD
Subjects
Patients were evaluated as part of the Iowa Prospective Longitudinal Study of recent-onset psychosis. Methods for selecting the overall population have been described in detail previously (59). Briefly, patients with schizophrenia spectrum diagnoses were recruited from patients consecutively admitted to the general psychiatric ward and to the Mental Health Clinical Research Center at the University of Iowa Hospitals and Clinics in Iowa City. Written informed consent was obtained from all participants.
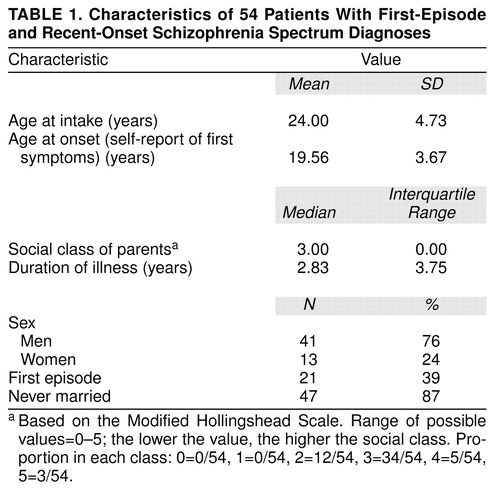
The patients in this report were entered into the study from 1987 to 1992. Four percent of the subjects who entered this longitudinal study did not complete 5 years in the study. At the time this analysis was begun, 65 patients had 5-year evaluations. Fifty-nine of these patients had clinical assessments and neuropsychological tests. Forty-nine met DSM-III or DSM-III-R criteria for schizophrenia, schizophreniform disorder, or a related schizophrenia spectrum disorder diagnosis at index assessment and had a primary diagnosis of DSM-III-R or DSM-IV schizophrenia confirmed at the 5-year evaluation. The remaining 16 patients had diagnoses of schizotypal disorder (N=3), psychotic disorder not otherwise specified (N=5), delusional disorder (N=1), affective spectrum disorders (N=5), substance-induced psychotic disorder (N=1), and psychotic disorder caused by a medical condition (N=1) at the 5-year follow-up assessment. Twenty-one of these patients were experiencing their first episode at the time of the index evaluation. Twenty-seven of these patients were included in a previous report (20). The five patients with affective spectrum diagnoses were omitted from all analyses. Characteristics of the 54 patients with schizophrenia spectrum disorders are shown in table 1.
Clinical Assessment
During the index hospitalization, all patients underwent evaluation with a structured interview, the Comprehensive Assessment of Symptoms and History (CASH) (60), which includes the Scale for the Assessment of Negative Symptoms (SANS) (61) and the Scale for the Assessment of Positive Symptoms (SAPS) (62). All symptoms were rated at their worst during the month before intake. All possible sources of information, including hospital records and interviews with informants, the patient, nurses, and social workers, were used in completing the CASH. Diagnosis was established by the clinical research team, using all available sources of information.
The PSYCH-Base (60) is a companion instrument to the CASH that includes an assessment of psychosocial functioning, as well as information on all past and present psychiatric treatment. Medication dose was recorded in the PSYCH-Base at the time of index assessment and in the PSYCH-UP at each longitudinal follow-up, which was completed at 6-month intervals over the 5-year period. During the index assessment, 45 of the 54 patients were treated with neuroleptic drugs; 39 received typical neuroleptics (fluphenazine: N=11, haloperidol: N=19, thiothixene: N=3, thioridiazine: N=3, perphenazine: N=2, molindone hydrochloride: N=1), and six received atypical neuroleptics (risperidone: N=3, olanzapine: N=2, clozapine: N=1). At the 5-year follow-up, 40 patients were receiving neuroleptics; 29 were receiving typical neuroleptics (fluphenazine: N=11, haldoperidol: N=11, thiothixene: N=3, thioridiazine: N=1, perphenazine: N=3), and 11 were receiving atypical neuroleptics (risperidone: N=1, olanzapine: N=2, clozapine: N=8). Neuroleptic doses were converted to chlorpromazine-equivalent doses (63) for use in data analysis.
