Markers of Glutamatergic Neurotransmission and Oxidative Stress Associated With Tardive Dyskinesia
Abstract
Objective: Tardive dyskinesia is a movement disorder affecting 20%–40% of patients treated chronically with neuroleptic drugs. The dopamine supersensitivity hypothesis cannot account for the time course of tardive dyskinesia or for the persistence of tardive dyskinesia and the associated structural changes after neuroleptics are discontinued. The authors hypothesized that neuroleptics enhance striatal glutamatergic neurotransmission by blocking presynaptic dopamine receptors, which causes neuronal damage as a consequence of oxidative stress.Method: CSF was obtained from 20 patients with schizophrenia, 11 of whom had tardive dyskinesia. Markers for oxidative stress, including superoxide dismutase, lipid hydroperoxide, and protein carbonyl groups, and markers for excitatory neurotransmission, including N-acetylaspartate, N-acetylaspartylglutamate, aspartate, and glutamate, were measured in the CSF specimens. Patients were also rated for tardive dyskinesia symptoms with the Abnormal Involuntary Movement Scale.Results: Tardive dyskinesia patients had significantly higher concentrations of N-acetylaspartate, N-acetylaspartylglutamate, and aspartate in their CSF than patients without tardive dyskinesia when age and neuroleptic dose were controlled for. The significance of the higher levels of protein-oxidized products associated with tardive dyskinesia did not pass Bonferroni correction, however. Tardive dyskinesia symptoms correlated positively with markers of excitatory neurotransmission and protein carbonyl group and negatively with CSF superoxide dismutase activity.Conclusions: These findings suggest that there are elevated levels of oxidative stress and glutamatergic neurotransmission in tardive dyskinesia, both of which may be relevant to the pathophysiology of tardive dyskinesia. Am J Psychiatry 1998; 155: 1207-1213
Tardive dyskinesia is a movement disorder that affects 20%–40% or more of patients treated chronically with neuroleptic drugs (1). The manifestations of tardive dyskinesia may include adventitious movements of the oral-facial region, choreoathetosis of the extremities, and lordotic posturing. Although the theory that striatal postsynaptic dopamine receptor supersensitivity causes tardive dyskinesia has been widely accepted for two decades, there is evidence that challenges this model (2). Although acute administration of neuroleptics temporarily increases the firing of dopamine neurons, chronic treatment with neuroleptics leads to a decrease in their firing rate caused by depolarization block (3)). Dopamine synthesis and release decrease after an acute elevation of dopamine turnover with haloperidol treatment; dopamine metabolite levels return to normal with chronic treatment (4). Notably, striatal dopamine metabolites are reduced in monkeys with dyskinesia caused by long-term neuroleptic treatment. Finally, postmortem neurochemical studies have not revealed a correlation between dopamine receptor up-regulation and tardive dyskinesia (5). Thus, an excessive activation of postsynaptic striatal dopamine receptors is not consistent with the time course or persistence of tardive dyskinesia.
An alternative hypothesis supports a neurodegenerative process affecting striatal efferents analogous to the process in Huntington’s disease. Tardive dyskinesia is similar to Huntington’s disease in that both diseases present with choreoathetoid movements and pathological changes in the striatum. Christensen et al. (6) reported neuronal loss in a postmortem brain study of the basal ganglia of patients with persistent tardive dyskinesia, and similar losses have been described in rats treated chronically with neuroleptics (7-9). Importantly, loss of the presynaptic markers for the striatal-pallidal and nigral γ-aminobutyric acid (GABA), GABAergic neurons, and glutamic acid decarboxylase have been observed in a primate model for tardive dyskinesia and in postmortem studies of patients with tardive dyskinesia (10-12).
