Serotonin Transporter Protein Gene Polymorphism and Personality Measures in African American and European American Subjects
Abstract
Objective:The SLC6A4 locus encodes the serotonin transporter, which in turn mediates the synaptic inactivation of the neurotransmitter serotonin. A polymorphism located in the 5′ promoter region of the gene is associated with altered transcriptional activity of SLC6A4; an earlier study reported an association of the polymorphism with anxiety- and depression-related traits, including harm avoidance and neuroticism. The authors attempted to replicate this finding. Method:They assessed genotype at the SLC6A4 promoter polymorphism, and an additional polymorphism in intron 2, in 322 American subjects of European and African ancestry, some with diagnoses of a personality disorder or substance dependence and some normal comparison subjects. Harm avoidance was measured by the Tridimensional Personality Questionnaire in all subjects, and neuroticism was measured by the NEO Five-Factor Inventory in 185 subjects. Allele frequencies in the groups were compared, and hierarchical multiple regression was used to examine the correlation of demographic features, psychiatric diagnostic group, and genotype with harm avoidance and neuroticism scores.Results:Although the demographic factors and psychiatric diagnoses had effects on harm avoidance and neuroticism scores, there was no main effect of genotype on these personality measures. In the context of these overall negative findings, interactions were observed between sex and promoter system genotype and between race and promoter system genotype which suggest that the present findings are not wholly inconsistent with those of the earlier study. Conclusions:The authors were unable to replicate the association finding. The specific phenotypic composition of the groups studied with respect to other behaviors could have influenced ability to detect association of SLC6A4 polymorphisms with personality measures; population stratification for this locus is also of potential importance. Am J Psychiatry 1998; 155: 1332-1338
Many neuropsychiatric disorders involve serotonergic neurotransmission, and therefore genes coding for proteins involved in serotonergic neurotransmission are considered candidates for a range of behavioral phenotypes. Several serotonin system genes have been reported to be associated with psychiatric phenotypes; for example, alleles at an intronic tryptophan hydroxylase polymorphism were reported to be associated with suicide-related behaviors or impulsivity in some subjects (1-3), and alleles at a silent exonic serotonin 2a receptor (HTR2A) polymorphism were reported to be associated with schizophrenia (4, 5).
The serotonin transporter protein mediates sodium-dependent presynaptic reuptake of serotonin, terminating serotonergic neurotransmission. The gene coding for this protein is designated SLC6A4 (“carrier family 6 [neurotransmitter transporter, serotonin], member 4”); it maps to chromosome 17q11.1-q12 (6) close to D17S98 (7). Several genetic polymorphisms at the SLC6A4 locus have been reported (8-10). The serotonin transporter protein has great importance for modulating serotonergic function. This protein is the primary known target of the selective serotonin reuptake inhibitor drugs. Genetic variation in the gene coding for this protein could have effects on any of the broad range of serotonin-related behaviors (11).
A reported association between genetic variation in the promoter region of SLC6A4 and anxiety-related traits (12) is noteworthy among reported associations with serotonin system genes because of the demonstrated functional effect of the polymorphism (10, 12). This SLC6A4 polymorphism consists of a series of repeats within the promoter region of the gene; two common alleles in this region differ in length by 44 base pairs (10). Heils et al. (10) demonstrated that the “l” (or 16-repeat) allele has higher basal transcriptional activity than the “s” (or 14-repeat) allele (by about a factor of three), and higher increases in activity induced by either cyclic AMP or protein kinase C, in a human choriocarcinoma cell line, JAR. Lesch et al. (12) studied human lymphoblast cell lines expressing the three common genotypes at this locus, “ll,” “ls,” and “ss,” and demonstrated higher serotonin transporter protein mRNA levels in “ll” cells than in “ls” or “ss” cells. Other in vitro experiments were also consistent with the formulation that the “l” allele has higher activity than the “s” allele, and the “s” allele acts nearly as a dominant.
