Spontaneous Dyskinesia in Subjects With Schizophrenia Spectrum Personality
Abstract
OBJECTIVE: The study of spontaneous dyskinesia in schizophrenia is confounded by the widespread use of neuroleptics. The authors hypothesized that spontaneous dyskinesia would be present in subjects with schizophrenia spectrum personality (schizoid, paranoid, or schizotypal). They also tested the hypothesis that dyskinetic-like movements would increase after repeated dextroamphetamine challenge to the dopaminergic system. METHOD: Dyskinetic-like movements were assessed in 34 spectrum subjects and 22 normal subjects; nine subjects from each group were administered both placebo and repeated dextroamphetamine challenges. RESULTS: Spectrum subjects had more dyskinetic-like movements than normal subjects. Spontaneous dyskinesia was present in 12% of the spectrum subjects but was not seen in the normal subjects. Subjects with schizotypal personality had more dyskinetic-like movements than subjects with schizoid personality or normal subjects. Dyskinesia was present in 24% of the schizotypal subjects but not in the other groups. Dyskinetic movement scores correlated with positive symptom scores. With repeated amphetamine challenge, normal subjects showed a pattern of behavioral sensitization (an increase in dyskinetic-like movements), but spectrum subjects showed an abnormal response (fewer dyskinetic-like movements). CONCLUSIONS: Dyskinesia and dyskinetic-like movements are more common in subjects with schizophrenia spectrum personality (primarily schizotypal) than in normal subjects and are related to positive symptoms. A failure of normal behavioral sensitization mechanisms after dextroamphetamine challenge is seen in subjects with schizophrenia spectrum personality. (Am J Psychiatry 1998; 155:70–75)
Spontaneous dyskinesia has been recognized since the earliest descriptions of schizophrenia (1–3). The prevalence of spontaneous choreiform movements led some early schools of psychopathology to subclassify these patients as choreiform (parakinetic) and to propose that this subtype had a distinct neuropathology that affected both motor and mental systems (4, 5). It is known that dyskinesia and other extrapyramidal movement disorders have primary neuropathology in the basal ganglia (6). In the modern era, widespread use of drugs that block dopamine receptors and the effects of these drugs in the basal ganglia confound the study of spontaneous dyskinesia.
In a recent retrospective study, 15% of preneuroleptic era schizophrenic patients showed oral-facial dyskinesia, and 23% had some dyskinetic-like movements (7). In another study, never-medicated schizophrenic patients had significantly greater motor force instability (strongly associated with dyskinesia), and 14% had dyskinesia (8). A recent study of never-medicated schizophrenic patients in Morocco reported that 14% exhibited spontaneous dyskinesia, and 36% experienced some dyskinetic-like movements (9). However, earlier reports from Morocco found only two of 70 never-medicated schizophrenic patients who displayed spontaneous dyskinesia (10). No cases of spontaneous dyskinesia were found among 12 never-medicated Nigerian patients with schizophrenia (11). Among 89 never-medicated, first-episode schizophrenic patients, only one experienced dyskinesia, while 15 displayed parkinsonism (12).
Never-medicated schizophrenic patients are difficult to find for observation. However, subjects with milder manifestations of schizophrenic-like symptoms may be studied. Some children at risk for schizophrenia have been observed to have dyskinetic-like (choreiform) movements (13, 14). We hypothesized that subjects with schizophrenia spectrum personality (i.e., paranoid, schizoid, or schizotypal) would have more dyskinetic-like movements than normal subjects. These spectrum disorders have phenomenologic similarity to schizophrenia and may have biologic and genetic relatedness (15–17). Quantifiable schizophrenic-like symptoms are reliably present without confounds of neuroleptic exposure, advanced age, institutionalization, or medical comorbidity. From human and animal models of behavioral sensitization, which provide models for dyskinesia and psychosis (18–21), we hypothesized that dyskinetic-like movements would increase after repeated amphetamine challenge to the dopaminergic system.
