Low Phosphoinositide-Specific Phospholipase C Activity and Expression of Phospholipase C β1 Protein in the Prefrontal Cortex of Teenage Suicide Subjects
Abstract
OBJECTIVE: The enzyme phosphoinositide-specific phospholipase C (PI-PLC) is a component of the phosphoinositide signal transduction system. Other components of this system have been found to be abnormal in adults and adolescents who have committed suicide, and so the authors examined whether PI-PLC activity and protein expression of PLC isozymes are abnormal in postmortem brains of teenage suicide subjects. METHOD: PI-PLC activity and protein expression of the PLC β1, δ1, and γ1 isozymes were examined in Brodmann’s areas 8 and 9 of postmortem brains obtained from 18 teenage suicide subjects and 18 matched comparison subjects. PI-PLC activity was determined by enzymatic assay, and protein expression of the PLC isozymes was determined by the Western blot technique. RESULTS: Compared with the normal subjects, the teenage suicide subjects had significantly lower PI-PLC activity and immunolabeling of the specific PLC β1 isozyme in both membrane and cytosol fractions of Brodmann’s areas 8 and 9 combined (prefrontal cortex). There was also a significant correlation between PI-PLC activity and protein levels of the PLC β1 isozyme in the brains of the teenage suicide subjects. There was no significant difference in PI-PLC activity or level of PLC β1 protein between the suicide subjects with a history of mental disorders and those with no history of mental disorders; however, both groups had significantly lower PI-PLC activity and expression of PLC β1 protein than the normal subjects. CONCLUSIONS: Low PI-PLC activity and expressed levels of the PLC β1 isozyme in postmortem brains of suicide subjects may have clinical relevance in the pathophysiology of suicidal behavior.
A large body of evidence indicates that there are abnormalities in serotonin (5-HT) receptor subtypes in the postmortem brains of people who have committed suicide. Several studies of postmortem brain samples have indicated high numbers of 5-HT2A receptors in the prefrontal cortex of suicide subjects, although this finding has not been replicated in some other studies (see reference 1 for review). 5-HT2A receptors are linked to the phosphoinositide signaling system, and in the phosphoinositide signaling pathway, agonist-induced activation of 5-HT2A receptors causes the hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) by the enzyme phosphoinositide-specific phospholipase C (PI-PLC), which results in the formation of two second messengers: diacylglycerol and inositol 1,4,5-trisphosphate. Diacylglycerol activates protein kinase C and increases the affinity of the enzyme for calcium (2).
Since some evidence suggests that persistent activation and overactivity of receptors linked to the phosphoinositide signaling system may cause changes in other components of the signaling system, it is possible that G proteins, PLC, and/or protein kinase C may also be abnormal in postmortem brains of suicide subjects. For example, Pacheco et al. (3) and Cowburn et al. (4) have shown that there are abnormalities in G protein levels in postmortem brains of suicide subjects. On the other hand, Friedman and Wang (5), Young et al. (6), and Mathews et al. (7) showed abnormal levels of G proteins in postmortem brains of patients with bipolar disorder.
Suicide by teenagers is a major public health concern, and little is known about its neurobiology. The neurobiology of suicide by teenagers may be different from that of suicide by adults, since the observations of many investigators indicate that response to antidepressant treatment, especially to the tricyclics, differs between adolescents and adults with depression (8, 9). Because there have been so few neurobiological studies in adolescent suicide subjects, it is not clear whether the neurochemical abnormalities observed in adults are also present in teenage suicide subjects.
Recently we reported (10) that [3H]phorbol dibutyrate binding to protein kinase C, one of the key components of the phosphoinositide signaling system, is abnormal in the postmortem brains of teenage suicide subjects. To examine whether abnormalities exist in other components of the phosphoinositide signal transduction cascade, in the present investigation we studied PI-PLC activity and protein expression of PLC isozymes in combined Brodmann’s areas 8 and 9 of teenage suicide subjects and deceased normal teenage subjects.