Lesch et al. (12) studied the relationship between polymorphic variation at this locus and personality in a group of 505 subjects consisting of male siblings and other relatives as well as unrelated individuals. Eighty-seven percent were European American, and the remainder were African American, Hispanic, or from other populations. Personality was evaluated with use of the revised NEO Peronality Inventory (13) and Cattell’s 16-PF personality inventory. An association was demonstrated between the “s” allele and higher NEO neuroticism scores, reflecting anxiety- and depression-related factors; subjects with the “ss” and “ls” alleles had significantly higher scores on this measure than subjects with the “ll” allele. Significant differences, ranging from p=0.002 to p=0.027, by genotype were seen for four of the six facets of this factor. Lesch et al. also derived scores from the NEO that were analogous to the harm avoidance measure of the Tridimensional Personality Questionnaire (14), and genotype was also associated with this measure (p=0.023). An association with anxiety was also demonstrated, as measured by the Cattell instrument (p=0.023). The authors concluded that this polymorphism accounts for 7%–9% of the genetic variance for these personality measures and 3%–4% of the total variance. Results for sibling pairs were consistent and were used to exclude population stratification as a mechanism for a false positive finding. This study population was composed of several subgroups, including a collection of homosexual male siblings.
Since the Lesch et al. report (12) of an association with anxiety, neuroticism, and harm avoidance, Cook et al. (15), using the transmission disequilibrium test, have demonstrated linkage disequilibrium between the “s” allele and autism, and McDougle et al. (16) have used the transmission disequilibrium test to demonstrate linkage disequilibrium between the “l” allele and obsessive-compulsive disorder.
A polymorphism in intron 2 of SLC6A4, also consisting of a variable number of repeats (usually 9, 10, or 12) of a 17 base-pair segment (8), has not been shown to have a direct effect on gene expression. We previously addressed the issues of population variation for each system and linkage disequilibrium between these two SLC6A4 systems (17). Allele frequencies for both systems showed variation among European American, African American, and Japanese subjects, with significant differences overall for each system and significant differences between each pair of populations for both systems. Linkage disequilibrium also varied among the populations, with nearly significant linkage disequilibrium in a control European American population and significant linkage disequilibrium in an alcohol-dependent European American population. We also reported the existence of two promoter system alleles even longer than the “l” allele; both(inferred to contain 18 or 20 repeats and designated “vl” and “xl”) were observed in Japanese subjects, and the longest of these (20 repeats) was observed in African American subjects also (17). Functional characteristics of these alleles have not been investigated.
Functional effects of the promoter polymorphism have been demonstrated both by in vitro measures (10, 12) and through association or linkage with phenotype (12, 15, 16). However, the potential for population stratification has also been demonstrated (17), and therefore association studies with this marker are expected to be potentially subject to population stratification error. Ability to detect an association could also depend on the other phenotypic characteristics of the populations studied. The present study was an attempt to replicate some of the findings of Lesch et al. (12) and to extend them by diagnostic category (by studying subjects with a known diagnosis of substance dependence or personality disorder, as well as normal comparison subjects) and by population group (by studying European American and African American subjects separately). In addition, we studied the intron 2 system, which was not studied by Lesch et al. (12) in this context. These are the only two reported SLC6A4 polymorphisms that are available in PCR format; one additional polymorphism, 3′ to these, which must be typed by Southern blot techniques (9), was used to place this gene in the genetic linkage map (7).
METHOD
Subjects
Subjects were assessed at one of three sites, as described below. All subjects gave written informed consent, after the procedure had been fully explained, before their study participation. Overall, there were 322 subjects, of whom 221 were European American and 101 African American. Two hundred one subjects were assessed at the University of Connecticut (139 of whom were substance dependent, five of whom had personality disorders, and 57 of whom were comparison subjects), 77 subjects (52 with personality disorders and 25 comparison subjects) were assessed at the Medical College of Pennsylvania, and 44 subjects (40 with personality disorders, two with substance dependence, and two comparison subjects) were assessed at Mt. Sinai School of Medicine, Bronx Veterans Affairs Medical Center. Allele frequencies for both SLC6A4 systems have been reported previously (17) for a group of subjects overlapping those assessed at the University of Connecticut described here.
Of the 322 subjects who participated in the study, 190 (59.0%) were male. A significantly greater proportion of the personality disorder patients (72.2%) were male, compared with the normal subjects (53.6%) and the substance-dependent patients (53.2%) (χ2=9.94, df=2, p=0.007). A significantly greater proportion of the comparison subjects (45.2%) were African American, compared with the substance-dependent patients (24.8%) and the personality disorder patients (28.9%) (χ2=10.59, df=2, p=0.005). The mean age of all subjects was 35.5 years (SD=9.9); the comparison subjects were significantly younger (mean age=32.9 years, SD=9.0) than both the substance-dependent patients (mean=36.8 years, SD=9.6) and the personality disorder patients (mean=36.0 years, SD=10.8) (F=4.35, df=2, 319, p=0.01).