METHOD
Subjects
Subjects were recruited from the community through separate newspaper advertisements that requested participation of normal volunteers and participation of individuals who had psychic or ESP experiences or who were loners (22). Family members of patients consecutively admitted to our clinic were also invited to participate. All subjects were screened with a 10-minute structured telephone interview to exclude those with major medical illness, psychiatric illness, or history of drug abuse. Subjects with a lifetime diagnosis of a DSM-III-R axis I disorder or who had been exposed to neuroleptic drugs were excluded from the study. Exceptions to this rule were subjects with only one lifetime episode of major depression that had not occurred in the previous 2 years and was not recurrent or treated with antidepressants, electroconvulsive therapy, or hospitalization. Subjects whose history of substance abuse (not dependence) occurred more than 2 years before recruitment could be included. After complete description of the study to the subjects, written informed consent was obtained.
Clinical Assessments
Subjects underwent the following clinical assessments: 1) the Structured Clinical Interview for DSM-III-R (SCID) (23) or the Schedule for Affective Disorders and Schizophrenia—Lifetime Version (SADS-L) (24); 2) Structured Interview for DSM-III Personality Disorders or Structured Interview for DSM-III-R Personality, Revised (25); and 3) for the community subjects, the Modified Family History Research Diagnostic Criteria interview (22, 26). Subjects who consented to the amphetamine challenge completed a medical history and underwent a physical examination, ECG, and blood work to rule out any condition or medication use that could compromise safety or confound the assessment of mental status or involuntary movements. All interviews were conducted by master's-level trained clinicians. All diagnostic data were reviewed at a best-estimate diagnostic meeting that was chaired by a psychiatrist (G.K.T.). Positive and negative symptom scores were derived by summation of the symptom scores from the Structured Interview for DSM-III Personality Disorders. All schizoid personality symptoms were counted as negative, and all paranoid personality symptoms were counted as positive. Schizotypal symptoms were divided into positive (ideas of reference, excessive social anxiety, odd beliefs, unusual perceptual experiences, odd or eccentric behavior, odd speech, and suspiciousness) or negative (no close friends and constricted affect). Schizotypal symptoms were not counted twice in the total if they had been previously counted among the paranoid or schizoid symptoms.
Subject Groups
We assembled a group of subjects with schizophrenia spectrum personality (spectrum group) and a group of normal comparison subjects (normal group). The normal group had no family history of schizophrenia or psychosis, no axis II diagnosis, and no more than two traits from the spectrum list (and a severity rating of less than 2 if present). For inclusion in the spectrum group, we used a threshold of one criterion point less than that required for diagnosis of DSM-III-R paranoid, schizoid, or schizotypal personality disorder. This permitted subjects with moderately severe schizophrenia-related psychopathology (three to four traits in one or more categories but not sufficient for a DSM diagnosis) to be included. The groups included 34 spectrum subjects (mean age=32.1 years, SD=8.9) and 22 normal subjects (mean age=32.1, SD=8.2). Among the spectrum subjects were 23 with schizotypal personality, 21 with schizoid personality, and eight with paranoid personality (note concurrence in 47% of the group). On the basis of relative excess of symptoms required to meet criteria, we divided the spectrum group into two groups: a primarily schizoid group (N=15, mean age=31.7, SD=9.7) and a primarily schizotypal group (N=17, mean age=31.6, SD=8.5). Two spectrum subjects, including one with paranoid personality, were not categorized because neither schizoid nor schizotypal symptoms predominated. Five of the subjects with paranoid personality were placed in the schizotypal group, and two were placed in the schizoid group. The group with more schizotypal symptoms had higher positive symptom scores than the group with more schizoid symptoms (mean=6.6 [SD=2.0] versus mean=2.3 [SD=1.5], respectively; t=–6.85, df=30, p<0.00001).