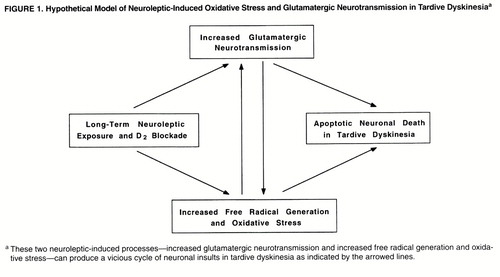
Presynaptic dopamine D2 receptors inhibit the release of glutamate from excitatory cortical striatal projections (13). Therefore, neuroleptic blockade of these receptors increases the synaptic release of aspartate and glutamate in the striatum (14, 15). Persistent activation of glutamate ionotropic receptors has long been known to cause neuronal degeneration (16). Oxidative damage mediates the delayed neuronal degeneration caused by activation of N-methyl-D-aspartic acid (NMDA) and non-NMDA glutamate ionotropic receptors (17). Oxyradicals can damage cellular proteins, membranes, and DNA, depending on their source, causing cell death. An important aspect of the linkage between delayed glutamate-induced degeneration and oxidative stress is that it provides a mechanism whereby persistent low levels of glutamate receptor stimulation can cause cumulative damage to neurons, ultimately leading to degeneration (figure 1). This form of neurodegeneration exhibits many of the characteristics of “apoptosis.” It provides an important pathological link between moderate levels of excessive glutamate ionotropic receptor stimulation and delayed neuronal degeneration.
Notably, Mitchell et al. (18) have reported that elimination of striatal dopaminergic afferents leads to striatal neuronal apoptotic death (i.e., program cell death). In addition, elevated oxyradicals can inhibit presynaptic glutamate uptake, inactivate the enzymatic defenses against cellular oxidants (19), disrupt mitochondrial electron transport (20, 21), stimulate nitric oxide synthase by the influx of calcium mediated by NMDA receptor, and interact with superoxide to form the highly reactive peroxynitrite radical (22), which results in an increased generation of redox-active species and extraneuronal excitatory amino acids. These secondary interactions can, thereby, produce a vicious cycle promoting glutamate mediated oxidative damage in the striatum (figure 1).
The present study was designed to examine the hypothesis that enhanced excitatory amino acid neurotransmission and oxidative damage are present in patients with schizophrenia who received neuroleptic treatment and developed tardive dyskinesia. We also examined the hypothesis that there is a reciprocal relationship between the glutamatergic markers and oxidative stress defense mechanisms (figure 1). Accordingly, several markers for excitatory neurotransmission (i.e., N-acetylaspartylglutamate, N-acetylaspartate, aspartate, and glutamate) and for oxidative damage (i.e., superoxide dismutase, protein carbonyl content, and lipid hydroperoxides) were measured in the CSF of patients with schizophrenia who had histories of chronic neuroleptic treatment, about half of whom exhibited the symptoms of tardive dyskinesia. Specifically, we hypothesized that concentrations of excitatory neurotransmitters (aspartate, glutamate, N-acetylaspartate, and N-acetylaspartylglutamate) are higher in tardive dyskinesia but that superoxide dismutase activity, a defense enzyme for oxidative damage, is attenuated, which results in increased production of oxidized molecules. We also examined the relationships among the glutamatergic markers, oxidative stress markers, and tardive dyskinesia symptoms.
METHOD
Case control sampling was applied to 20 patients selected from our study group of about 500 outpatients with schizophrenia treated at the Freedom Trail Mental Health Clinic affiliated with Massachusetts General Hospital in Boston. The majority of the patients had chronic schizophrenia; the mean duration of illness was about 20 years (table 1). After complete description of the study in a protocol approved by our institutional review board, written informed consent was obtained from all subjects. The patients’ tardive dyskinesia status was stratified according to the Schooler criteria (23) before they enrolled in the study. The severity of tardive dyskinesia was assessed by using the Abnormal Involuntary Movement Scale (AIMS) (24). The selected subjects were diagnosed by our research psychiatrist (G.T.), and all of them fulfilled DSM-III-R diagnostic criteria for schizophrenia. Exclusion criteria included history of any neurological disorder other than tardive dyskinesia, illicit drug use in the previous 6 months, or any unstable medical condition.
Overall, the patients with and without tardive dyskinesia were similar in their demographics, clinical features, Parkinsonian symptoms, and chlorpromazine-equivalent neuroleptic dose (25) (table 1). All of the patients had chronic schizophrenia and had taken neuroleptics for a long time; however, the exact duration of tardive dyskinesia and neuroleptic exposure as well as the life-long doses of neuroleptic could not be ascertained. All of the patients received neuroleptics and had been receiving stable doses during the previous 2 months. In the tardive dyskinesia group, the neuroleptics were fluphenazine (N=5), fluphenazine and thioridazine (N=2), haloperidol (N=2), trifluoperazine (N=1), and thioridazine (N=1). Six tardive dyskinesia patients received benztropine, and one received propranolol. In the non-tardive-dyskinesia group, the neuroleptics were fluphenazine (N=6), fluphenazine and chlorpromazine (N=1), haloperidol (N=1), and haloperidol and trifluoperazine (N=1). Four non-tardive-dyskinesia patients received benztropine, two received trihexyphenidyl, one received clonazepam, and two received lorazepam. Scores on the Scale for the Assessment of Negative Symptoms and the Scale for the Assessment of Positive Symptoms of the two groups were similar (table 1).