University of Connecticut Health Center, Farmington
At this site, all comparison subjects were assessed with the Structured Clinical Interview for DSM-III-R (SCID) (18), and the majority (N=52, or 91%) were also interviewed with the Structured Clinical Interview for DSM-III-R Personality Disorders (19). Comparison subjects were screened to exclude those with alcohol or drug dependence and those with mood, anxiety, or (for most subjects) personality disorders. (Comparison subjects were recruited from the community in the Greater Hartford, Conn., area through use of a reverse telephone directory, by focusing on neighborhoods from which the substance-dependent patients were drawn.) Substance-dependent subjects were diagnosed according to the SCID with alcohol dependence, drug dependence (most had cocaine dependence; some had opiate dependence), or both. Harm avoidance was assessed with the Temperament and Character Inventory (20), in which the Tridimensional Personality Questionnaire (14) is embedded. The Temperament and Character Inventory is a 226-item, true-false questionnaire that measures seven dimensions of personality, one of which is harm avoidance. Neuroticism was assessed with the NEO Five-Factor Inventory (13), which is a 60-item, self-report instrument.
Medical College of Pennsylvania—Hahnemann School of Medicine, Philadelphia
At this site, subjects were systematically evaluated as part of a larger program designed to study the biological correlates of various personality dimensions in subjects with personality disorders and in healthy normal volunteers. Subjects were recruited from the outpatient psychiatry clinic and by newspaper and public service announcements. The diagnostic procedures are summarized elsewhere (21). Briefly, diagnoses were made according to DSM-III-R and assigned by a best-estimate process based on information from interviews in which the Schedule for Affective Disorders and Schizophrenia (SADS) (22) and the Structured Interview for the DSM-III-R Personality Disorders (23) were used, from clinical interviews by a research psychiatrist, and from all other available clinical data. The Structured Interview for the DSM-III-R Personality Disorders is an instrument that assesses lifetime personality traits independent of affective symptoms. Harm avoidance was assessed with the Tridimensional Personality Questionnaire (14).
Mt. Sinai School of Medicine, New York
Subjects were recruited from the outpatient clinics at the Mt. Sinai and Bronx VA Medical Centers as well as from community advertisements. Potential subjects were evaluated for axis I pathology according to the Research Diagnostic Criteria by two experienced raters using the SADS (22). Persons meeting the criteria for schizophrenia were excluded from the study. All subjects with personality disorders were studied as outpatients and were interviewed by one rater with the Structured Interview for the DSM-III-R Personality Disorders (23). A second rater administered the same interview independently to an individual close to each subject. Consensus diagnoses were reached in a meeting of all raters with an expert diagnostician. Harm avoidance was assessed with the Tridimensional Personality Questionnaire (14).
Genotyping
DNA was extracted from whole blood by standard techniques. Genotyping was accomplished at the VA Connecticut Healthcare System, West Haven Campus. PCR primers for the intron 2 polymorphism were described by Lesch et al. (8); conditions were modified from theirs as described previously (17). Alleles at this system are designated for the number of repeats, STin2.9, STin2.10, and STin2.12. PCR primers and conditions for the promoter system have been described previously (17). Alleles at this system are designated according to their relative size, “l” (16 repeats), “s” (14 repeats), and “xl” (20 repeats).