Assessment of Dyskinesia
A structured examination for involuntary movements was videotaped and later rated blind to group and treatment condition. Involuntary movements were rated with the Maryland Psychiatric Research Center Involuntary Movement Scale (27), which was developed for rating tardive dyskinesia. Greater sensitivity at the minimal rating level was allowed to capture subtle and brief choreiform movements, since these subjects were expected to exhibit few signs. However, involuntary movements above minimal were consistent with the anchors used in rating drug-induced signs. Stereotypies and mannerisms are not rated on this scale. Global dyskinetic (and parkinsonian) ratings have high face validity (28); overall severity is rated from 0 to 7. A diagnosis of dyskinesia (or parkinsonism) was made if the global rating equaled or exceeded 3. Any lesser rating was considered within normal limits. This conservative threshold was set so that potential overrepresentation of dyskinesia due to greater sensitivity would be minimized. Total dyskinetic movement score, another independent measure, was determined from the summation of individual items in the dyskinesia subscale and is highly sensitive to change in pharmacologic and neurophysiologic studies (27). Interrater reliabilities (intraclass correlation coefficients) for global dyskinetic ratings, total dyskinetic scores, and global parkinsonian ratings were 0.78, 0.81, and 0.90, respectively.
Amphetamine Challenge
Nine spectrum and nine normal subjects participated in the dextroamphetamine challenge. We did not challenge any subject for whom the clinical presentation suggested premorbid schizophrenia or risk of decompensation. The 18 subjects were challenged twice with 30 mg of oral dextroamphetamine and once with matching placebo in a double-blind, randomized administration. Each challenge occurred on one of three separate days with at least 7 days (mean=10.3 days, SD=5.6) between challenges. Adjusted for weight, the average dextroamphetamine dose was 0.41 mg/kg (SD=0.07); there was no significant difference between the spectrum and normal groups (0.39 and 0.43 mg/kg, respectively). Subjects arrived at the Maryland Psychiatric Research Center at 9:00 a.m., had a standard breakfast, and received the oral dose at 10:00 a.m. Vital signs were monitored hourly. Motor examination was videotaped 2.5–3.5 hours later. Plasma dextroamphetamine levels have been shown to peak 3 hours after oral dose (29), and significant effects have remained after 4 hours (30). The Brief Psychiatric Rating Scale (BPRS) was completed after motor examination (31). All subjects returned home at 5:00 p.m. without distress. Before the challenge, baseline assessments were performed to familiarize subjects with the testing procedures, personnel, and facility.
Data Analysis
Global dyskinetic and parkinsonian movement ratings, ordered categorical variables, were tested (corrected for ties) for group differences by using nonparametric Wilcoxon rank sum or Kruskal-Wallis (three groups) tests. However, total dyskinetic scores were normally distributed (Kolmogorov D=0.93), and parametric tests were used. Associations between total dyskinetic score and age, gender, and history of major depression were tested because these factors may be associated with greater risk of spontaneous dyskinesia or tardive dyskinesia. Associations between dyskinetic-like movements and positive or negative symptom scores were explored. The repeated amphetamine challenge data were analyzed by using repeated measures analysis of variance (ANOVA, multivariate analogue), with two groups and three conditions. Post hoc ANOVA, two-tailed t (alpha=0.05), or Wilcoxon tests were performed when appropriate. Positive change in total dyskinetic score represents an increase in dyskinetic-like movement above placebo. BPRS anxiety and activation factor scores were used as control measures.
RESULTS
The involuntary movements observed in this study were generally infrequent and within the range of normal. The most frequently endorsed signs included side to side and undulating tongue movement, lip puckering, and muscle twitches (chorea-like) in fingers and feet/toes. Other signs included tic-like facial contraction, lateral jaw deviation, and muscle twitches in arm or shoulder. Clinically significant involuntary movements were noted in a few cases because of frequency, amplitude, or multiplicity of signs and were similar to mild cases of drug-induced tardive dyskinesia. Statistical analysis showed a pattern of greater global dyskinetic ratings in the spectrum subjects than in the normal subjects (Wilcoxon z=2.23, p=0.03) (table 1). We compared schizoid subjects (N=15), schizotypal subjects (N=17), and normal subjects (N=22). Significant differences occurred among groups (Kruskal-Wallis χ2=9.57, df=2, p=0.008). Schizotypal subjects had greater dyskinetic ratings than schizoid subjects (Wilcoxon z=2.12, p=0.03) and normal subjects (Wilcoxon z=3.02, p=0.003). Dyskinesia (global rating of 3 or higher) was diagnosed in 12% of all spectrum subjects; all four were in the schizotypal group. Thus, 24% of schizotypal subjects had dyskinesia. No schizoid or normal subject had dyskinesia. Dyskinetic scores were not associated with age, gender, or history of major depression. There were no qualitative differences in movement form and no significant chi-square (one-way) differences among groups in specific dyskinetic items. However, spectrum subjects were three to seven times more likely to show finger and foot/toe chorea-like movements. No significant group differences were seen for global parkinsonian movement scores, although four spectrum subjects (12%) met criteria for parkinsonism (global rating of 3 or higher), including one subject with dyskinesia.
