Longitudinal Population-Based Twin Study of Retrospectively Reported Premenstrual Symptoms and Lifetime Major Depression
Abstract
Objective: While family and twin studies suggest that retrospectively reported premenstrual symptoms are heritable, these studies have not accounted for the unreliability of such measures. In addition, we know little about the relationship of the familial risk factors for premenstrual symptoms and major depression. Method: Lifetime major depression and premenstrual-related tiredness, sadness, and irritability were assessed twice over 6 years in 1,312 menstruating female twins ascertained from a population-based twin register. A twin-measurement model—which permits estimation of the etiologic roles of genetic and environmental factors with correction for errors of measurement or short-term temporal fluctuations—was applied to these data.Results: A single premenstrual symptom factor was found that was moderately stable over time. The best-fitting twin-measurement model estimated the heritability of the stable component of premenstrual symptoms at 56% and showed no impact of family-environmental factors. A bivariate twin-measurement model estimated that the genetic and environmental risk factors for lifetime major depression contributed only modestly to the etiology of premenstrual syndrome. No evidence was found for significant biases in the twin method.Conclusions: Retrospectively reported premenstrual-related symptoms of depression and anxiety are moderately stable over time and, when correction is made for this level of stability, substantially heritable. The genetic and environmental risk factors for these premenstrual symptoms and lifetime major depression are not closely related. Am J Psychiatry 1998; 155: 1234-1240
Psychological symptoms experienced during the premenstrual phases of the female reproductive cycle are common and sometimes disabling (1-5). Estimates of the prevalence of premenstrual symptoms vary substantially, in part because of differences in instruments, definitions, and populations (5-10). However, all studies show that many women experience no premenstrual symptoms, a substantial proportion experience mild symptoms, and a few women report severe or disabling symptoms. What is responsible for this variation?
The seven family and twin studies of premenstrual symptoms published to date (11-16), with one exception (12), suggest that familial factors contribute substantially to individual differences in the vulnerability to premenstrual symptoms. However, of the four twin studies of premenstrual symptoms (13-16), only one, with 31 pairs, relied on prospective evaluations (13). The three larger studies (14-16) used single retrospective reports of premenstrual symptoms, the reliability and the validity of which have both been questioned (17-19) and defended (20, 21). In twin studies using one occasion of measurement, the impact of genetic and environmental risk factors may be attenuated by measurement error. However, by using two occasions of measurement, the importance of both genetic and environmental variation can be assessed with correction for unreliability of measurement (22).
PREMENSTRUAL SYMPTOMS AND DEPRESSION
The relationship between premenstrual symptoms and major depression has been controversial (3, 23). Some studies suggest that severe forms of premenstrual symptoms are “manifestations of an underlying depressive disorder” (24). Other investigators have concluded that the association between severe premenstrual symptoms and major depression is weak (25). Since most studies of this question have used clinical samples, the possibility of referral bias—whereby women with major depression and premenstrual symptoms are more likely to seek treatment—cannot be excluded. Furthermore, given strong evidence for the importance of familial/genetic factors in the etiology of both premenstrual symptoms and major depression (26, 27), examining the interrelationship of the genetic and environmental risk factors for these two syndromes in a genetically informative population might further clarify their etiologic relationship.
GOALS
In this paper we use longitudinal information on female twin pairs from a population-based registry to address two questions. First, using a “twin-measurement model,” we examine the etiologic importance of genetic and environmental factors in retrospectively reported premenstrual symptoms, correcting for the effect of measurement error. Second, using a bivariate twin-measurement model, we examine, correcting for measurement error, the relationship between the genetic and environmental risk factors for premenstrual symptoms and lifetime major depression.
METHOD
Sample
The data for this report come from a study of common psychiatric disorders in female-female twin pairs from the Virginia Twin Registry, which is formed from a systematic review of all birth records from 1915 onward in the Commonwealth of Virginia. In 1987–1988 questionnaires were sent to members of Caucasian female same-sex twin pairs born between 1935 and 1971 for whom, at prior contact, we had usable addresses. The rate of response to this questionnaire was around 64%. This is an underestimation of the true cooperation rate as an unknown proportion of the twins never received the questionnaire because of such factors as errors of address or incorrect forwarding. In our first interview we assessed 92% of the eligible individuals who had returned questionnaires (N=2,163), 90% face to face and the rest by telephone. Zygosity was determined blindly by standard questions (28), photographs, and when necessary, DNA (27, 29). We performed two additional waves of telephone interviews, completing interviews for 2,001 (92.5%) and 1,898 (87.7%) of the original sample, respectively. The mean age of the participating twins in the third wave of interviews was 34.6 years (SD=7.5, range=22–59). All of the interviewers were blind to information about the co-twin. Written informed consent was obtained before the face-to-face interviews, and oral assent was obtained before the telephone interviews.