Statistical Analysis
Hierarchical multiple regression was used to examine the correlations of demographic features, psychiatric diagnostic group, and genotype with harm avoidance and neuroticism scores. In the analysis involving harm avoidance scores, the following independent variables were entered, in this order: sex, race, age, diagnostic group, promoter system genotype, intron 2 system genotype, and all two-way and three-way interactions involving sex, race, diagnostic group, and the two genotypes. Demographic variables were centered or effect coded (–1, 1) and two orthogonal dummy variables were created from the three-level diagnosis variable (comparison subjects, substance-dependent patients, personality disorder patients). Two orthogonal contrasts (comparison subjects versus patients and substance-dependent patients versus personality disorder patients) were used to examine the differences in harm avoidance and neuroticism scores among the diagnostic groups. The promoter genotype was dichotomized on the basis of the presence or absence of the “s” (14-repeat) allele (consistent with the report by Lesch et al. [12] of a dominant-like effect of this allele). Subjects with the rare “xl” (20-repeat) allele for this locus were excluded from the regression analyses. The intron 2 genotype was trichotomized (homozygous for the STin2.10 or STin2.12 alleles and heterozygous). Subjects with the rare STin2.9 allele for this locus were excluded from the regression analyses. Because neuroticism scores were not available for the majority of patients with a personality disorder diagnosis, regression analysis for this dependent measure was conducted with use of only two levels of the diagnosis variable (comparison subjects versus substance-dependent patients).
The use of hierarchical regression made it possible to control for the effects of each of the demographic and diagnosis predictor variables before examining the effect of the two genotypes on harm avoidance and neuroticism scores. This approach also made it possible to examine the contribution to both harm avoidance and neuroticism scores of all two-way and three-way interactions involving either of the polymorphic systems. However, given the small number of subjects with rare alleles in the two polymorphic systems, their effects on harm avoidance and neuroticism scores were examined by using univariate analysis of variance.
RESULTS
Allele Frequencies
Allele observations and frequencies for both systems in each racial group are shown in table 1. Within each group (European Americans or African Americans) there was no heterogeneity by allele system for either polymorphism by diagnosis (2×3 chi-square statistic): for European Americans, intronic system (χ2=1.88, df=2, p=0.39); for African Americans, intronic system (χ2=0.31, df=2, p=0.86); for European Americans, promoter system (χ2=2.50, df=2, p=0.29); and for African Americans, promoter system (χ2=1.97, df=2, p=0.37). STin2.9 alleles were collapsed together with STin2.10 alleles and “xl” alleles were collapsed together with “l” alleles wherever they occurred. There were significant differences by racial group (2×2 chi-square statistic): European Americans versus African Americans, promoter system: χ2=19.10, df=1, p<0.00002; intronic system: χ2=4.17, df=1, p<0.05. (As noted above, the comparison and substance dependence groups overlap with those for which we previously described similar racial differences [17].)
Harm Avoidance
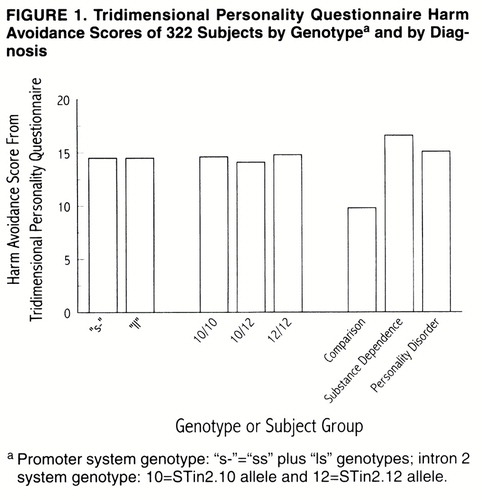
These analyses used the full set of subjects (N=322). Of the demographic variables, sex was a and by Diagnosis. Patients had higher harm avoidance scores (mean=16.0, SD=8.1) than comparison subjects (mean=9.8, SD=5.1) (F=43.06, df=1, 306, R2=0.114, p<0.001). The two patient groups, however, did not differ significantly on this measure: for substance-dependent patients, the mean score was 16.6 ( SD=8.1); for personality disorder patients, the mean score was 15.1 (SD=7.9).
Neither of the polymorphisms had a significant main effect on harm avoidance (figure 1). For the promoter system, subjects with one or two copies of the “s” allele had a mean harm avoidance score of 14.5 (SD=7.7), compared with a mean harm avoidance score of 14.5 (SD=8.2) among subjects who were homozygous for the “l” allele (F=0.03, df=1, 305, p=0.87). For the intron 2 system, harm avoidance scores did not differ between subjects who were homozygous for the STin2.10 allele (mean=14.6, SD=7.4), those who were homozygous for the STin2.12 allele (mean=14.8, SD=8.2), and those who were heterozygous (mean=14.1, SD=7.8) (F=0.63, df=1, 304, p=0.43). The only significant interaction was between sex and the promoter system (F=4.22, df=1, 303, p=0.04). Among male subjects, those with one or two copies of the “s” allele had a mean harm avoidance score of 13.6 (SD=7.5), compared with 11.8 (SD=7.4) for subjects homozygous for the “l” allele. Among female subjects, those with one or two copies of the “s” allele had a mean harm avoidance score of 15.9 (SD=7.9), compared with 17.8 (SD=7.8) for subjects homozygous for the “l” allele.