For all subjects, positive symptom score was associated with global dyskinetic rating (rs=0.34, df=55, p=0.01) and was also correlated with total dyskinetic score (r=0.45, df=55, p=0.0001). The association between positive symptom score and global dyskinetic rating remained significant for spectrum subjects alone (rs=0.35, df=33, p=0.05). The positive symptom scores of the spectrum subjects also significantly correlated with total dyskinetic score (figure 1). The association between positive symptom score and total dyskinetic score remained significant after age, gender, and history of depression were entered as covariates. Negative symptom scores of the spectrum subjects were not significantly correlated with total dyskinetic score (r=–0.29, df=33, p=0.09) but were inversely correlated with positive symptom score (r=–0.59, df=33, p=0.0002). Subjects with schizophrenia spectrum personality who had a family history of schizophrenia or psychotic illness (N=11) did not differ from spectrum subjects without a family history (N=23) on global dyskinetic ratings (Wilcoxon z=0.15, p=0.88). Positive or negative symptom scores did not differ by family history of schizophrenia or psychotic illness.
After repeated challenges with amphetamine, a significant interaction was found between group and condition for total dyskinetic score (repeated measures ANOVA F=3.85, df=2,15, p=0.05) (figure 2). Post hoc comparison showed a significant group difference between placebo and second amphetamine condition (ANOVA F=7.20, df=1,16, p=0.02). Normal subjects had a positive change in dyskinetic score and spectrum subjects had a negative change in dyskinetic score that were significantly different (t=2.68, df=16, p=0.02). Changes in dyskinetic scores were not associated with age, gender, weight, administration order, history of stimulant use, or BPRS measures of anxiety and activation. Simple t tests between various mean scores were not significant. No qualitative differences in movement were noted among groups. A significant inverse correlation occurred between changes in dyskinetic scores and negative symptom scores (r=–0.53, df=55, p=0.02); a similar association was also noted within the nine spectrum subjects (r=–0.41, p=0.27). The more negative symptoms present, the more likely a subject had reduced dyskinetic-like movements (reversed behavioral sensitivity) rather than the normal augmentation after repeated challenges with amphetamine.
DISCUSSION
We found that subjects with schizophrenia spectrum personality experience dyskinetic-like movements and spontaneous dyskinesia more often than normal subjects. These results are in broad agreement with studies that have shown that approximately 14%–15% of never-medicated schizophrenic patients experience spontaneous dyskinesia and that a greater percentage exhibit some dyskinetic-like movement (7–9). As with schizophrenia-like symptoms, the spectrum personality group has dyskinetic-like movements that border the normal range. The use of a sensitive method and the Maryland Psychiatric Research Center Involuntary Movement Scale (27) allowed assessment of spontaneous dyskinetic-like movements at multiple levels within the normal range. This sensitivity improved our ability of finding the small effect, since a simple 2×2 chi-square with a diagnostic cutoff global score of 3 or greater yields Fischer's exact p=0.126. Confirmation awaits larger numbers of subjects and the use of instrumental measures of dyskinetic-like movements.
The severity of dyskinetic-like movements appears to be related to positive symptom score, and the effect is seen primarily in subjects with schizotypal symptoms. The association remained after the two high scorers were excluded from analysis. Spontaneous dyskinetic-like motor disturbances have recently been shown to correlate with positive symptoms in never-medicated schizophrenic patients (8), and positive symptoms have been associated with tardive dyskinesia (11, 32, 33). These results fit the hypothesis that hyperdopaminergia underlies both positive symptoms and dyskinetic movements. Because of covariance, it is not clear if negative symptoms mitigate against dyskinetic-like movements. Potentially conflicting with our results are reports of positive associations between negative or deficit symptoms and spontaneous dyskinesia (7) or tardive dyskinesia (34–36). The relative prominence of dyskinetic-like movements in the extremities of spectrum personality subjects is in agreement with findings from younger, never-medicated schizophrenic patients with spontaneous dyskinesia (37). Follow-up and cross-sectional studies may clarify any higher risk for deteriorating course, poor prognosis, or deficit-like syndrome in spontaneously dyskinetic schizotypal subjects.