METHOD
PI-PLC activity and the protein expression of PLC isozymes were determined in prefrontal cortex (Brodmann’s areas 8 and 9 combined) obtained from 18 teenage suicide subjects and 18 psychiatrically normal teenage subjects, herein referred to as normal subjects (table 1). Postmortem brain tissue was obtained from the Brain Collection Program at the Maryland Psychiatric Research Center in Baltimore, in collaboration with the Medical Examiner’s Office of the State of Maryland. The brain samples were free of neuropathological abnormalities and HIV antibodies. Toxicological data were obtained by analyzing urine and blood samples.
Diagnostic Method
All subjects for this study were diagnosed in the following manner: after giving written informed consent, at least one family member underwent an interview based on the Diagnostic Evaluation After Death (11) and the Structured Clinical Interview for DSM-III-R (12). When there was a prior history of mental health treatment and in all cases of suicide, permission was obtained from family members for access to clinical records from mental health treatment providers. An attempt was made to collect all the available records on each case, and then the appropriate data were extracted from the records and compiled by using the Diagnostic Evaluation After Death. Independent DSM-III-R diagnoses made by two senior psychiatrists (R.R.C. and C.A.T.) were compared, and discrepancies were resolved through a consensus conference. Data on the suicide cases were collected, and the circumstances of each suicide were determined by using the Diagnostic Evaluation After Death during the same interview process. Cases were considered to be suicides only if the manner of death was determined to be suicide by the medical examiner. Similarly, the normal subjects were verified as free from mental illnesses by means of these same diagnostic procedures.
Preparation of Membrane and Cytosol Fractions
Tissues obtained from Brodmann’s areas 8 and 9 were homogenized by using the Polytron (Brinkman Instruments, Westbury, N.Y.) in homogenizing buffer containing 20 mM Tris-HCl, 2 mM ethylene glycol-bis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid, 5 mM ethylenediamine tetraacetic acid, 1.5 mM pepstatin, 2 mM leupeptin, 0.5 mM phenylmethylsulfonyl fluoride, 0.2 U/ml aprotinin, and 2 mM dithiothreitol. The homogenate was centrifuged at 2,800 rpm for 10 minutes at 4˚C to remove nuclei and unbroken cells. The supernatant was centrifuged at 100,000 g for 60 minutes at 4˚C. The resulting supernatant was the cytosol fraction, and the pellet thus obtained was resuspended in the same homogenizing buffer containing 0.2% Triton X-100 (Sigma Chemical Co., St. Louis). The homogenate was held on ice for 60 minutes with intermittent shaking and was centrifuged again at 100,000 g for 60 minutes. The resultant supernatant was the membrane fraction. The protein concentrations in the membrane and cytosol fractions were determined by the procedure of Lowry et al. (13) with bovine serum albumin used as the standard.
Determination of PI-PLC Activity
PI-PLC activity was measured in the membrane and cytosol fractions by the procedure of Pandey (14). The reaction was initiated by adding membrane or cytosol fraction (10 µg protein) to an incubation buffer (20 mM Tris-HCl, 1 mM CaCl2, and 100 mM KCl, pH=7.4) containing 10 mM LiCl and PIP2 substrate (50 µM unlabeled PIP2, 2.0 µCi/ml [3H]PIP2, and 0.5 mg/ml cetrimide) in a total volume of 100 µl, and the reaction was carried out at 37˚C for 10 minutes. The reaction was terminated by addition of 500 µl of 1 M HCl and 500 µl of a mixture of chloroform and methanol (1:1 vol/vol). The tubes were vigorously mixed and then centrifuged at 1000 g for 10 minutes. The aqueous phase was transferred to a scintillation vial containing scintillation liquid, and the radioactivity was counted in a liquid scintillation counter. Each experiment had its blank, wherein the protein suspension was added after the reaction was stopped with the chloroform-methanol mixture (1:1 vol/vol). PI-PLC activity was expressed as the amount of [3H]inositol 1,4,5-trisphosphate formed, in disintegrations per minute per milligram of protein.