For subjects with the “xl” allele in the promoter system (N=7), the mean harm avoidance score was 11.7 (SD=4.5), compared with a mean of 14.5 (SD=7.9) for subjects without this allele (N=315) (F=0.84, df=1, 320, p=0.36). For subjects with the STin2.9 allele in the intron 2 system (N=5), the mean harm avoidance score was 11.4 (SD=5.1), compared with a mean of 14.5 (SD=7.9) for subjects without the STin2.9 allele (N=317) (F=0.75, df=1, 320, p=0.39).
Neuroticism
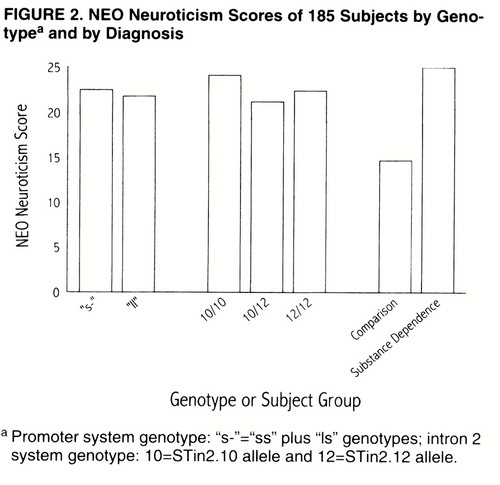
These analyses used comparison and substance-dependent subjects assessed at the University of Connecticut (N=185). None of the demographic variables was significantly correlated with the measure of neuroticism. However, psychiatric diagnosis accounted for a significant proportion of the variance in neuroticism scores (figure 2); substance-dependent patients had higher neuroticism scores (mean=25.0, SD=9.5) than comparison subjects (mean=14.7, SD=6.0) (F=50.22, df=1, 182, R2=0.216, p<0.001).
Neither of the polymorphisms exerted a main effect on neuroticism score (figure 2). For the intron 2 system, neuroticism scores did not differ between subjects who were homozygous for the STin2.10 allele (mean=24.1, SD=10.5), those who were homozygous for the STin2.12 allele (mean=22.4, SD=10.5), and those who were heterozygous (mean=21.2, SD=8.3) (F=0.006, df=1, 181, p=0.94). For the promoter system, subjects with one or two copies of the “s” allele had a mean neuroticism score of 22.5 (SD=10.2), compared with a mean neuroticism score of 21.8 (SD=9.3) among subjects who were homozygous for the “l” allele (F=0.53, df=1, 180, p=0.47). The only significant interaction was between race and the promoter system genotype (F=3.91, df=1, 179, p<0.05). Among European Americans, individuals with one or two copies of the “s” allele had a mean neuroticism score of 23.4 (SD=10.3), while those who were homozygous for the “l” allele had a mean neuroticism score of 21.2 (SD=9.6). In contrast, among African Americans, the individuals with one or two copies of the “s” allele had a mean neuroticism score of 18.9 (SD=8.6), compared with individuals homozygous for the “l” allele, whose mean neuroticism score was 22.7 (SD=9.1).
For subjects with the “xl” allele at the promoter system (N=5, all of whom were African American), the mean neuroticism score was 13.8 (SD=6.5), compared with a mean of 22.2 (SD=9.8) for subjects without this allele (N=189) (F=3.62, df=1, 192, p<0.06). For subjects with the 9-repeat allele in the intron system (N=4), the mean harm avoidance score was 22.1 (SD=9.8), compared with a mean of 17.3 (SD=10.3) for subjects without the 9-repeat allele (N=190) (F=0.95, df=1, 192, p=0.33).