Parkinsonian symptoms were measured by the Simpson-Angus Rating Scale. All of the AIMS and Simpson-Angus Rating Scales were administered by the same investigator. To ascertain the diagnosis, tardive dyskinesia and AIMS scores were assessed once within 6 months before the lumbar puncture and again when lumbar puncture was performed. The AIMS scores at the two assessment periods did not reveal significant changes over time in these patients (table 1).
Lumbar punctures were performed between 8:00 and 9:00 a.m., following a 12-hour fast. A total of 10 ml of CSF was collected from each patient. Routine analyses were done to confirm that the protein concentration was in the normal range and the cell count was smaller than 5/mm3. CSF specimens were immediately transferred to a –80˚C freezer. All biochemical analyses were performed blind to diagnosis.
To provide a reference group for our study, CSF was obtained from 20 age-matched and sex-matched normal subjects at the Biochemistry Laboratory, Massachusetts General Hospital, to control for psychiatric disorder and neuroleptic treatment. Medical record review was performed to exclude subjects who had histories of neurological disorders, psychiatric disorders, major medical illness, neuroleptic exposure, or illicit drug use. Subjects who had family histories of movement disorders were also excluded.
Amino Acids and Peptide Analysis
Amino acids were measured by o-phthalaldehyde pre-column derivatization coupled with reverse-phase C-18 column high performance liquid chromatography separation and fluorescent detection (26). Absolute concentrations of the amino acids were determined by using computer analysis (Maxima 820, Waters, Mass.) of peak height with internal and external standards. The aspartate levels were also reported in another study of this group of patients (25). N-Acetylaspartylglutamate and N-acetylaspartate were measured by anion-exchange high performance liquid chromatography separation and ultraviolet detection at the wavelength of 214 nm (26). Their concentrations were determined by peak height calculation.
Superoxide Dismutase Analysis
Total superoxide dismutase activity in the CSF was analyzed according to a modification of a spectrophotometric method (27). The reduction of cytochrome c by superoxide was used to detect and measure superoxide dismutase activity by the change in absorbance at 550 nm. Aliquots of CSF were added to 20 mM of bicarbonate buffer containing 10 M sodium azide, 10 M cytochrome c, 100 M xanthine, and 1 M EDTA. The reduction of acetylated cytochrome c was initiated by the addition of xanthine oxidase.
Lipid Hydroperoxide
The levels of lipid hydroperoxides were measured according to an adaptation of the ferrous oxidation/xylenol orange method described by Puttfarcken et al. (27). Ten l of CSF was added to 90 l of the reaction mixture (100 M xylene orange (o-cresolsulfonphthalein-3"3"-bis(methyl-iminodiacetic acid sodium salt), 250 M ferrous chloride, 25 mM H2SO4, and 4 mM BHT in 90% methanol and incubated at 30˚C for 30 minutes. The absorbance of the sample was determined at 560 nm against a blank, which consists of all the reagents and 10 l of water. The final concentration of lipid hydroperoxides was calculated using an extinction coefficient of 4.3×104 M–1cm–1.
Protein Carbonyl Assay
The method for protein carbonyl assay was adapted from Vevine et al. (28). Briefly, 90 l of CSF was added to 10 l of 10% (weight/volume) streptomycin sulfate in 50 mM of HEPES, pH=7.2, and then centrifuged at 11,000 g for 10 minutes after 15 minutes of incubation. The supernatant was precipitated with an equivalent volume of 20% trichloroacetic acid by centrifuge at 11,000 g for 10 minutes. The protein pellet was redissolved in 50 l of water. Then 6 l of 1 M Tris-HCl, 10 mM of EDTA, and 14 l of 100 mM [3H]NaBH4 with a specific activity of 100 mCi/mmol was added to the sample and incubated at 37˚C for 30 minutes. The sample was again precipitated with 1 ml of 10% trichloroacetic acid and centrifuged at 11,000 g for 10 minutes. The pellet was again dissolved in 6 M of guanidine solution. The radioactivity was determined by liquid scintillation counting after incubation at 37˚C for 30 minutes. The data were expressed as picamoles of carbonyl group/g of protein.