Immunolabeling of PLC β1, γ1, and δ1 Isozymes
Details of the immunolabeling procedure have been described earlier by us (15). Briefly, the procedure is as follows: equal volumes (20 µl) of membrane or cytosol fraction, containing 30 µg of protein, were subjected to 7.5% (weight/vol) polyacrylamide gel by using a gel electrophoresis apparatus. The proteins were subsequently transferred electrophoretically to an enhanced chemiluminescent nitrocellulose membrane (Amersham, Arlington Heights, Ill.) by using a protein transfer unit. The membranes were washed with TBST (10 mM Tris-base, 0.15 M NaCl, and 0.05% [vol/vol] Tween 20) for 10 minutes. The blots were blocked by incubating with a blocking solution (5% [weight/vol] powdered nonfat milk in TBST, 2 ml nonidet P-40, and 0.02% [weight/vol] sodium dodecyl sulfate [pH=8.0]). Then the blots were incubated with primary monoclonal antibody (anti-PLC β1, γ1, or δ1) diluted at 1:1000 in the blocking solution for 90 minutes. The membranes were then thoroughly washed with TBST and incubated with horseradish-peroxidase-linked secondary antibody (antimouse IgG; diluted at 1:3000 in the blocking solution) for 60 minutes. The membranes were then washed with TBST and exposed to enhanced chemiluminescent autoradiography nitrocellulose film. β-Actin antibody was probed in the same membrane at a concentration of 1:5000 for 90 minutes and then probed with antimouse IgG as the secondary antibody at a concentration of 1:5000 for 90 minutes. The bands on the autoradiogram were quantified by using the Loats Image Analysis System (Loats Associates, Westminster, Md.), and the optical density of each sample was corrected by the optical density of the corresponding β-actin band. The values are presented as percentages of comparison values.
Statistical Analyses
Data analyses were performed by using the SPSS 8.0 (SPSS, Chicago) statistical software package. Group differences between the comparison and suicide subjects with or without a history of mental disorders were examined by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison procedure when significant main effects were present. The PI-PLC activity and expressed levels of the PLC β1, δ1, and γ1 isozymes in the suicide subjects and normal subjects were compared by using independent t test samples. Bonferroni corrections were used to test for statistically significant differences between the two subject groups (a p value of 0.05 was divided by the number of variables examined to calculate appropriate p values for statistical significance). The relationships of postmortem interval, age, race, and gender to PI-PLC activity and protein expression of PLC isozymes were determined by Pearson product moment correlation analyses. Significance was set at an alpha level of ≤0.05.
RESULTS
Clinical and demographic characteristics of the suicide subjects and normal subjects are shown in table 1. There was no significant difference in age or postmortem interval between the two groups.
The correlations of age, sex, race, and postmortem interval to PI-PLC activity and PLC isozymes in the membrane and cytosol fractions of the postmortem brains are shown in table 2. None was significant.
PI-PLC Activity
The mean PI-PLC activity in Brodmann’s areas 8 and 9 combined was significantly lower in both the membrane (mean=13,149 disintegrations/min per mg protein, SD=5,659) and cytosol (mean=11,978, SD=5,314) fractions of the prefrontal cortex of the teenage suicide subjects than in the normal subjects (membrane: mean=23,679, SD=6,925; cytosol: mean=21,450, SD=5,905) (table 3).
We then examined the effect of a history of mental illness on PI-PLC activity (table 3). Among the 18 suicide subjects, six had no known history of mental disorders, 10 had a history of mental disorders, and two had a history of alcohol abuse but not mental disorders. Our one-way ANOVA showed that there was a significant group difference in protein kinase C activity in both the membrane (F=11.5, df=2, 31, p<0.001) and cytosol (F=12.3, df=2, 31, p<0.001) fractions. The mean PI-PLC activity in the membrane and cytosol fractions of the subjects with a history of mental disorders was similar to that in the suicide subjects with no history of mental disorders (membrane: t=0.33, df=14, p=0.75; cytosol: t=0.88, df=14, p=0.39). However, PI-PLC activity in each subgroup—with a history of mental disorders and with no history of mental disorders—was significantly lower than in the normal subjects (table 3). Among the 10 subjects with mental disorders, three had major depression, three had adjustment or conduct disorders, and three had depression with comorbid adjustment or conduct disorders. There were no significant differences in PI-PLC activity among these three diagnostic subgroups of the subjects with mental disorders.