DISCUSSION
We were unable to confirm a relationship between genotype at the serotonin transporter protein promoter polymorphic system and measures of neuroticism (measured by the NEO) or harm avoidance (measured by the Tridimensional Personality Questionnaire). The groups with psychiatric diagnoses (personality disorders or substance dependence) had significantly higher harm avoidance scores than the normal comparison group; there were also significant effects of sex and race. Our European American group was probably generally comparable ethnically to the one described by Lesch et al. (12), that is, a mixed American population. Although significant variation has been demonstrated previously for allele frequency for both of the polymorphic systems studied, and haplotypes, between different populations (17), the use of a case-control design is supported by the prior demonstrations that the promoter system polymorphism is functional.
We observed an interaction between sex and promoter system genotype, the nature of which suggests that our findings are not wholly inconsistent with those of Lesch et al. (12), since their study group was predominantly male. That is, among the male subjects in our study group, Tridimensional Personality Questionnaire harm avoidance was higher in those with one or two “s” alleles, compared to “ll” subjects; the difference among female subjects was in the opposite direction. There was an interaction of race by promoter genotype on the primary dependent measure used by Lesch et al. (i.e., NEO neuroticism). The finding of higher neuroticism scores in European Americans with one or two “s” alleles is also consistent with the report by Lesch et al. (12), whose study group was predominantly European American. However, it would be difficult to account for mechanisms of physiological effects for this polymorphism in opposite directions by sex or race or detectable only in males or European Americans which would be consistent with the regulatory differences for the different alleles that have been described (10, 12).
The trend for the subjects with an “xl” promoter system allele to have lower neuroticism scores than those without that allele did not reach significance and was based on a very small group; it would be of interest to assess this possible relationship in a larger group, which would obviously have to be composed of African Americans, Japanese, or some other population in which this allele is present (we have never observed it in European Americans). The observation that subjects with this allele might have lower levels of neuroticism than other subjects is consistent with speculating that this allele may have even higher activity than the “l” allele.
In a smaller subgroup overlapping the present study group, composed of patients evaluated for personality disorders who were recruited clinically at one of the sites (Mt. Sinai; N=66), there was a significant relationship between patients having the “ss” genotype and increased harm avoidance scores (24), so it is possible that heterogeneity in the patient groups recruited from different sites might have contributed to our negative finding. Investigation of a larger group recruited at that site would be required to test this possibility.
We found higher frequency of the SLC6A4 promoter system “l” allele in African American subjects with substance abuse and those with personality disorder than in European American subjects of similar phenotype, as we had previously demonstrated for control groups (17). If, as was suggested by Lesch et al. (12), the “s” allele is functionally dominant to the “l” allele, the major phenotypic groups 1) “ll” and 2) “ls” plus “ss” would differ greatly by population. If this polymorphism really contributes directly to the population variance for the traits of anxiety, neuroticism, and harm avoidance, as suggested by Lesch et al. (12), the population difference carries the implication that higher rates of anxiety and neuroticism should be seen in the European American population than the African American population, assuming that other genetic and environmental factors influencing this phenotype are roughly equivalent between the populations. (Whether such influence would even be directly measurable, in the context of many other factors that could differentially influence anxiety in different populations and confound comparisons, is open to question.) The existence of these population differences raises interesting questions about the evolutionary significance of variation in genetically determined anxiety levels, as we noted previously (17).
Overall, we were not able to replicate the findings of Lesch et al. (12) of a relationship between serotonin transporter protein promoter system alleles and neuroticism and harm avoidance. We also did not find any consistent relationship between these phenotypic measures and genotype at another SLC6A4 polymorphic system, the intron 2 system. These data could reflect either the absence of a true relationship or an effect size too small to detect even in a group of 322 subjects (as for our harm avoidance analyses). The specific phenotypic composition of the groups studied with respect to other behaviors could also influence whether an effect of this polymorphism on personality measures is detectable. Other positive findings from family-controlled studies with this system (references 15 and 16 and the sibling pair results from the Lesch et al. study [12]) strongly support the existence of somephenotypic effect of polymorphic variation at this system, while two other recent reports did not support an association with Tridimensional Personality Questionnaire harm avoidance (25) or with peer-rated neuroticism (26). It is unclear at this point what phenotypic construct may best capture the effect of this variation; there may well be an effect on anxiety and neuroticism that is measurable in some samples, together with a larger effect in some other behavioral domain. Our results also suggest that it might be helpful to make behavioral assessments of larger groups of subjects with “xl” alleles and to assess the in vitro function of this allele.