Statistics
Because of the small number of patients in the study group, the nonparametric Kruskal-Wallis one-way analysis of variance was computed for each molecule or enzyme activity in CSF between patients with schizophrenia who did or did not have tardive dyskinesia. Bonferroni correction was applied to reduce type I error (table 2). To test the a priori hypothesis of a vicious cycle promoting glutamate-mediated oxidative damage in tardive dyskinesia, Spearman correlation coefficients were calculated between oxidative stress and glutamatergic markers and also between AIMS scores and CSF markers.
RESULTS
Excitatory Neurotransmitters
After controlling for age and stable dose of neuroleptics, we found that the patients with tardive dyskinesia had higher concentrations of N-acetylaspartate, N-acetylaspartylglutamate, and aspartate than the patients without tardive dyskinesia (table 2, figure 2). The elevation in glutamate did not pass Bonferroni correction (U=75, N=20, p=0.05). Levels of glycine and GABA were not significantly different among the three groups (the 20 patients with schizophrenia who did or did not have tardive dyskinesia and the 20 normal subjects from the Biochemistry Laboratory). Benzodiazepine may affect the excitatory neurotransmitters, but the number of subjects receiving benzodiazepine was too small (N=3) to determine its effect.
Oxidative Stress Markers
The CSF of patients with tardive dyskinesia had higher levels of protein carbonyl oxidation products than the CSF of patients without tardive dyskinesia, but this difference did not pass Bonferroni correction (U=81, N=20, p=0.002) (table 2). Superoxide dismutase activity was lower in the tardive dyskinesia group but did not pass the statistical test (U=47, N=20, p=0.09). However, no difference in the level of lipid hydroperoxides was observed among the patients with schizophrenia who did or did not have tardive dyskinesia and the normal subjects.
Relation Between Oxidative Stress and Excitatory Neurotransmission
In the CSF of schizophrenia patients as a group, superoxide dismutase activity was inversely correlated with aspartate concentration (r=–0.49, N=20, p=0.03); carbonyl group levels positively correlated with aspartate (r=0.45, N=20, p=0.05).
Relation Between Tardive Dyskinesia Presentations and Oxidative Stress and Excitatory Neurotransmission
For all schizophrenic subjects, total scores on the AIMS were positively correlated with N-acetylaspartate (r=0.68, N=20, p=0.001), N-acetylaspartylglutamate (r=0.79, N=20, p=0.0003), aspartate (r=0.63, N=20, p=0.003), glutamate (r=0.44, N=20, p=0.05), and protein carbonyl (r=0.41, N=20, p=0.07) but inversely correlated with superoxide dismutase activity (r=–0.41, N=20, p=0.07). The significant correlations result from the bimodal distribution of AIMS scores in the schizophrenic patients; within the tardive dyskinesia group, AIMS scores did not correlate with any neurochemical markers.
DISCUSSION
Our findings demonstrate higher levels of N-acetylaspartate, N-acetylaspartylglutamate, and aspartate in the CSF of patients with schizophrenia who had tardive dyskinesia than in the CSF of patients with schizophrenia who did not have tardive dyskinesia (table 2). It appears that the elevation in markers for excitatory amino acid neurotransmission relates to the presence of tardive dyskinesia rather than current neuroleptic exposure or underlying psychopathology of schizophrenia because the neuroleptic dose and the status of schizophrenia (subtype as well as positive and negative symptoms) were similar between the patients with and without tardive dyskinesia (table 1).
Our findings support our a priori hypothesis. However, since all of our subjects had chronic schizophrenia, our findings may not be generalizable to all patients suffering from tardive dyskinesia. In addition, the number of subjects studied was small and statistical power is therefore limited. As a result, there may be type II error. Nevertheless, our findings did reveal the elevation of several excitatory markers (table 2).
The relatively small number of patients studied did not permit us to examine the effects of the biochemical variables simultaneously, but only in isolation. Therefore, we applied Bonferroni correction to reduce type I error. In addition, the biochemical assays we performed were cross-sectional analyses. The temporal sequence of the onset of tardive dyskinesia and the biochemical changes is not clear, and the case-control design cannot demonstrate a causative relationship.