Immunolabeling of PLC Isozymes
To examine whether the lower PI-PLC activity in the teenage suicide subjects was due to a lower level of one or more specific PLC isozymes, we determined the immunolabeling of PLC β1, δ1, and γ1 in both the membrane and cytosol fractions of combined Brodmann’s areas 8 and 9 in the postmortem brain samples obtained from teenage suicide and normal subjects. Representative autoradiograms showing the immunolabeling of PLC β1, δ1, and γ1 isozymes are shown in Figure 1. As reported in the literature, PLC β1 migrated to 150 kDa, whereas PLC γ1 and δ1 migrated to 145 and 85 kDa, respectively (16). The molecular weight of the β-actin band was 46 kDa. We determined the ratios of the PLC isozyme bands to their respective β-actin bands. For the membrane and cytosol fractions of the postmortem brains, the ratios for PLC β1 were 1.1 (membrane) and 1.2 (cytosol), respectively; for PLC δ1 they were 1.3 (membrane) and 1.4 (cytosol), and for PLC γ1 they were 1.5 (membrane) and 1.6 (cytosol).
As shown in Figure 2 and in table 3, the immunolabeling of PLC β1 was significantly lower in both the membrane and cytosol fractions of the teenage suicide subjects than in the normal subjects, whereas no significant difference was observed in the immunolabeling of either PLC δ1 or PLC γ1 between the suicide and normal subjects (Figure 2).
Our one-way ANOVA showed that there was a significant group difference in the immunolabeling of PLC β1 in both the membrane (F=15.4, df=2, 31, p<0.001) and cytosol (F=26.2, df=2, 31, p<0.001) fractions. When we compared levels of the PLC β1 isozyme in the postmortem brains of the teenage suicide subjects who had a history of mental disorders (N=10) and those without a history of mental disorders (N=6), we observed that the protein expression of the PLC β1 isozyme was nonsignificantly different in both the membrane (t=1.02, df=14, p=0.32) and cytosol (t=0.23, df=14, p=0.82) fractions (table 3).
Relationship of PI-PLC Activity and PLC Isozymes
We next determined the relationship of PI-PLC activity to the expressed levels of the PLC isozymes. There was a significant correlation between PI-PLC activity and the expression of the PLC β1 isozyme in both the membrane (r=0.73, p<0.0001) and cytosol (r=0.68, p<0.0001) fractions (Brodmann’s areas 8 and 9 combined) of the total study group (suicide and normal subjects); however, we did not observe any significant correlations between PI-PLC activity and the expression of the PLC δ1 isozyme (membrane: r=0.16, p<0.33; cytosol: r=0.37, p<0.07) or PLC γ1 isozyme (membrane: r=0.04, t=0.80; cytosol: r=0.04, t=0.79) in the total group. When we examined the suicide subjects and normal subjects separately, we found a significant correlation between PI-PLC activity and PLC β1 protein in both the membrane (r=0.81, p<0.0001) and cytosol (r=0.56, p=0.02) fractions of the postmortem brains of the teenage suicide subjects but not in the membrane (r=0.12, p=0.61) or cytosol (r=0.17, p=0.49) fraction of the normal subjects, which indicates that abnormalities exist in both PI-PLC activity and in the expression of the PLC β1 isozyme in teenage suicide subjects.
DISCUSSION
We found that in both membrane and cytosol fractions of the prefrontal cortex, PI-PLC activity and expression of a specific PLC isozyme (PLC β1) were significantly lower in teenage suicide subjects than in normal teenage subjects. Moreover, PI-PLC activity was significantly correlated with the PLC β1 isozyme level in the prefrontal cortex of the teenage suicide subjects. PI-PLC activity and the PLC β1 isozyme level were significantly lower than normal in both the suicide subjects with and without a history of mental disorder, but there were no significant differences in either measure between these subgroups.
We also examined the effect of antidepressant treatment on PI-PLC activity and on expression of the PLC β1 isozyme. Two of the suicide subjects had a history of treatment with antidepressants, but their PI-PLC activity and PLC β1 expression were similar to the mean values of the entire suicide group; however, one subject who had been treated with verapamil expressed a very low level of PLC β1.
Thus, overall our study suggests that adolescents who commit suicide have significantly lower than normal PI-PLC activity in the prefrontal cortex and that this low level is due to selectively lower protein expression of the PLC β1 isozyme. Moreover, these results also suggest that the low PI-PLC activity and expression of the PLC β1 isozyme in suicide subjects is independent of a history of mental illness.