A three-dimensional model of the interrelationships among the symptoms of schizophrenia has been constructed on the basis of previous factor analytic studies from this research group (64–66). The dimensions were composed in the following way: global SANS ratings of affective flattening, alogia, avolition/apathy, attention, and anhedonia/asociality were summed to provide an index of total negative symptoms; global SAPS ratings of bizarre behavior, formal thought disorder, and inappropriate affect were summed as an index of disorganized symptoms; and global SAPS ratings of hallucinations and delusions were summed to characterize the psychotic dimension. These symptom dimension scores were used in the statistical analyses described below.
Cognitive Assessment
The first cognitive assessment was conducted during the patients’ index hospitalization. Testing took place at a time when patients were demonstrably capable of attending to the testing. Patients were subsequently followed at 6-month intervals over a 5-year period with the PSYCH-UP. The cognitive tests were repeated at the 5-year follow-up. No subject was unable to successfully complete the cognitive battery because of illness or noncooperation. The cognitive battery was updated over the course of the longitudinal study, and tests were added or replaced, and, as a result, some subjects do not have scores on all tests.
The test scores analyzed for the present study are as follows: IQ (WAIS-R [67]), including verbal IQ, performance IQ, and full-scale IQ; a composite of both verbal IQ and performance IQ; motor speed score (finger-tapping test [68]); speeded visual motor scanning and mental set shifting score (Trail Making B [69]); verbal associative fluency score (MAE Oral Word Association Test [70]); flexibility of cognitive set score (Wisconsin Card Sorting Test [71]); delayed visual memory score (Rey-Osterrieth Complex Figure Delay [72]); free recall score on the logical memory test (73); and visual search and attention score (Circle A letter-cancellation task [74]).
Statistical Analysis
Paired t tests were conducted to assess significant changes in symptoms, medication dose, and cognitive function from index hospitalization to follow-up. A nonparametric counterpart to the paired t test, the Wilcoxon signed ranks test, was used to analyze the data. Since the results did not differ among the tests, they are reported from the parametric analysis. To assess the relationship between test performance at the two time periods, intraclass correlation coefficients were calculated. All statistical tests were two-tailed.
Kendall’s tau-b statistic was used to examine whether changes in cognitive performance were related to clinical symptom patterns over the 5-year period. Symptoms were rated every 6 months throughout the 5-year follow-up period, and a median slope for each subject was calculated from the ratings. For the analysis, change scores for each cognitive test were correlated with the symptom slope.
It was assumed that symptoms at the time of testing would have the most influence on cognitive performance. To test our assumption, we compared change scores of the index assessment and the 5-year follow-up for each cognitive test with change scores for each symptom dimension at the two time periods. The relationship between changes in medication dose (i.e., neuroleptic dose in chlorpromazine-equivalent doses) and changes in cognitive function was also evaluated. Pearson’s product-moment correlation and the Spearman rank-order correlation were used to assess the degree of association. Since the results did not differ among the tests, we reported the parametric results.
Finally, stepwise multiple regression analyses were conducted to identify the importance of different dimensions of schizophrenia in predicting cognitive performance over time. Regression analyses were conducted for three dependent variables—verbal, performance, and full-scale IQ—to determine whether medication and symptoms at the 5-year follow-up could predict changes in performance. A cut-off value of p<0.01 was used for the regression analyses.
RESULTS
Longitudinal Course of Cognitive Performance
Table 2 shows changes in mean clinical status, medication dose, and cognitive performance from index hospitalization to the 5-year follow-up. Significant improvements in clinical status were observed in all three symptom dimensions. Performance IQ and full-scale IQ significantly improved, whereas verbal IQ did not change. Mean performance significantly improved in the time to completion of the Circle A letter-cancellation task, free recall (i.e., without a time delay) on the logical memory test, and the number of categories correctly completed in the Wisconsin Card Sorting Test. Finger-tapping speed significantly worsened over time for both the right and the left hands. Performance on the remaining cognitive tests did not change over time. Although some variables showed a mean change, only performance on the IQ measures showed high stability in baseline to follow-up correlations; 37 subjects improved their performance, 13 subjects performed worse, and four subjects showed no change. Most of the other measures showed moderate stability (table 3).