Previous failures to find spontaneous dyskinesia among never-medicated patients with schizophrenia may have been due to a sampling bias. Schizophrenic patients in Morocco and Nigeria who had never been medicated may have had less severe positive symptoms, since treatment resources are limited (37). Natural selection would bias an untreated group toward fewer positive symptoms and perhaps less dyskinesia. Akinesia and tremor, frequently observed in first-episode patients (12, 38), may themselves mask or restrict expression of spontaneous dyskinesia and may predict risk of later development of spontaneous dyskinesia, similar to findings in tardive dyskinesia (39).
No relationship between spontaneous dyskinetic-like movements and family history of psychosis was found. Family history of psychosis increased vulnerability to drug-induced tardive dyskinesia in some studies (40–42) but not all (43). Our recruitment of nonfamilial cases with negative symptoms appears to have eliminated the commonly seen association of negative symptoms to family membership (22, 44). Future studies on the association of spontaneous dyskinesia with family history of psychosis should control for positive and negative symptoms.
After a sensitizing dose of amphetamine, the dopaminergic response to amphetamine in the spectrum group did not follow the normal pattern of behavioral sensitization (an increase in dyskinetic movements); instead the opposite occurred (reduced dyskinetic movements). An animal model of preexisting excitotoxic injury to dopaminergic pathways appears curiously more consistent with our findings (45, 46). Animals with prior stimulant-induced dopamine depletion and dopaminergic axonal degeneration in the striatum and other projection areas showed a reverse behavioral sensitization response after repeated challenges with amphetamine (47–49). Furthermore, lesions specific to the prefrontal cortex or amygdala have reduced (but not eliminated) the degree of behavioral sensitization in rats (50). The failure of normal behavioral sensitization may be related to deficit-like symptoms (negative symptom score) and might explain the reduced vulnerability to drug abuse in schizophrenic patients with deficit syndrome (51). Although potentially important, these are preliminary results with weaknesses due to the limited number of subjects. Furthermore, spectrum subjects who have had difficulty with stress or overstimulation in the past may have selected themselves out of the amphetamine challenge, which would leave an unrepresentative subgroup with less dopaminergic reactivity.
 |
Presented in part at the Fifth International Congress on Schizophrenia Research, Warm Springs, Va., April 8–12, 1995. Received Feb. 28, 1997; revision received July 11, 1997; accepted July 18, 1997. From the Schizophrenia-Related Disorders Program, Maryland Psychiatric Research Center. Address reprint requests to Dr. Cassady, Schizophrenia-Related Disorders Program, Maryland Psychiatric Research Center, P.O. Box 21247, Baltimore, MD 21228. Supported in part by NIMH grants MH-40279 and MH-49826 and by Harford County Mental Health and Addictions, Bel Air, Md. The authors thank the staff of the Schizophrenia-Related Disorders Program for their work and Dr. Deborah Medoff and Xiaonong Gu for statistical assistance.

FIGURE 1. Correlation Between Positive Symptom Score and Dyskinetic Score in Subjects With Schizophrenia Spectrum Personalitya
ar=0.53, df=33, p=0.001.