There have been some studies of PI-PLC in postmortem brains of adult suicide subjects. For example, Jope et al. (17) did not find any significant abnormalities in Ca2+-stimulated PI-PLC activity in postmortem brain tissue obtained from adult bipolar patients. Pacheco et al. (3) studied PI-PLC activity and the expression of the PLC β1 isozyme in postmortem brains of depressed adult suicide subjects but did not observe any significant abnormalities in PI-PLC activity or in PLC β1 immunolabeling in prefrontal or occipital cortical areas. In a recent publication, Mathews et al. (7) reported a nonsignificantly higher than normal level of the PLC β1 isozyme in the occipital cortex, but not the frontal or temporal cortex, of bipolar subjects.
Thus, our finding of low PI-PLC activity and a low level of the PLC β1 isozyme in the postmortem brains of teenage suicide subjects is noteworthy. The reason that PLC β1 expression is abnormal in teenage but not adult suicide subjects is unclear, but it may be that neurobiological factors and clinical characteristics differ between adults and teenagers.
Even though serotonergic mechanisms have not been studied in postmortem brain samples obtained from depressed or suicidal youths or adolescents, the results of several studies suggest the presence of abnormal 5-HT mechanisms in depressed adolescents. Some investigators have reported that CSF 5-hydroxyindoleacetic acid is strongly correlated with aggression in adolescent subjects (18). Ryan et al. (19) reported abnormal prolactin and cortisol response to 5-hydroxytryptophan in depressed children, although they did not find any correlation with aggression or suicidality. It has also been reported that depressed children and adolescents have low numbers of platelet serotonin transporter sites (20, 21) and that these low numbers are associated with suicidality (21). Neurodevelopmental studies suggest that, whereas the serotonergic system is fully developed in adolescents, the noradrenergic system does not develop fully until early adulthood (22), and this could be why adolescents are more responsive to selective serotonin reuptake inhibitors than to noradrenergic antidepressants (9).
The mechanisms associated with the low PI-PLC activity and expression of the PLC β1 isozyme in teenage suicide are not clear. Since we found low PI-PLC activity and PLC β1 expression in both membrane and cytosol fractions of brain tissue, it is unlikely that the low PLC values are caused by abnormal translocation of PLC. These low levels do not appear to be related to the postmortem interval or to degradation of PLC enzymes by proteolysis, since the low PLC values were observed in only one of the isozymes, i.e., PLC β1; however, it is possible that the low PI-PLC activity may be related to a higher number of 5-HT2A receptors, as has been found in postmortem brains of suicide subjects (1), which cause stimulation of the phosphoinositide signaling system. It is possible that a sustained increase in phosphoinositide metabolism may cause desensitization of activity in both PLC and protein kinase C and may also cause a decrease in the expression of the PLC β1 isozyme, to which 5-HT2A receptors are linked through Gq proteins. There is some evidence in the literature that agonist stimulation of receptors and G proteins can cause desensitization of G proteins and effectors (23). It has also been observed that agonist stimulation decreases PI-PLC activity in turkey erythrocytes (24).
Our observations of low PI-PLC activity and expression of PLC β1, together with our previous report of a low number of [3H]phorbol dibutyrate binding sites in postmortem brains of teenage suicide subjects (10), indicate that abnormalities and disturbances in various components of the phosphoinositide signaling system may be associated with the pathophysiology of teenage suicide. Questions remain: Are the abnormalities that are observed downstream in the signaling system related to the abnormalities in the receptors that are linked to the phosphoinositide signaling system or are they independently abnormal? and Do these abnormalities represent genetic vulnerabilities to suicide?
Received Dec. 8, 1998; revision received April 28, 1999; accepted June 4, 1999. From the Psychiatric Institute, Department of Psychiatry, University of Illinois at Chicago; and the Maryland Psychiatric Research Center, Baltimore. Address reprint requests to Dr. Ghanshyam Pandey, Psychiatric Institute, University of Illinois at Chicago, 1601 West Taylor St., Chicago, IL 60612; [email protected] (e-mail). Supported by NIMH grant MH-48153 to Dr. Ghanshyam Pandey. Brain collection was supported in part by NIMH grant MH-40279. The authors thank John Smialek, M.D., and Dennis Chute, M.D., for cooperation in the collection of brain samples, Boris Lapidus, M.D., and Mr. Christopher Grove for dissections, Ms. Terri U’Prichard for help in performing psychological autopsies, and Mr. Paresh Savani for technical assistance.