Longitudinal Relationship Between Cognitive Performance and Symptom Dimensions
Symptom scores were obtained at 6-month intervals throughout the 5-year follow-up period. For the three symptom dimensions, median symptoms were at the highest at the index hospitalization and showed improvement in the subsequent months before leveling off. Verbal IQ (tau-b=–0.28, N=54, p<0.003) and full-scale IQ (tau-b=–0.25, N=54, p<0.008) were significantly correlated with negative symptoms. Improvement in IQ performance was associated with improvement in negative symptoms. There were no other significant correlations between cognitive performance and any of the symptom dimensions.
The results of the change score correlations for IQ were similar to the results obtained by using the symptom data from each of the 6-month intervals. Significant correlations with negative symptoms were observed for verbal IQ (r=–0.34, df=53, p<0.02) and full-scale IQ (r=–0.35, df=53, p<0.01). Improvement in performance on the Trail Making B score (i.e., faster time to completion) was correlated with improvement in psychotic symptoms (r=0.40, df=49, p<0.007). Slower finger tapping with the right hand was associated with an increase in disorganized symptoms (r=0.31, df=50, p=0.03). Only the Trail Making B score was significantly associated with medication changes (r=0.52, df=49, p<0.0004). That is, a decrease in medication was related to improvement. This likely reflects that only the most ill patients received increases in medication dose.
Negative symptoms at the time of the 5-year follow-up were significantly related to the change in performance in verbal IQ (F=12.73, df=2, 53, p<0.0008; R2=0.62). Improvement in negative symptoms accounted for 10% of the improvement in performance. Medication dose at the 5-year follow-up significantly predicted improvement in performance IQ (F=6.73, df=2, 53, p<0.01; R2=0.63). Medication accounted for 7% of the variance in improvement in performance IQ. Symptom dimensions did not significantly predict change in performance IQ. For full-scale IQ, improvement in negative symptoms predicted improvement in performance (F=11.84, df=2, 53, p<0.001; R2=0.68), accounting for 8% of the variance in full-scale IQs. No other variables significantly predicted performance changes.
DISCUSSION
The objective of the present study was to examine the longitudinal course of cognitive function and its relationship with clinical symptoms in a cohort of young patients with recent-onset schizophrenia who received standard neuroleptic treatment over most of a 5-year period. Overall, the findings are more consistent with a neurodevelopmental model of schizophrenia than with a neurodegenerative model. The results indicate that there was no cognitive deterioration between the two test periods. Rather, improvements in mean test performance were observed for performance IQ, full-scale IQ, the Circle A letter-cancellation task, free recall on the logical memory test, and the number of categories completed on the Wisconsin Card Sorting Test. Performance on the remaining tests did not change over time, with the exception of a significant decrease in group performance on the finger-tapping test, an index of motor speed.
Although mean performance IQ and full-scale IQ showed a statistically significant improvement, the improvement was modest. Overall performance for verbal IQ was within the normal range of functioning at time one and time two. Both mean performance IQ and full-scale IQ were in the low normal range at the first test session and within the normal range at the second testing. Mean performance IQ showed a significant improvement of almost 0.5 standard deviations. Although this improvement could reflect practice effects, a change of this magnitude is unlikely over a 5-year interval. Further, it is also unlikely because schizophrenic subjects often demonstrate impairment in tests of learning and recall memory. In general, the results indicate that IQ, a measure of general cognitive function, improved from the index testing to the 5-year follow-up.
Individuals with improvement in negative symptoms had improvement in verbal IQ and full-scale IQ. That is, a change in verbal IQ and full-scale IQ was significantly related to parallel changes in negative symptoms but not to psychotic or disorganized symptoms. This finding lends support to previous findings in the literature that the negative dimension is more closely related to neuropsychological test performance than the psychotic or disorganized dimension (75, 76), represents a core feature of schizophrenia (77), and is associated with a poorer long-term cognitive outcome.