Previous studies indicated that neuroleptic blockade of presynaptic dopamine D2 receptors located on corticostriatal glutamatergic afferents enhances the release of glutamate. Ultrastructural study reveals that neuroleptic treatment increases the number of glutamatergic corticostriatal synapses (29). In fact, Gattaz et al. (30) reported that the CSF levels of glutamate were higher in patients receiving neuroleptics than those not taking neuroleptics. Consistent with this pathogenic mechanism for elevated striatal excitatory neurotransmission in tardive dyskinesia, clozapine, an atypical neuroleptic rarely causingtardive dyskinesia, increases extracellular aspartate and glutamate in the prefrontal cortex without affecting their levels within thestriatum (31). The inability of clozapine to increase striatal glutamatergic activity may explain the fact that it is less likely to produce tardive dyskinesia (14).
Our study also demonstrates a reduction in superoxide dismutase activity and an increase in protein carbonyl concentration in the CSF of tardive dyskinesia patients. Although they did not pass the statistical test, it is important to discuss the findings in the context of the oxidative stress hypothesis of tardive dyskinesia. Superoxide dismutase is an enzyme critical in the detoxification of superoxide, a normal byproduct of oxidative metabolism (32). Attenuated activity of superoxide dismutase may contribute to the increase of oxidized protein. Chronic treatment of rats with fluphenazine has been reported to cause a decrease in superoxide dismutase and catalase activities in the nervous system (33). Decreased superoxide dismutase renders neurons more vulnerable to oxyradical injury, consistent with the elevated levels of protein carbonyl groups. Although we did not observe an elevation in lipid hydroperoxides in the CSF of patients with tardive dyskinesia, elevated levels of conjugated dienes and thiobarbituric acid reactive products in the CSF of tardive dyskinesia patients have been reported (34, 35). The discrepant findings may be due to the different types of patients or analytical methodologies applied.
Superoxide dismutase also protects against neuronal degeneration induced by glutamatergic mechanisms (36). The inverse correlations between CSF levels of aspartate, protein carbonyl groups, and superoxide dismutase activity in tardive dyskinesia suggest an etiologic relationship between enhanced excitatory amino acid neurotransmission and oxidative damage associated with tardive dyskinesia (figures 1 and 2). Low peripheral superoxide dismutase activity has been reported in drug-naive patients during a first episode of schizophrenia (37). In addition to oxidative damage enhanced by neuroleptic-induced hyperglutamatergic neurotransmission, the other plausible model for the development of tardive dyskinesia is that lower activity of superoxide dismutase renders the striatal neurons more vulnerable to excitatory neurotransmission, which is exacerbated by neuroleptics, in a subgroup of patients with schizophrenia.
Our study needs to be replicated with larger groups of patients. The relationships between the glutamatergic and oxidative stress markers can be noncausal. The strong correlations between oxidative stress and glutamatergic neurotransmission suggest that their effects on the occurrence of tardive dyskinesia may be confounded; their strong correlations may preclude separation of their effects statistically even in a large-series study. Also, the findings in CSF may represent only part of the neuronal activity as far as the oxidative damage and enhanced glutamatergic neurotransmission are concerned.
Both preclinical and clinical studies point to degeneration of striatal efferent neurons, especially GABAergic neurons, in tardive dyskinesia. Although inconclusive, one brain imaging study has revealed reduction in the volume of the caudate nuclei in patients with tardive dyskinesia compared with patients without tardive dyskinesia and normal control subjects (38). In the primate model of experimental tardive dyskinesia, monkeys with neuroleptic-induced dyskinesis exhibit reductions in presynaptic GABAergic markers in the subthalamic nucleus, the medial segment of the globus pallidus, and the rostral part of the substantia nigra (12). Rodent models of tardive dyskinesia have also revealed significantly lower density of large neurons in the striatum (39) and decreased glutamic acid decarboxylase activity in the substantia nigra (11, 40). Finally, Mitchell et al. (18) demonstrated in the rat apoptotic neuronal death in the striatum as a consequence of a lesion to the nigrostriatal dopaminergic pathway. The elevated levels of CSF N-acetylaspartate in tardive dyskinesia are consistent with a neuronal degenerative process because CSF N-acetylaspartate, a marker for neuronal integrity, is elevated in amyotrophic lateral sclerosis (41) and because tissue N-acetylaspartate levels decrease in areas involved in active neuronal degeneration in amyotrophic lateral sclerosis, Huntington’s disease, and Alzheimer’s disease (for a review see reference 42)).