 |
 |
 |
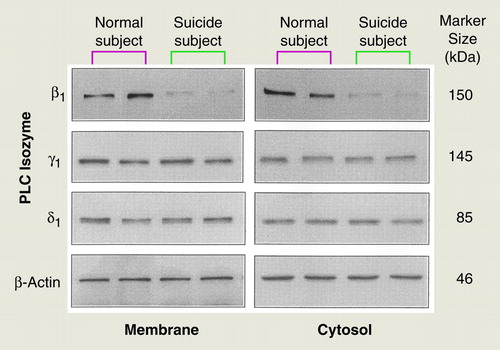
FIGURE 1. Representative Autoradiograms Showing Immunolabeling of Phospholipase C (PLC) β1, δ 1, and γ 1 Isozymes in Brodmann’s Areas 8 and 9 in Membrane and Cytosol Fractions of Postmortem Brains From Normal Teenage Subjects and Teenage Suicide Subjectsa
aAliquots of proteins were applied to 7.5% sodium dodecyl sulfate/polyacrylamide gels. Proteins were transferred to intracellular membranes and were probed with antibodies specific for PLC β1, δ1, and γ1 isozymes.
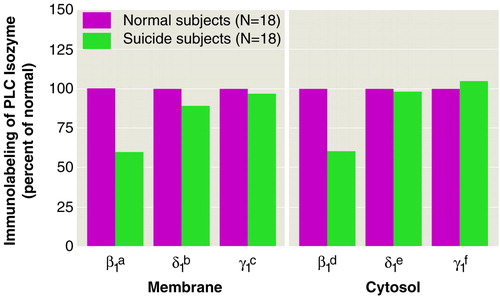
FIGURE 2. Mean Expressed Levels of Phospholipase C (PLC) β1, δ1, and γ1 Isozymes in Brodmann’s Areas 8 and 9 in Membrane and Cytosol Fractions of Postmortem Brains From Normal Teenage Subjects and Teenage Suicide Subjects
at=6.02, df=34, p<0.001.
bt=1.41, df=34, p<0.16.
ct=0.62, df=34, p<0.54.
dt=7.52, df=34, p<0.001.
et=0.32, df=34, p<0.74.
ft=0.27, df=34, p<0.78.
1. Gross-Isseroff R, Biegon A, Voet H, Weizman A: The suicide brain: a review of postmortem receptor/transporter binding studies. Neurosci Biobehav Rev 1998; 22:653–661Crossref, Medline, Google Scholar
2. Casabona G: Intracellular signal modulation: a pivotal role for protein kinase C. Prog Neuropsychopharmacol Biol Psychiatry 1997; 21:407–425Crossref, Medline, Google Scholar
3. Pacheco MA, Stockmeier C, Meltzer HY, Overholser JC, Dilley GE, Jope RS: Alterations in phosphoinositide signaling and G-protein levels in depressed suicide brain. Brain Res 1996; 723:37–45Crossref, Medline, Google Scholar
4. Cowburn RF, Marcusson JO, Eriksson A, Wiehager B, O’Neill C: Adenylyl cyclase activity and G-protein subunit levels in postmortem frontal cortex of suicide victims. Brain Res 1994; 633:297–304Crossref, Medline, Google Scholar
5. Friedman E, Wang H-Y: Receptor-mediated activation of G proteins is increased in postmortem brains of bipolar affective disorder subjects. J Neurochem 1996; 67:1145–1152Google Scholar
6. Young LT, Li PP, Kish SJ, Siu KP, Warsh JJ: Postmortem cerebral cortex Gs α-subunit levels are elevated in bipolar affective disorder. Brain Res 1991; 553:323–326Crossref, Medline, Google Scholar
7. Mathews R, Li PP, Young LT, Kish SJ, Warsh JJ: Increased Gq/11α immunoreactivity in postmortem occipital cortex from patients with bipolar affective disorder. Biol Psychiatry 1997; 41:649–656Crossref, Medline, Google Scholar
8. Hazell P, O’Connell D, Heathcote D, Robertson J, Henry D: Efficacy of tricyclic drugs in treating child and adolescent depression: a meta-analysis. Br Med J 1997; 310:897–901Crossref, Google Scholar
9. Emslie GJ, Rush AJ, Weinberg WA, Kowatch RA, Hughes CW, Carmody T, Rintelmann J: A double-blind, randomized, placebo-controlled trial of fluoxetine in children and adolescents with depression. Arch Gen Psychiatry 1997; 54:1031–1037Google Scholar
10. Pandey GN, Dwivedi Y, Pandey SC, Conley RR, Roberts RC, Tamminga CA: Protein kinase C in postmortem brain of teenage suicide subjects. Neurosci Lett 1997; 228:111–114Crossref, Medline, Google Scholar