The results are not consistent with a neurodegenerative model. Overall, clinical symptoms, including negative, psychotic, and disorganized symptom dimensions, improved from index hospitalization to the 5-year follow-up. Negative and psychotic symptoms were prominent at the time of the index evaluation and declined at the 5-year follow-up. Disorganized symptoms were somewhat less prominent than the other symptom domains at the index evaluation and declined as well over the 5-year period.
The finding of motor slowing could be related to long-term neuroleptic exposure. The majority of patients were on maintenance regimens of neuroleptics throughout the 5-year follow-up period, with dose adjustments as needed. Most were taking typical neuroleptics at both index and follow-up evaluations, since data for this study antedated the widespread availability of atypical neuroleptics. Mean medication dose did not significantly change between time one and time two. This finding may also have resulted from dose changes in anticholinergic medication. However, the patients treated with anticholinergics were on stable doses that did not change over time for the most part. When the change score analyses were used for those patients treated with anticholinergic medications (i.e., changes in dose were correlated with changes in cognitive performance to examine a possible relationship between the two), no significant relationships were observed between anticholinergic treatment and cognitive performance. Although past research has highlighted the possibility of adverse cognitive effects from medication treatment, it seems likely that stable medication dose over time contributes to improvements or may prevent deterioration in cognitive performance.
This study has some limitations. Treatment was not experimentally controlled in this study, and patients were receiving a variety of neuroleptics in variable doses. This was thus a naturalistic study, where those sources of variation in treatment were allowed to fluctuate freely. Further, the treatment was usually with older, typical neuroleptics. On the other hand, a naturalistic study group may be a more representative group of patients with schizophrenia in general. It will be interesting to see if long-term outcome is better in patients treated with atypical neuroleptics. Another limitation was that we performed many statistical tests and did not control for multiple comparisons. Were we to use a more stringent significance criterion, the correlations between medication dose change and cognitive performance change would fail to remain significant. However, the performance changes would still remain significant, with the exception of the Circle A letter-cancellation task score and the free recall score on the logical memory test. The correlations between negative symptoms and verbal IQ and full-scale IQ would also have remained significant. Finally, our group size was only moderately large. It provided good statistical power (>0.7) with a two-tailed test, a significance level of p<0.05, and an effect size of 0.48 (calculated from the data in the present study).
There are numerous studies in the literature attempting to delineate the course of cognitive performance across the life span of schizophrenia. However, a convergence in findings has not been established. This study supports the view that cognitive function does not deteriorate over the course of the illness and that it may, in fact, improve in a cohort of patients who remain in treatment, even with typical neuroleptics. Symptom pattern does, however, have a relationship with cognitive performance. Negative symptoms are associated with performance on full-scale IQ and verbal IQ, tests of general intellectual ability, and, in particular, verbal ability.
Future longitudinal studies of cognitive and neuropsychological function, particularly with newer atypical neuroleptics, will help to further characterize the course of schizophrenia and continue to provide clues that may lead to more effective treatments. Overall, the results suggest that clinicians should adopt an attitude of guarded optimism concerning cognitive outcome in schizophrenia, even in patients treated with typical neuroleptics. Future studies of cognitive outcome in patients treated primarily with atypical neuroleptics will provide an interesting and clinically important comparison.
Presented in part at the Sixth International Congress on Schizophrenia Research, Colorado Springs, April 12, 1997. Received Dec. 1, 1997; revision received April 12, 1999; accepted May 11, 1999. From the Mental Health Clinical Research Center, Department of Psychiatry, and the Department of Preventive Medicine and Environmental Health, University of Iowa. Address reprint requests to Dr. Gold, MH-CRC, 2911 JPP, University of Iowa Hospitals and Clinics, 200 Hawkins Dr., Iowa City, IA 52242-1057; [email protected] (e-mail). Supported by NIMH grants MH-43271, MH-40856, and MH-31593 and a National Alliance for Research on Schizophrenia and Depression Young Investigator Award (Dr. Gold).