Measures
Both in the initial questionnaire mailed to the twins and in the third wave of interviews, we asked the twins four questions about the psychological aspects of their premenstrual experiences. In this paper we will refer to these two assessments of premenstrual symptoms as “time 1” and “time 2.” These four items, which were similar to those used in other general population surveys (10), were as follows:
A lot of women experience changes in their health and mood BEFORE they have their periods. Just BEFORE the start of your period, do you:
a) have less energy than usual or get tired more easily?
b) feel more sad, blue, or depressed?
c) feel more irritable or get upset more easily?
d) have any other changes in health or mood?
We called these items, respectively, “TIRED,” “SAD,” “IRRITABLE,” and “OTHER.” Four possible responses were provided: “A Lot,” “Some,” “A Little,” and “No.” Twins who reported that their menstrual symptoms had stopped completely (9.8% of the time 1 sample and 13.0% of the time 2 sample) were excluded from these analyses. Women who were pregnant, however, were asked to respond for their typical premenstrual experiences. At time 1 and time 2, 28.3% and 27.6% of the twins, respectively, reported currently using oral contraceptives. Individuals were included in this sample if they answered at least three of the four premenstrual items at both times of assessment. For those missing a single item at time 1 or time 2 (N=8 and N=4, respectively), the score on the missing item was imputed from theanswered items.
Diagnoses of lifetime major depression were based on DSM-III-R criteria, as determined with an adaptation of the Structured Clinical Interview for DSM-III-R (30) conducted by carefully trained interviewers with prior professional mental health experience; this assessment was made both during the initial face-to-face interview and during the third telephone interview. The interviewers were blind to the psychiatric history of the co-twin. For these analyses, individuals assessed at the third interview who reported an onset of major depression after the first interview were considered to be unaffected, so that we were obtaining two reports on each subject’s lifetime history for major depression before the first interview.
Statistical Analysis
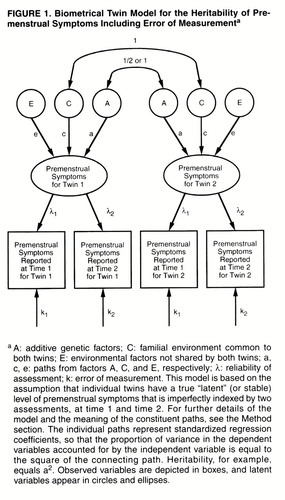
We performed a principal components analysis factor analysis of the four premenstrual symptom items at both waves by using the PROCFACTOR procedure in SAS (31). The number of factors was determined by an eigenvalue criterion. The twin-measurement model used in this investigation has been described previously (32). Briefly, we assume that the variation in premenstrual symptoms can be ascribed to three sets of factors: 1) additive genetic factors (A), which contribute twice as much to the correlation in monozygotic twins as dizygotic twins (because monozygotic twins share all their genes identical by descent, while dizygotic twins share on average one-half of their genes); 2) family or “common” environment (the familial factors, such as parental attitudes, that are shared by members of a twin pair) (C), which contributes equally to the correlation in monozygotic and dizygotic twins; and 3) individual specific environment (E), which traditionally reflects environmental experiences not shared by both members of a twin pair and therefore contributes to differences between them in their reported levels of premenstrual symptoms. (We also explored the impact of adding dominance genetic variance in model fitting but do not report these results here as they did not result in improvements in overall fit.)
As outlined in figure 1, the twin-measurement model uses the premenstrual symptom scores of twins at both time 1 and time 2. The model assumes that each twin has a true but unobserved (or “latent”) level of premenstrual symptoms. We measured premenstrual symptoms twice in these twins, but each measurement is fallible in that it partially reflects the true level of premenstrual symptoms and partly reflects short-term temporal fluctuations or error. The paths λ1 and λ2 represent the degree to which the assessments of premenstrual symptoms obtained at time 1 and time 2, respectively, reflect this true level of symptoms. The higher the value of λ, the more accurately any one measure of premenstrual symptoms reflects anindividual’s true level of symptoms. The other path to assessments of premenstrual symptoms at the two time points (k1 and k2, respectively) represents error in the individual assessments. By definition, λ2+k2=1.0. These models assume that the errors of measurement are uncorrelated within time. The true, or latent, level of premenstrual symptoms is then modeled in a standard twin design, as already outlined, with the sources of variance in liability divided between additive genetic, common environmental, and individual specific environmental factors.