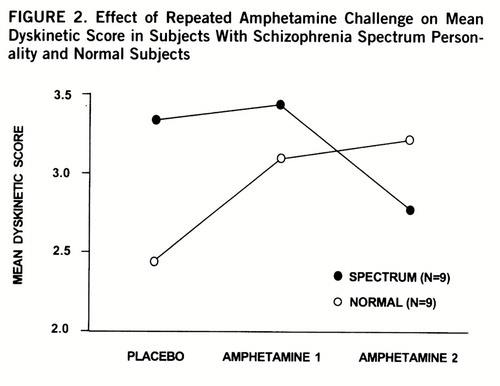
FIGURE 2. Effect of Repeated Amphetamine Challenge on Mean Dyskinetic Score in Subjects With Schizophrenia Spectrum Personality and Normal Subjects
1. Griesinger W: Mental Pathology and Therapeutics, 2nd ed. Translated by Robertson CL, Rutherford J. New York, William Wood, 1882Google Scholar
2. Kraepelin E: Dementia Praecox and Paraphrenia (1919). Translated by Barclay RM; edited by Robertson GM. Birmingham, Ala, Classics of Medicine Library, 1989Google Scholar
3. Waddington JL, Crow TJ: Abnormal involuntary movements and psychosis in the preneuroleptic era and in unmedicated patients: implications for the concept of tardive dyskinesia, in Tardive Dyskinesia: Biological Mechanisms and Clinical Aspects. Edited by Wolf ME, Mosnaim AD. Washington, DC, American Psychiatric Press, 1988, pp 49–66Google Scholar
4. Kleist K: Schizophrenic symptoms and cerebral pathology. J Ment Sci 1960; 106:246–255Crossref, Medline, Google Scholar
5. Leonhard K: Die Defektschizophrenen Krankheitsbilder. Leipzig, Germany, Georg Thieme, 1936Google Scholar
6. Cote L: Basal ganglia, the extrapyramidal motor system, and diseases of transmitter metabolism, in Principles of Neural Science. Edited by Kandel ER, Schwartz JH. New York, Elsevier/North-Holland, 1981, pp 347–357Google Scholar
7. Fenton WS, Wyatt RJ, McGlashan TH: Risk factors for spontaneous dyskinesia in schizophrenia. Arch Gen Psychiatry 1994; 51:643–650Crossref, Medline, Google Scholar
8. Caligiuri MP, Lohr JB: A disturbance in the control of muscle force in neuroleptic-naive schizophrenic patients. Biol Psychiatry 1994; 35:104–111Crossref, Medline, Google Scholar
9. Hoffman WF, Kadri N, Fenn DS, Moussaoui D, Bentounsi B, Tilane A, Khomeis M, Casey DE: Abnormal movements in never medicated schizophrenics (abstract). Schizophr Res 1995; 15:208Google Scholar
10. Moussaoui D, Douki S, Bentounsi B, Otarid A, Chorfi M, Mamou A, Benamor L: Epidemiologie des dyskinesies tardive au Maghreb. L'Encephale 1988; 14:203–208Medline, Google Scholar
11. McCreadie RG, Ohaeri JU: Movement disorder in never and minimally treated Nigerian schizophrenic patients. Br J Psychiatry 1994; 164:184–189Crossref, Medline, Google Scholar
12. Chatterjee A, Chakos M, Koreen A, Geisler S, Sheitman B, Woerner M, Kane JM, Alvir J, Lieberman JA: Prevalence and clinical correlates of extrapyramidal signs and spontaneous dyskinesia in never-medicated schizophrenic patients. Am J Psychiatry 1995; 152:1724–1729Link, Google Scholar
13. Marcus J, Hans SL, Lewow E, Wilkinson L, Burack CM: Neurological findings in high-risk children: childhood assessment and 5-year followup. Schizophr Bull 1985; 1:85–100Crossref, Google Scholar
14. Walker E, Savoie T, Davis D: Neuromotor precursers of schizophrenia. Schizophr Bull 1994; 3:441–451Crossref, Google Scholar
15. Siever LJ: The biology of the boundaries of schizophrenia, in Schizophrenia Research, vol 1: Advances in Neuropsychiatry and Psychopharmacology. Edited by Tamminga CA, Schultz SC. New York, Raven Press, 1991, pp 181–191Google Scholar
16. Kendler KS, Gruenberg AM: Genetic relationship between paranoid personality disorder and the “schizophrenia spectrum” disorders. Am J Psychiatry 1982; 139:1185–1186Link, Google Scholar
17. Kendler KS, McGuire M, Gruenberg AM, O'Hare A, Spellman M, Walsh D: The Roscommon family study, III: schizophrenia-related personality disorders in relatives. Arch Gen Psychiatry 1993; 50:781–788Crossref, Medline, Google Scholar