 |
 |
 |
1. Bleuler E: Dementia Praecox or the Group of Schizophrenias (1911). Translated by Zinkin J. New York, International Universities Press, 1950Google Scholar
2. Kraepelin E: Dementia Praecox and Paraphrenia. Translated by Barclay RM; edited by Robertson GM. Edinburgh, E & S Livingstone, 1919Google Scholar
3. Pollack M, Woerner M, Klein DF: A comparison of childhood characteristics of schizophrenics, personality disorders, and their siblings, in Life History Research in Psychopathology. Edited by Roff M, Ricks DF. Minneapolis, University of Minnesota Press, 1970, pp 208–225Google Scholar
4. Parnas J, Schulsinger F, Schulsinger H, Mednick S, Teasdale TW: Behavioral precursors of schizophrenia spectrum: a prospective study. Arch Gen Psychiatry 1982; 39:658–664Crossref, Medline, Google Scholar
5. Aylward E, Walker E, Bettes B: Intelligence in schizophrenia: meta-analysis of the research. Schizophr Bull 1984; 10:430–459Crossref, Medline, Google Scholar
6. Mirsky AF, Silberman EK, Latz A, Nagler S: Adult outcomes of high-risk children. Schizophr Bull 1985; 11:150–154Crossref, Medline, Google Scholar
7. Parnas J, Schulsinger H: Continuity of formal thought disorder from childhood to adulthood in a high-risk sample. Acta Psychiatr Scand 1986; 74:246–251Crossref, Medline, Google Scholar
8. Done DJ, Crow TJ, Johnstone EV, Sacker A: Childhood antecedents of schizophrenia and affective illness. BMJ 1994; 309:699–703Crossref, Medline, Google Scholar
9. Jones P, Rodgers B, Murray R: Child developmental risk factors for schizophrenia in the British 1946 birth cohort. Lancet 1994; 344:1398–1402Google Scholar
10. Payne R: Cognitive abnormalities, in Handbook of Abnormal Psychology. Edited by Eysenck HJ. New York, Basic Books, 1961, pp 193–261Google Scholar
11. Schwartzman A, Douglas V, Muir R: Intellectual loss in schizophrenia, part II. Can J Psychol 1962; 16:161–168Crossref, Medline, Google Scholar
12. Smith A: Mental deterioration in chronic schizophrenia. J Nerv Ment Dis 1964; 139:479–487Crossref, Medline, Google Scholar
13. Bleuler M: The Schizophrenic Disorders: Long-Term Patient and Family Studies. New Haven, Conn, Yale University Press, 1972Google Scholar
14. Silverberg-Shalev R, Gordon HW, Bentin S, Aranson A: Selective language deterioration in chronic schizophrenia. J Neurol Neurosurg Psychiatry 1981; 44:547–551Crossref, Medline, Google Scholar
15. Abrahamson D: Schizophrenic deterioration. Br J Psychiatry 1983; 143:82–83Medline, Google Scholar
16. Bilder RM, Lipschutz-Broch L, Reiter G, Geisler SH, Mayerhoff DI, Lieberman JA: Intellectual deficits in first-episode schizophrenia: evidence for progressive deterioration. Schizophr Bull 1992; 18:437–448Crossref, Medline, Google Scholar
17. Haywood H, Moelis I: Effect of symptom change on intellectual function in schizophrenia. J Abnorm Psychol 1963; 67:76–78Crossref, Google Scholar
18. Klonoff H, Fibiger CH, Hutton GH: Neuropsychological patterns in chronic schizophrenia. J Nerv Ment Dis 1970; 150:291–300Crossref, Medline, Google Scholar
19. Heaton RK, Crowley TJ: Effects of psychiatric disorders and their somatic treatments on neuropsychological test results, in Handbook of Clinical Neuropsychology, vol 1. Edited by Felskov SB, Boll TJ. New York, John Wiley & Sons, 1981, pp 481–525Google Scholar
20. Nopoulos P, Flashman L, Flaum M, Arndt S, Andreasen N: Stability of cognitive functioning early in the course of schizophrenia. Schizophr Res 1994; 14:29–37Crossref, Medline, Google Scholar