Three differences between a measurement model and the standard twin model, based on a single time of assessment, are noteworthy. First, this model provides separate estimates for error of measurement (k) and true individual specific environment (e), which are confounded in the standard twin model. Second, it provides a direct estimate of the reliability of the phenotypic assessment support (λ). Third, while a standard twin model estimates the heritability of the observed phenotype (including error), the measurement model estimates the heritability of the latent phenotype, correcting for the effects of error. The latter will always be greater than the former.
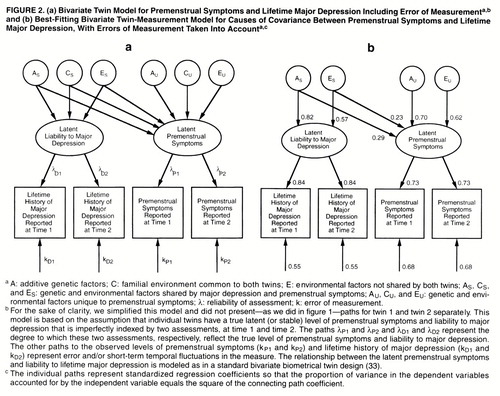
We present here, for the first time to our knowledge, a bivariate measurement model that combines features of the measurement model with those of a standard bivariate twin model (figure 2a) (33, 34). Basically, in a bivariate model we try to decompose the covariation between two disorders into its genetic and environmental components. However, in the bivariate measurement model, instead of decomposing covariation between observed disorders, we explore the etiologic relationship between two latent disorders, which are each, in turn, assessed at two occasions of measurement. Thus, this model permits us to assess the genetic and environmental covariance between two disorders while correcting for the effects of measurement error. As outlined in figure 2a, we use a Cholesky decomposition (34) that includes two “sets” of genetic and environmental factors—those that are shared by major depression and premenstrual symptoms (AS, CS, and ES) and those that are unique to premenstrual symptoms (AU, CU, and EU). Lower-case letters (a, c, and e) are used to label the paths from these factors.
These analyses are based on the assumption that the exposure to environmental factors that influence premenstrual symptoms are similarly correlated in monozygotic and dizygotic twins. We tested these two assumptions by using regression analysis to examine whether, with controls for zygosity, measures of childhood or adult environmental similarity could predict the difference in premenstrual symptoms in members of a twin pair.
Modeling fitting was performed with the program Mx (35) by using weighted least squares to fit to matrices of polychoric correlations computed by PRELIS 2.14 (36). Because the full-weight matrices were almost singular, we used only the diagonal of the weight matrix to fit the model. The best-fit model was selected by using Akaike’s information criterion (AIC) (37).
RESULTS
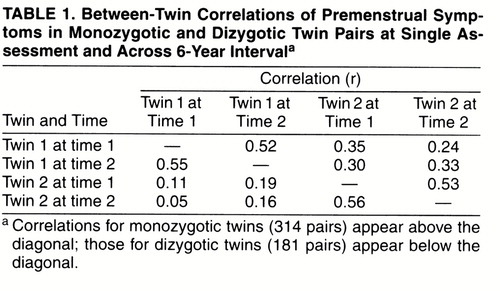
Data on premenstrual symptoms were available on two occasions for 1,312 twins and both members of 314 monozygotic and 181 dizygotic pairs. The twins’ reports were separated by an average of 71.8 months (SD=27.7). Premenstrual symptom scores at time 1 did not significantly predict participation in the second assessment.
Phenotypic Factor Analysis
We performed a factor analysis of the four premenstrual items as assessed at time 1, which produced a single factor with an eigenvalue of greater than 1.0, accounting for 65.6% of the variance. All four items loaded highly on this factor: TIRED=0.77, SAD=0.88, IRRITABLE=0.85, and OTHER=0.73. A factor analysis of the time 2 items produced nearly identical results (congruency coefficient=0.99 [38]). Further analyses were performed by using this “premenstrual symptoms” factor as defined at time 1.
Stability and Potential Confounders
Test-retest stability of premenstrual symptoms for the 1,312 twins, as assessed by a Pearson product-moment correlation, was r=0.53 (df=1310, p<0.001). Levels of premenstrual symptoms did not change over time (paired t=0.06, df=1311, p=0.95). The interaction between the premenstrual score at time 1 and the length of the interval between time 1 and time 2 did not predict the time 2 premenstrual score (t=0.54, df=2307, p=0.57).
We examined between-twin correlations in premenstrual symptoms at time 1 and time 2 after controlling for age and zygosity and then adding, sequentially, oral contraceptive use and parity. No appreciable differences were seen (for instance, at time 1 the three correlations were, respectively, 0.247, 0.245, and 0.247).