11. Salzman S, Endicott J, Clayton P, Winokur G: Diagnostic Evaluation After Death (DEAD). Rockville, Md, National Institute of Mental Health, Neuroscience Research Branch, 1983Google Scholar
12. Spitzer RL, Williams JBW, Gibbon M, First MB: The Structured Clinical Interview for DSM-III-R (SCID), I: history, rationale, and description. Arch Gen Psychiatry 1992; 49:624–629Crossref, Medline, Google Scholar
13. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ: Protein measurement with folin phenol reagent. J Biol Chem 1951; 193:265–275Medline, Google Scholar
14. Pandey SC: Acute and chronic ethanol consumption effects on the immunolabeling of Gq/11α subunit protein and phospholipase C isozymes in the rat brain. J Neurochem 1996; 67:2355–2361Google Scholar
15. Dwivedi Y, Pandey GN: Administration of dexamethasone upregulates protein kinase C activity and the expression of γ and ε protein kinase C isozymes in the rat brain. J Neurochem 1999; 72:380–387Crossref, Medline, Google Scholar
16. Cockcroft S, Thomas GMH: Inositol-lipid-specific phospholipase C isoenzymes and their differential regulation by receptors. Biochem J 1992; 288:1–14Crossref, Medline, Google Scholar
17. Jope RS, Song L, Li PP, Young LT, Kish SJ, Pacheco MA, Warsh JJ: The phosphoinositide signal transduction system is impaired in bipolar affective disorder brain. J Neurochem 1996; 66:2402–2409Google Scholar
18. Kruesi MJ, Hibbs ED, Zahn TP, Keysor CS, Hamburger SD, Bartko JJ, Rapoport JL: A 2-year prospective follow-up study of children and adolescents with disruptive behavior disorders: prediction by cerebrospinal fluid 5-hydroxyindoleacetic acid, homovanillic acid, and autonomic measures? Arch Gen Psychiatry 1992; 49:429–435Google Scholar
19. Ryan ND, Birmaher B, Perel JM, Dahl RE, Meter V, Al-Shabout M, Iyengar S, Puig-Antich J: Neuroendocrine response to L-5-hydroxytryptophan challenge in prepubertal depression. Arch Gen Psychiatry 1992; 49:843–851Crossref, Medline, Google Scholar
20. Sallee FR, Hilal R, Dougherty D, Beach K, Nesbitt L: Platelet serotonin transporter in depressed children and adolescents: 3H-paroxetine platelet binding before and after sertraline. J Am Acad Child Adolesc Psychiatry 1998; 37:777–784Crossref, Medline, Google Scholar
21. Ambrosini PJ, Metz C, Arora RC, Lee JC, Kregel L, Meltzer HY: Platelet imipramine binding in depressed children and adolescents. J Am Acad Child Adolesc Psychiatry 1992; 31:298–305Crossref, Medline, Google Scholar
22. Goldman-Rakic PS, Brown RM: Postnatal development of monoamine content and synthesis in the cerebral cortex of Rhesus monkeys. Brain Res Dev Brain Res 1982; 4:339–349Crossref, Google Scholar
23. Milligan G: Agonist regulation of cellular G protein levels and distribution: mechanisms and functional implications. Trends Pharmacol Sci 1993; 14:413–418Crossref, Medline, Google Scholar
24. Galas M-C, Harden TK: Receptor-induced heterologous desensitization of receptor-regulated phospholipase C. Eur J Pharmacol Mol Pharmacol 1995; 291:175–182Crossref, Medline, Google Scholar