21. DeLisi LE, Tew W, Xie S, Hoff AL, Sakuma M, Kushner M, Lee G, Shedlack K, Smith AM, Grimson R: A prospective follow-up study of brain morphology and cognition in first-episode schizophrenic patients: preliminary findings. Biol Psychiatry 1995; 38:349–360Crossref, Medline, Google Scholar
22. Sweeney JA, Haas GL, Keilp JG, Long M: Evaluation of the stability of neuropsychological functioning after acute episodes of schizophrenia: one-year followup study. Psychiatry Res 1991; 38:63–76Crossref, Medline, Google Scholar
23. Centis DM, Ragland JD, Gur RC, Gur RE: Neuropsychological evidence supporting a neurodevelopmental model of schizophrenia: a longitudinal study. Schizophr Res 1997; 24:289–298Crossref, Medline, Google Scholar
24. Hoff AL, Riordan H, O’Donnell DW, Morris L, DeLisi LE: Neuropsychological function of first-episode schizophreniform patients. Am J Psychiatry 1992; 149:898–903Link, Google Scholar
25. Calev A, Venables PH, Monk AF: Evidence for distinct verbal memory pathologies in severely and mildly disturbed schizophrenics. Schizophr Bull 1983; 9:247–264Crossref, Medline, Google Scholar
26. Levin S, Yurgelun-Todd D, Craft S: Contributions of clinical neuropsychology to the study of schizophrenia. J Abnorm Psychol 1989; 98:341–356Crossref, Medline, Google Scholar
27. Green M, Walker E: Neuropsychological performance and positive and negative symptoms in schizophrenia. J Abnorm Psychol 1985; 94:460–469Crossref, Medline, Google Scholar
28. Green M, Walker E: Attentional performance in positive- and negative-symptom schizophrenia. J Nerv Ment Dis 1986; 174:208–213Crossref, Medline, Google Scholar
29. Saykin AJ, Gur RC, Gur RE, Mozley PD, Mozley LH, Resnick SM, Kester DB, Stafiniak P: Neuropsychological function in schizophrenia: selective impairment in memory and learning. Arch Gen Psychiatry 1991; 48:618–624Crossref, Medline, Google Scholar
30. Goldberg TE, Hyde TM, Kleinman JE, Weinberger DR: Course of schizophrenia: neuropsychological evidence for a static encephalopathy. Schizophr Bull 1993; 19:797–804Crossref, Medline, Google Scholar
31. Nuechterlein KH, Dawson ME: Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophr Bull 1984; 10:160–203Crossref, Medline, Google Scholar
32. Liddle PF: Schizophrenic syndromes, cognitive performance and neurological dysfunction. Psychol Med 1987; 17:49–57Crossref, Medline, Google Scholar
33. Strauss ME: Relations of symptoms to cognitive deficits in schizophrenia. Schizophr Bull 1993; 19:215–232Crossref, Medline, Google Scholar
34. Cuesta MJ, Peralta V: Cognitive disorders in the positive, negative, and disorganization syndromes of schizophrenia. Psychiatry Res 1995; 58:227–235Crossref, Medline, Google Scholar
35. Braff DL: Sensory input deficits and negative symptoms in schizophrenic patients. Am J Psychiatry 1989; 146:1006–1011Google Scholar
36. Hammer MA, Katsanis J, Iacono WG: The relationship between negative symptoms and neuropsychological performance. Biol Psychiatry 1995; 37:828–830Crossref, Medline, Google Scholar
37. Strauss ME, Buchanan RW, Hale J: Relations between attentional deficits and clinical symptoms in schizophrenic outpatients. Psychiatry Res 1993; 47:205–213Crossref, Medline, Google Scholar
38. Nuechterlein KH, Edell WS, Norris M, Dawson ME: Attentional vulnerability indicators, thought disorder, and negative symptoms. Schizophr Bull 1986; 12:408–426Crossref, Medline, Google Scholar
39. Cornblatt BA, Lenzenweger MF, Dworkin RH, Erlenmeyer-Kimling L: Positive and negative schizophrenic symptoms, attention, and information processing. Schizophr Bull 1985; 11:397–408Crossref, Medline, Google Scholar