The hypothesis of oxidative damage to striatal neurons mediated by neuroleptic enhancement of glutamatergic neurotransmission is supported by reports that vitamin E reverses the symptoms of tardive dyskinesia; the anecdotal reports have been sustained by double-blind, placebo-controlled studies with vitamin E (43-45). Notably, patients are more responsive to treatment with vitamin E earlier in the course of their disorder, consistent with the model that the oxidative damage is cumulative over time and would involve functional impairment before frank degeneration. Similarly, in a double-blind, placebo-controlled study of vitamin E treatment for Huntington’s disease, patients who were less symptomatic at the initiation of treatment exhibited the most favorable response (46). Inasmuch as centrally active free radical scavengers may not only reverse the oxidative damage but also correct an impairment in the inactivation of glutamate, additional studies on the glutamatergic neurotransmission and oxidative stress in tardive dyskinesia as well as efficacy of prevention and treatment with centrally active free radical scavengers on the surrogates of central excitatory neurotransmission and oxidative stress in tardive dyskinesia need to be carried out.
Received Nov. 10, 1997; revision received Feb. 27, 1998; accepted March 24, 1998. From the Laboratory of Molecular and Developmental Neuroscience, Department of Psychiatry, Harvard Medical School and Massachusetts General Hospital, Boston.. Address reprint requests to Dr. Coyle, Department of Psychiatry, Harvard Medical School, 115 Mill St., Belmont, MA 02178. Supported in part by grant MH-51290 from NIMH, grant NS-13584 from the National Institute of Neurological and Communicative Disorders and Stroke, and a Senior Investigator Award from the National Alliance for Research on Schizophrenia and Depression (Dr. Coyle); and by Public Health Service grant NS-13584-17, a Young Investigator Award from the National Alliance for Research on Schizophrenia and Depression, a Stanley Foundation Research Award, the Canavan Foundation, and the Peter and Elizabeth Tower Foundation (Dr. Tsai).
 |
 |

1. Morgenstern H, Glazer WM: Identifying risk factors for tardive dyskinesia among long-term outpatients maintained with neuroleptic medications. Arch Gen Psychiatry 1993; 50:723–733Crossref, Medline, Google Scholar
2. Jenner P, Marsden CD: Is the dopamine hypothesis of tardive dyskinesia completely wrong? Trends Neurosci 1986; 9:259–260Google Scholar
3. Cadet JL, Lohr JB: Possible involvement of free radicals in neuroleptic-induced movement disorders: evidence from treatment of tardive dyskinesia with vitamin E. Ann NY Acad Sci 1989; 570:176–185Crossref, Medline, Google Scholar
4. Rastogi SK, Rastogi RB, Singhal RL, Lapierre YD: Behavioral and biochemical alterations following haloperidol treatment and withdrawal: the animal model of tardive dyskinesia reexamined. Prog Neuropsychopharmacol Biol Psychiatry 1983; 7:153–164Crossref, Medline, Google Scholar
5. Crow TJ, Cross AJ, Johnstone EC, Owen F, Owens DG, Waddington JL: Abnormal involuntary movements in schizophrenia: are they related to the disease process or its treatment? are they associated with changes in dopamine receptors? J Clin Psychopharmacol 1982; 2:336–340Google Scholar
6. Christensen E, Moller JE, Faurbye A: Neuropathological investigation of 28 brains from patients with dyskinesia. Acta Psychiatr Scand 1970; 46:14–23Crossref, Medline, Google Scholar
7. Gunne LM, Andren PE: An animal model for coexisting tardive dyskinesia and tardive parkinsonism: a glutamate hypothesis for tardive dyskinesia. Clin Neuropharmacol 1993; 16:90–95Crossref, Medline, Google Scholar