18. Klawans HL, Margolin DI: Amphetamine-induced dopaminergic hypersensitivity in guinea pigs: implications in psychosis and human movement disorders. Arch Gen Psychiatry 1975; 32:725–732Crossref, Medline, Google Scholar
19. Segal DS, Janowsky DS: Psychostimulant-induced behavioral effects: possible models of schizophrenia, in Psychopharmacology: A Generation of Progress. Edited by Lipton MA, DiMascio A, Killam KF. New York, Raven Press, 1978, pp 1113–1123Google Scholar
20. Robinson TE: Behavioral sensitization: characterization of enduring changes in rotational behavior produced by intermittent injections of amphetamine in male and female rats. Psychopharmacology (Berl) 1984; 84:466–475Crossref, Medline, Google Scholar
21. Robinson TE, Jurson PA, Bennett JA, Bentgen KM: Persistent sensitization of dopamine neurotransmission in ventral striatum (nucleus accumbens) produced by prior experience with (+)-amphetamine: a microdialysis study in freely moving rats. Brain Res 1988; 462:211–222Crossref, Medline, Google Scholar
22. Thaker GK, Cassady S, Adami H, Moran M, Ross DE: Eye movements in spectrum personality disorders: comparison of community subjects and relatives of schizophrenic patients. Am J Psychiatry 1996; 153:362–368Link, Google Scholar
23. Spitzer RL, Williams JBW, Gibbon M, First MB: Instruction Manual for the Structured Clinical Interview for DSM-III-R (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1989Google Scholar
24. Spitzer RL, Endicott J: Schedule for Affective Disorders and Schizophrenia—Lifetime Version, 3rd ed. New York, New York State Psychiatric Institute, Biometrics Research, 1979Google Scholar
25. Pfohl B, Blum N, Zimmerman M, Stangl D: Structured Interview for DSM-III-R Personality, Revised (SIDP-R). Iowa City, University of Iowa College of Medicine, Department of Psychiatry, 1989Google Scholar
26. Adami HM, Thaker GK, Moran MJ, Cassady SL, Heckner CL, Lahti AC, Tamminga CA: Improved diagnosis of schizophrenia spectrum disorders using a modified FH-RDC (abstract). Biol Psychiatry 1990; 27:112AGoogle Scholar
27. Cassady SL, Thaker GK, Tamminga CA: The MPRC scale and the characterization of involuntary movements. Psychiatry Res 1997; 70:21–37Crossref, Medline, Google Scholar
28. Gardos G, Cole JO, LaBrie R: The assessment of tardive dyskinesia. Arch Gen Psychiatry 1977; 134:763–768Google Scholar
29. Angrist B, Corwin J, Bartlik B, Cooper T: Early pharmacokinetics and clinical effects of oral d-amphetamine in normal subjects. Biol Psychiatry 1987; 22:1357–1368Crossref, Medline, Google Scholar
30. Smith RC, Davis JD: Comparative effects of d-amphetamine, l-amphetamine, and methylphenidate on mood in man. Psychopharmacology (Berl) 1977; 53:1–12Crossref, Medline, Google Scholar
31. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976Google Scholar
32. Crow TJ: The two syndrome concept: origins and current status. Schizophr Bull 1985; 11:471–486Crossref, Medline, Google Scholar
33. Sandyk R, Kay SR: The relationship of tardive dyskinesia to positive schizophrenia. Int J Neurosci 1991; 56:107–139Crossref, Medline, Google Scholar
34. Guy W, Ban TA, Wilson WH: The prevalence of abnormal involuntary movements among chronic schizophrenics. Int Clin Psychopharmacol 1986; 1:134–144Crossref, Medline, Google Scholar
35. Waddington JL, Youssef HA, Dolphin C, Kinsella A: Cognitive dysfunction, negative symptoms, and tardive dyskinesia in schizophrenia: their association in relation to topography of involuntary movements and the criterion of the abnormality. Arch Gen Psychiatry 1987; 44:907–912Crossref, Medline, Google Scholar