40. Green M, Walker E: Susceptibility to backward masking in schizophrenic patients with positive or negative symptoms. Am J Psychiatry 1984; 141:1273–1275Google Scholar
41. Heaton R, Paulsen JS, McAdams LA, Kuck J, Zisook S, Braff D, Harris J, Jeste DV: Neuropsychological deficits in schizophrenics. Arch Gen Psychiatry 1994; 51:469–476Crossref, Medline, Google Scholar
42. Hoff AL, Harris D, Faustman WO, Beal M, DeVilliers D, Mone RD, Moses JA, Csernansky JG: A neuropsychological study of early onset schizophrenia. Schizophr Res 1996; 20:21–28Crossref, Medline, Google Scholar
43. Cleghorn JM, Kaplan RD, Szechtman B, Szechtman H, Brown GM: Neuroleptic drug effects on cognitive function in schizophrenia. Schizophr Res 1990; 3:211–219Crossref, Medline, Google Scholar
44. Gold JM, Hurt SW: The effects of haloperidol on thought disorder and IQ in schizophrenia. J Pers Assess 1990; 54:390–400Crossref, Medline, Google Scholar
45. Smith RC, Largen J, Vroulis G, Ravichandran GK: Neuropsychological test scores and clinical response to neuroleptic drugs in schizophrenia patients. Compr Psychiatry 1992; 33:139–145Crossref, Medline, Google Scholar
46. Goldberg TE, Greenberg RD, Griffin SJ, Gold JM, Pickar D, Schulz SC, Weinberger DR: The effect of clozapine on cognition and psychiatric symptoms in patients with schizophrenia. Br J Psychiatry 1993; 162:43–48Crossref, Medline, Google Scholar
47. Verdoux H, Magnin E, Bourgeois M: Neuroleptic effects on neuropsychological test performance in schizophrenia. Schizophr Res 1995; 14:133–139Crossref, Medline, Google Scholar
48. Johnstone EC, Crow TJ, Frith CD, Husband J, Kreel L: Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet 1976; 2:924–926Crossref, Medline, Google Scholar
49. Donnelly EF, Weinberger DR, Walman IM, Wyatt RJ: Cognitive impairment associated with morphological brain abnormalities on computed tomography in chronic schizophrenia patients. J Nerv Ment Dis 1980; 168:305–308Crossref, Medline, Google Scholar
50. Andreasen NC, Smith MR, Jacoby CG, Dennert JW, Olsen SA: Ventricular enlargement in schizophrenia: definition and prevalence. Am J Psychiatry 1982; 139:292–296Link, Google Scholar
51. Golden CJ, MacInnes WD, Ariel RN, Ruedrich SL, Chu CC, Coffman JA, Graber B, Bloch S: Cross-validation of the ability of the Luria-Nebraska Neuropsychological Battery to differentiate chronic schizophrenics with and without ventricular enlargement. J Consult Clin Psychol 1982; 50:87–95Crossref, Medline, Google Scholar
52. Kemali D, Maj M, Galderisi S, Ariano MG, Cesarelli M, Milici N, Salvati A, Valente A, Volpe M: Clinical and neuropsychological correlates of cerebral ventricular enlargement in schizophrenia. J Psychiatr Res 1985; 19:587–596Crossref, Medline, Google Scholar
53. Pandurangi AK: A comprehensive study of chronic schizophrenic patients. Acta Psychiatr Scand 1986; 73:161–171Crossref, Medline, Google Scholar
54. Bilder RM, Degreef G, Pandurangi AK, Reider RO, Sackheim HA, Mukherjee S: Neuropsychological deterioration and CT findings in chronic schizophrenia. Schizophr Res 1988; 1:37–45Crossref, Medline, Google Scholar
55. DeQuardo JR, Tandon R, Goldman R, Meador-Woodruff JH, McGrath-Giroux M, Brunberg JA, Loraine K: Ventricular enlargement, neuropsychological status, and premorbid function in schizophrenia. Biol Psychiatry 1994; 35:517–524Crossref, Medline, Google Scholar