8. Nielsen EB, Lyon M: Evidence for cell loss in corpus striatum after long-term treatment with a neuroleptic drug (flupenthixol) in rats. Psychopharmacology (Berl) 1978; 59:85–89Crossref, Medline, Google Scholar
9. Pakkenberg H, Fog R, Nilakantan B: The long-term effect of perphenazine enanthate on the rat brain: some metabolic and anatomical observations. Psychopharmacologia 1973; 29:329–336Crossref, Medline, Google Scholar
10. Anderson U, Haggstrom JE, Levin ED, Bondesson U, Valverius M, Gunne LM: Reduced glutamate decarboxylase activity in the subthalamic nucleus in patients with tardive dyskinesia. Mov Disord 1989; 4:37–46Crossref, Medline, Google Scholar
11. Gunne LM, Haggstrom JE: Experimental tardive dyskinesia. J Clin Psychiatry 1985; 46:48–50Medline, Google Scholar
12. Gunne LM, Haggstrom JE, Sjoquist B: Association with persistent neuroleptic-induced dyskinesia of regional changes in brain GABA synthesis. Nature 1984; 309:347–349Crossref, Medline, Google Scholar
13. Carlsson M, Carlsson A: Interaction between glutamatergic and monoaminergic systems within the basal ganglia—implications for schizophrenia and Parkinson’s disease. Trends Neurosci 1990; 13:272–276Crossref, Medline, Google Scholar
14. Bardgett ME, Wrona CT, Newcomer JW, Csernansky JG: Subcortical excitatory amino acid levels after acute and subchronic administration of typical and atypical neuroleptics. Eur J Pharmacol 1993; 230:245–250Crossref, Medline, Google Scholar
15. Perry TL, Hansen S, Kish SJ: Effects of chronic administration of antipsychotic drugs on GABA and other amino acids in the mesolimbic area of rat brain. Life Sci 1979; 24:283–288Crossref, Medline, Google Scholar
16. Olney JW: Excitotoxic amino acids and neuropsychiatric disorders. Annu Rev Pharmacol Toxicol 1990; 30:47–71Crossref, Medline, Google Scholar
17. Coyle JT, Puttfarcken P: Oxidative stress, glutamate, and neurodegenerative disorders. Science 1993; 262:689–695Crossref, Medline, Google Scholar
18. Mitchell IJ, Lawson S, Moser B, Laidlaw SM, Cooper AJ, Walkinshaw G, Waters CM: Glutamate-induced apoptosis results in a loss of striatal neurons in the parkinsonian rat. Neuroscience 1994; 63:1–5Crossref, Medline, Google Scholar
19. Volterra A, Trotti D, Tromba C, Floridi S, Racagni G: Glutamate uptake inhibition by oxygen free radicals in rat cortical astrocytes. J Neurosci 1994; 14:2924–2932Crossref, Medline, Google Scholar
20. Burkhardt C, Kelly JP, Lim YH, Filley CM, Parker WD Jr: Neuroleptic medications inhibit complex I of the electron transport chain. Ann Neurol 1993; 33:512–517Crossref, Medline, Google Scholar
21. Jackson-Lewis V, Przedborski S: Neuroleptic medications inhibit complex I of the electron transport chain. Ann Neurol 1994; 35:244–245Crossref, Medline, Google Scholar
22. Simonian NA, Coyle JT: Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol 1996; 36:83–106Crossref, Medline, Google Scholar
23. Schooler NR, Kane JM: Research diagnoses for tardive dyskinesia (letter). Arch Gen Psychiatry 1982; 39:486–487Medline, Google Scholar
24. Simpson GM, Angus JWS: A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 1970; 212:11–19Crossref, Medline, Google Scholar
25. Goff DC, Tsai G, Beal MF, Coyle JT: Tardive dyskinesia and substrates of energy metabolism in CSF. Am J Psychiatry 1995; 152:1730–1736Link, Google Scholar
26. Tsai G, Cork LC, Stauch B, Coyle JT: Abnormal acidic amino acids and N-acetylaspartylglutamate in hereditary canine motor neuron disease. Brain Res 1993; 629:305–309Crossref, Medline, Google Scholar
27. Puttfarcken PS, Getz RL, Coyle JT: Kainic acid-induced lipid peroxidation: protection with butylated hydroxytoluene and U78517F in primary cultures of cerebellar granular cells. Brain Res 1993; 624:223–232Crossref, Medline, Google Scholar