36. Brown KW, White T: Sub-syndromes of tardive dyskinesia and some clinical correlates. Psychol Med 1992; 22:923–927Crossref, Medline, Google Scholar
37. Moussaoui D: Tardive dyskinesia in North Africa and the Middle East, in Neuroleptic-Induced Movement Disorders. Edited by Yassa R, Nair NPV, Jeste DV. New York, Cambridge University Press, 1997, pp 267–273Google Scholar
38. Caligiuri MP, Lohr JB, Jeste DV: Parkinsonism in neuroleptic-naive schizophrenic patients. Am J Psychiatry 1993; 150:1343–1348Link, Google Scholar
39. Chouinard G, Annable L, Ross-Chouinard A, Mercier P: A 5-year prospective longitudinal study of tardive dyskinesia: factors predicting appearance of new cases. J Clin Psychopharmacol 1988; 8:21S–26SCrossref, Medline, Google Scholar
40. O'Callahan E, Larkin C, Waddington JL: Obstetric complications, the putative familial-sporadic distinction, and tardive dyskinesia in schizophrenia. Br J Psychiatry 1990; 157:578–584Crossref, Medline, Google Scholar
41. Richardson MA, Haugland G, Craig TJ: Neuroleptic use, parkinsonian symptoms, tardive dyskinesia, and associated factors in child and adolescent psychiatric patients. Am J Psychiatry 1991; 148:1322–1328Link, Google Scholar
42. McCreadie RG, Hall DJ, Berry IJ, Robertson LJ, Ewing JI, Geals MF: The Nithsdale schizophrenia surveys, X: obstetric complications, family history and abnormal movements. Br J Psychiatry 1992, 161:799–805Google Scholar
43. Roy M-A, Flaum MA, Gupta S, Jaramillo L, Andreasen NC: Epidemiological and clinical correlates of familial and sporadic schizophrenia. Acta Psychiatr Scand 1994; 89:324–328Crossref, Medline, Google Scholar
44. Thaker G, Moran M, Adami H, Cassady S: Psychosis proneness scales in schizophrenia spectrum personality disorders: familial vs nonfamilial samples. Psychiatry Res 1993; 46:47–57Crossref, Medline, Google Scholar
45. Robinson TE, Becker JB: Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res Brain Res Rev 1986; 11:157–198Crossref, Google Scholar
46. Lieberman JA, Kinon BJ, Loebel AD: Dopaminergic mechanisms in idiopathic and drug-induced psychoses. Schizophr Bull 1990; 16:97–110Crossref, Medline, Google Scholar
47. Ricuarte GA, Schuster CR, Seiden LS: Long-lasting effects of repeated methamphetamine administration on dopamine and serotonin neurons in the rat brain: a regional study. Brain Res 1980; 193:153–160Crossref, Medline, Google Scholar
48. Lucot JB, Wagner GC, Schuster CR, Seiden LS: The effects of dopaminergic agents on the locomotor activity of rats after high doses of methylamphetamine. Pharmacol Biochem Behav 1980; 13:409–413Crossref, Medline, Google Scholar
49. Finnegan KT, Ricuarte G, Seiden JL, Schuster CR: Altered sensitivity to d-methylamphetamine, apomorphine and haloperidol in rhesus monkeys depleted of caudate dopamine by repeated administration of d-methylamphetamine. Psychopharmacology (Berl) 1982; 77:43–52Crossref, Medline, Google Scholar
50. Wolf ME, Dahlin SL, Hu XT, Xue CJ, White K: Effects of lesions of prefrontal cortex, amygdala, or fornix on behavioral sensitization to amphetamine: comparison with n-methyl-d-aspartamine antagonists. Neuroscience 1995; 69:417–439Crossref, Medline, Google Scholar
51. Kirkpatrick B, Amador XF, Flaum M, Yale SA, Gorman JM, Carpenter WT, Tohen M, McGlashan T: The deficit syndrome in the DSM-IV field trial, I: alcohol and other drug abuse. Schizophr Res 1996; 20:69–77Crossref, Medline, Google Scholar