Received Aug. 14, 1997; revisions received Feb. 3 and March 20, 1998; accepted April 23, 1998. From the Department of Psychiatry, VA Connecticut Healthcare System, West Haven Campus; the Division of Molecular Psychiatry, Department of Psychiatry, Yale University School of Medicine, New Haven, Conn.; the Department of Psychiatry, University of Connecticut School of Medicine, Farmington; the Medical College of Pennsylvania at the Eastern Pennsylvania Psychiatric Institute, Philadelphia; and the Mount Sinai School of Medicine and Bronx VA Medical Center, New York.. Address reprint requests to Dr. Gelernter, Psychiatry 116A2, VA Connecticut Healthcare System, West Haven Campus, 950 Campbell Ave., West Haven, CT 06516; [email protected] (e-mail). Supported in part by grants MH-00931, MH-49351, MH-30929, and MH-01387 from NIMH; grants AA-11330, AA-00143, and AA-03510 from the National Institute on Alcohol Abuse and Alcoholism; grant RR-06192 to the General Clinical Research Center of the University of Connecticut Health Center and grant RR-00071 to the Mt. Sinai School of Medicine General Clinical Research Center from NIH; grants DA-05592 and DA-10242 from the National Institute on Drug Abuse; the VA Medical Research Program (Merit Review grant to Dr. Gelernter); the VA National Center for Schizophrenia Research; and the VA National Center for Alcoholism Research.The authors thank Ann Marie Wantroba, Louis Song, Harold Landis, and Pamela Fall for technical assistance and Joseph Burleson, Ph.D., for statistical consultation.
 |
1. Nielsen DA, Goldman D, Virkkunen M, Tokola R, Rawlings R, Linnoila M: Suicidality and 5-hydroxyindolacetic acid concentration associated with a tryptophan hydroxylase polymorphism. Arch Gen Psychiatry 1994; 51:34–38Crossref, Medline, Google Scholar
2. Mann JJ, Malone KM, Nielsen DA, Goldman D, Erdos J, Gelernter J: Possible association of a polymorphism of the tryptophan hydroxylase gene with suicidal behavior in depressed patients. Am J Psychiatry 1997; 154:1451–1453Link, Google Scholar
3. New AS, Gelernter J, Yovell Y, Trestman RL, Nielsen DA, Silverman J, Mitropoulou V, Siever LJ: Tryptophan hydroxylase genotype is associated with impulsive aggression measures: a preliminary study. Am J Med Genet (Neuropsychiatr Genet) 1998; 81:13–17Crossref, Medline, Google Scholar
4. Inayama Y, Yoneda H, Sakai T, Ishida T, Nonomura Y, Kono Y, Takahata R, Koh J, Sakai J, Takai A, Inada Y, Asaba H: Positive association between a DNA sequence variant in the serotonin 2A receptor gene and schizophrenia. Am J Med Genet Neuropsychiatr Genet 1996; 67:103–105Crossref, Medline, Google Scholar
5. Williams J, Spurlock G, McGuffin P, Mallet J, Nöthen MM, Gill M, Aschauer H, Nylander P-O, Macciardi F, Owen MJ, EMASS Group: Association between schizophrenia and the T102C polymorphism of the 5-hydroxytryptamine type 2a-receptor gene. Lancet 1996; 347:1294–1296Crossref, Medline, Google Scholar
6. Ramamoorthy S, Bauman AL, Moore KR, Han H, Yang-Feng T, Chang AS, Ganapathy V, Blakely RD: Antidepressant- and cocaine-sensitive human serotonin transporter: molecular cloning, expression and chromosomal localization. Proc Natl Acad Sci USA 1993; 90:2542–2546Crossref, Medline, Google Scholar
7. Gelernter J, Pakstis AJ, Kidd KK: Linkage mapping of serotonin transporter protein gene on chromosome 17. Hum Genet 1995; 95:677–680Crossref, Medline, Google Scholar
8. Lesch K-P, Balling U, Gross J, Strauss K, Wolozin BL, Murphy DL, Riederer P: Organization of the human serotonin transporter gene. J Neural Transm 1994; 95:157–162Crossref, Medline, Google Scholar
9. Gelernter J, Freimer M: PstI STS RFLP at serotonin transporter protein (SERT) locus. Hum Mol Genet 1994; 3:383Crossref, Medline, Google Scholar