56. Nestor PG, Shenton ME, McCarley RW, Haimson J, Smith RS, O’Donnell B, Kimble M, Kikinis R, Jolesz FA: Neuropsychological correlates of MRI temporal lobe abnormalities in schizophrenia. Am J Psychiatry 1993; 150:1849–1855Google Scholar
57. Heaton RK, Drexler M: Clinical neuropsychological findings in schizophrenia and aging, in Schizophrenia and Aging: Schizophrenia, Paranoia, and Schizophreniform Disorders in Later Life. Edited by Miller NE, Cohen GD. New York, Guilford Press, 1987, pp 145–161Google Scholar
58. Woods BT: Is schizophrenia a progressive neurodevelopmental disorder? toward a unitary pathogenetic mechanism. Am J Psychiatry 1998; 155:1661–1670Google Scholar
59. Flaum MA, Andreasen NC, Arndt S: The Iowa prospective longitudinal study of recent-onset psychoses. Schizophr Bull 1992; 18:481–490Crossref, Medline, Google Scholar
60. Andreasen NC, Flaum M, Arndt S: The Comprehensive Assessment of Symptoms and History (CASH): an instrument for assessing diagnosis and psychopathology. Arch Gen Psychiatry 1992; 49:615–623Crossref, Medline, Google Scholar
61. Andreasen NC: Modified Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1984Google Scholar
62. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1984Google Scholar
63. Davis JM: Comparative doses and costs of antipsychotic medication. Arch Gen Psychiatry 1976; 33:858–861Crossref, Medline, Google Scholar
64. Andreasen NC, Arndt S, Alliger R, Miller D, Flaum M: Symptoms of schizophrenia: methods, meanings, and mechanisms. Arch Gen Psychiatry 1995; 52:341–351Crossref, Medline, Google Scholar
65. Arndt S, Alliger RJ, Andreasen NC: The distinction of positive and negative symptoms: the failure of a two-dimensional model. Br J Psychiatry 1991; 158:317–322Crossref, Medline, Google Scholar
66. Arndt S, Andreasen NC, Flaum M, Miller D, Nopoulos P: A longitudinal study of symptom dimensions in schizophrenia: prediction and patterns of change. Arch Gen Psychiatry 1995; 52:352–360Crossref, Medline, Google Scholar
67. Wechsler D (ed): Wechsler Adult Intelligence Scale—Revised, Manual. New York, Psychological Corp, 1981Google Scholar
68. Halstead WC: Brain and Intelligence. Chicago, University of Chicago Press, 1947Google Scholar
69. Reitan RM, Wolfson D: The Halstead-Reitan Neuropsychological Test Battery: Theory and Clinical Interpretation. Tucson, Ariz, Neuropsychology Press, 1975Google Scholar
70. Benton AL, Sivan AB, Des Hamsher K, Varney NR: Contributions to Neuropsychological Assessment: A Clinical Manual. Edited by Spreen O. New York, Oxford University Press, 1983Google Scholar
71. Berg EA: A simple objective test for measuring flexibility in thinking. J Gen Psychol 1948; 39:15–22Crossref, Medline, Google Scholar
72. Rey A: L’examen psychologique dans les cas d’encephalopathietraumatique. Arch Psychol 1941; 28:286–340Google Scholar
73. Wechsler D: A standardized memory scale for clinical use. J Psychol 1945; 19:87–95Crossref, Google Scholar
74. Talland GA, Schwab RS: Performance with multiple sets in Parkinson’s disease. Neuropsychologia 1964; 2:45–53Crossref, Google Scholar
75. Bilder RM, Mukherjee S, Rieder RO, Pandurangi AK: Symptomatic and neuropsychological components of defect states. Schizophr Bull 1985; 11:409–419Crossref, Medline, Google Scholar
76. Andreasen NC, Flaum M, Swayze VW II, Tyrrell G, Arndt S: Positive and negative symptoms in schizophrenia: a critical reappraisal. Arch Gen Psychiatry 1990; 47:615–621Crossref, Medline, Google Scholar
77. Carpenter WT Jr, Heinrichs DW, Wagman AMI: Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry 1988; 145:578–583Link, Google Scholar