28. Vevine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz A-G, Ahn BW, Shaltiel S, Stadtman ER: Determination of carbonyl content in oxidatively modified protein, in Methods in Enzymology. Edited by Packer L, Glazer AN. New York, Academic Press, 1990, pp 464–478Google Scholar
29. Meshul CK, Casey DE: Regional, reversible ultrastructural changes in rat brain with chronic neuroleptic treatment. Brain Res 1989; 489:338–346Crossref, Medline, Google Scholar
30. Gattaz WF, Gattaz D, Beckmann H: Glutamate in schizophrenics and healthy controls. Arch Psychiatr Nervenkr 1982; 231:221–225Crossref, Medline, Google Scholar
31. Daly DA, Moghaddam B: Actions of clozapine and haloperidol on the extracellular levels of excitotory amino acids in the prefrontal cortex and striatum of conscious rats. Neurosci Lett 1993; 152:61–64Crossref, Medline, Google Scholar
32. Halliwell B: Reactive oxygen species and the central nervous system. J Neurochem 1992; 59:1609–1623Crossref, Medline, Google Scholar
33. Cadet JL, Perumal AS: Chronic treatment with prolixin causes oxidative stress in rat brain. Biol Psychiatry 1990; 28:738–740Crossref, Medline, Google Scholar
34. Lohr JB, Kuczenski R, Bracha HS, Moir M, Jeste DV: Increased indices of free radical activity in the cerebrospinal fluid of patients with tardive dyskinesia. Biol Psychiatry 1990; 28:535–539Crossref, Medline, Google Scholar
35. Pall HS, Williams AC, Blake DR, Lunec J: Evidence of enhanced lipid peroxidation in the cerebrospinal fluid of patients taking phenothiazines. Lancet 1987; 2:596–599Crossref, Medline, Google Scholar
36. Schwartz PJ, Reaume A, Scott R, Coyle JT: Effects of over- and under-expression of Cu,Zn-superoxide dismutase on toxicity of glutamate analogs in transgenic mouse striatum. Brain Res 1998; 789:32–39Crossref, Medline, Google Scholar
37. Mukherjee S, Mahadik SP, Correnti EE, Scheffer R: The antioxidant defense system at the onset of psychosis. Biol Psychiatry 1994; 35:701–708Google Scholar
38. Mion CC, Andreasen NC, Arndt S, Swayze VW II, Cohen GA: MRI abnormalities in tardive dyskinesia. Psychiatry Res 1991; 40:157–166Crossref, Medline, Google Scholar
39. Jeste DV, Lohr JB, Manley M: Study of neuropathologic changes in the striatum following 4, 8, and 12 months of treatment with fluphenazine in rats. Psychopharmacology (Berl) 1992; 106:154–160Crossref, Medline, Google Scholar
40. Johansson PE: Methylazoxymethanol (MAM)-induced brain lesion and oral dyskinesia in rats. Psychopharmacology (Berl) 1990; 100:72–76Crossref, Medline, Google Scholar
41. Rothstein JD, Tsai G, Kuncl RW, Clawson L, Cornblath DR, Drachman DB, Pestronk A, Stauch B, Coyle JT: Abnormal excitatory amino acid metabolism in amyotrophic lateral sclerosis. Ann Neurol 1990; 28:18–25Crossref, Medline, Google Scholar
42. Tsai G, Coyle JT: N-Acetylaspartate in neuropsychiatric disorders. Prog Neurobiol 1995; 46:531–540Crossref, Medline, Google Scholar
43. Adler LA, Peselow E, Rotrosen J, Duncan E, Lee M, Rosenthal M, Angrist B: Vitamin E treatment of tardive dyskinesia. Am J Psychiatry 1993; 150:1405–1407Link, Google Scholar
44. Egan MF, Hyde TM, Albers GW, Elkashef A, Alexander RC, Reeve A, Blum A, Saenz RE, Wyatt RJ: Treatment of tardive dyskinesia with vitamin E. Am J Psychiatry 1992; 149:773–777Link, Google Scholar
45. Lohr JB, Caligiuri MP: A double-blind placebo-controlled study of vitamin E treatment of tardive dyskinesia. J Clin Psychiatry 1996; 57:167–173Medline, Google Scholar
46. Peyser CE, Folstein M, Chase GA, Starkstein S, Brandt J, Cockrell JR, Bylsma F, Coyle JT, McHugh PR, Folstein SE: A trial of d-α-tocopherol in Huntington’s disease. Am J Psychiatry 1995; 152:1771–1775Link, Google Scholar