10. Heils A, Teufel A, Petri S, Stöber G, Riederer P, Bengel D, Lesch KP: Allelic variation of human serotonin transporter gene expression. J Neurochem 1996; 66:2621–2624Crossref, Medline, Google Scholar
11. Coccaro EF, Murphy DL (eds): Serotonin in Major Psychiatric Disorders. Washington, DC, American Psychiatric Press, 1990Google Scholar
12. Lesch K-P, Bengel D, Heils A, Sabol SZ, Greenberg B, Petri S, Benjamin J, Müller CR, Hamer DH, Murphy DL: Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 1996; 274:1527–1531Crossref, Medline, Google Scholar
13. Costa PT, McCrae RR: Revised NEO Personality Inventory and NEO Five-Factor Inventory. Odessa, Fla, Psychological Assessment Resources, 1992Google Scholar
14. Cloninger CR, Przybeck TR, Svrakic DM: The Tridimensional Personality Questionnaire: US normative data. Psychol Rep 1991; 69:1047–1057Crossref, Medline, Google Scholar
15. Cook EH Jr, Courchesne R, Lord C, Cox NJ, Yan S, Lincoln A, Haas R, Courchesne E, Leventhal BL: Evidence of linkage between the serotonin transporter and autistic disorder. Molecular Psychiatry 1997; 2:247–250Crossref, Medline, Google Scholar
16. McDougle CJ, Epperson CN, Price LH, Gelernter J: Evidence for linkage disequilibrium between serotonin transporter protein and obsessive compulsive disorder. Molecular Psychiatry 1998; 3:270–274Crossref, Medline, Google Scholar
17. Gelernter J, Kranzler H, Cubells JF: Serotonin transporter protein (SLC6A4) allele and haplotype frequencies and linkage disequilibria in African and European American and Japanese populations and in alcohol-dependent subjects. Hum Genet 1997; 101:243–246Crossref, Medline, Google Scholar
18. Spitzer RL, Williams JBW, Gibbon M, First MB: The Structured Clinical Interview for DSM-III-R (SCID), I: history, rationale, and description. Arch Gen Psychiatry 1992; 49:624–629Crossref, Medline, Google Scholar
19. First MB, Spitzer RL, Gibbon M, Williams JBW: The Structured Clinical Interview for DSM-III-R Personality Disorders (SCID-II), part I: description. J Personality Disorders 1995; 9:83–91Crossref, Google Scholar
20. Cloninger CR, Svrakic DM, Przybeck TR: A psychobiological model of temperament and character. Arch Gen Psychiatry 1993; 50:975–990Crossref, Medline, Google Scholar
21. Coccaro EF, Kavoussi RJ, Sheline YI, Lish JD, Csernansky JG: Impulsive aggression in personality disorder: correlates with 3H-paroxetine binding in the platelet. Arch Gen Psychiatry 1996; 53:531–536Crossref, Medline, Google Scholar
22. Spitzer RL, Endicott J: Schedule for Affective Disorders and Schizophrenia (SADS). New York, New York, New York State Psychiatric Institute, Biometrics Research, 1975Google Scholar
23. Pfohl B, Blum N, Zimmerman M, Stangl D: Structured Interview for the DSM-III-R Personality Disorders, Revised (SIDP-R). Iowa City, University of Iowa College of Medicine, Department of Psychiatry, 1989Google Scholar
24. New AS, Gelernter J, Mitropoulou V, Siever LJ: Serotonin-related genes and impulsive aggression, in 1997 Annual Meeting New Research Program and Abstracts. Washington, DC, American Psychiatric Association, 1997, p 164Google Scholar
25. Ebstein RP, Gritsenko I, Nemanov L, Frisch A, Osher Y, Belmaker RH: No association between the serotonin transporter gene regulatory region polymorphism and the Tridimensional Personality Questionnaire (TPQ) temperament of harm avoidance. Molecular Psychiatry 1997; 2:224–226Crossref, Medline, Google Scholar
26. Ball D, Hill L, Freeman B, Eley TC, Strelau J, Riemann R, Spinath FM, Angleitner A, Plomin R: The serotonin transporter gene and peer-rated neuroticism. NeuroReport 1997; 8:1301–1304Crossref, Medline, Google Scholar