Testing Assumptions of the Twin Method
When zygosity was controlled for, neither similarity of childhood environment nor frequency of current contact predicted twin resemblance for premenstrual symptoms at time 1 (t=0.55, p=0.59, and t=0.44, p=0.66, respectively) or time 2 (t=1.46, p=0.15, and t=1.76, p=0.08, respectively) (df=393 in all cases). Frequency of contact at time 1 did not predict resemblance for premenstrual symptoms at time 2 (t=1.04, df=393, p=0.30).
Twin Correlations Within and Across Time
The correlations between monozygotic and dizygotic twins for the premenstrual symptom factor within and across the two times of measurement are seen in table 1. The within-time cross-twin correlations were similar at time 1 and time 2 for monozygotic twins (0.35 and 0.33, respectively) and dizygotic twins (0.11 and 0.16, respectively). The correlations in premenstrual symptoms, at both time points, were slightly more than twice as great in monozygotic as in dizygotic twins. Furthermore, the cross-twin cross-time correlations (e.g., the correlation in premenstrual symptoms for twin 1 at time 1 and twin 2 at time 2) were also substantially greater in monozygotic twins than in dizygotic twins.
Twin-Measurement Model for Premenstrual Symptoms
The full ACE model fit well (χ2=5.27, df=16, p=0.99, AIC=–26.73). We first simplified the model by setting the λ values equal across the two times of measurement (which had been estimated at 0.74 and 0.72 in the full model), thereby further improving the AIC (χ2=5.30, df=17, AIC=–28.70). Next, we dropped the parameters for common environment (which was estimated at zero in the full model). This change produced the model with the lowest AIC and hence the best balance of parsimony and fit (χ2=5-30, df=18, AIC= –30.70). This model estimated that the variability in latent premenstrual symptoms was due to genetic factors (with an estimated heritability of 56%) and individual specific environment (estimated at 44%). The latent level of symptoms was assessed with equal accuracy by the time 1 questionnaire and the time 2 telephone interview, with an estimated value of λ of 0.73. A model that postulated that the twin resemblance for premenstrual symptoms was due solely to family-environmental factors fit much worse (χ2=20.48, df=18, AIC=–15.52) and could be strongly rejected against the ACE model (difference test: c2=15.18, df=1, p<0.001).
Bivariate Twin-Measurement Model for Premenstrual Symptoms and Lifetime Major Depression
The full model (figure 2a) fit well (χ2=41.59, df=37, p=0.28, AIC=–32.41) and could be simplified by dropping the parameters for common environment for premenstrual symptoms and major depression and constraining the λ values to be equal across time for premenstrual symptoms and major depression. The model with the best balance of parsimony and fit (χ2=41.84, df=42, AIC=–42.16) is pictured in figure 2b and characterized in table 2 and has the following major features. First, the roles of additive genetic and environmental risk factors in the etiology of premenstrual symptoms are identical, within rounding error, to those seen when the premenstrual symptoms were examined alone. Second, in accord with findings from our previous study of major depression with the measurement model (22), the latent liability to lifetime major depression is highly heritable, with the remaining variance in liability due to individual specific environmental factors. Third, genetic factors that influence major depression also affect the liability to premenstrual symptoms. However, their impact is modest, accounting for 8% of the total and 14% of the genetic variance of premenstrual symptoms. Fourth, while environmental risk factors that influence premenstrual symptoms also influence the liability to major depression, their impact is slight, accounting for only 5% of the total variance in liability and 12% of the total environmental variance. The third and fourth points deserve restatement: 86% of the genetic variance and 88% of the environmental variance for premenstrual symptoms are not shared with major depression.
DISCUSSION
Heritability of Premenstrual Symptoms
In accord with the four previous twin studies that have examined this issue (13-16), this investigation showed a substantial heritable influence on premenstrual symptoms. We examined several possible biases in our sample but found no evidence that would undermine confidence in these results. Specifically, cooperation was independent of prior premenstrual symptoms, and twin resemblance for these symptoms could not be predicted by childhood or adult environmental similarity nor by similarity in oral contraceptive use or parity.
The reports on only two of the previous twin studies of premenstrual symptoms provided model-fitting results to which our findings could be compared. Van den Akker et al. (14) examined premenstrual symptoms as assessed by self-report questionnaires in female twin pairs from two British twin registries from London (364 pairs) and Birmingham (98 pairs). In the larger London sample, they found that the familial aggregation of premenstrual symptoms was largely due to genetic factors, with a heritability of 30%, but they also noted a modest contribution of the familial environment. In the small Birmingham sample, however, they found much higher heritability for premenstrual symptoms (80%) with no evidence for family environment. In our prior analysis of the data on premenstrual symptoms at time 1 for 827 pairs (15), the best-fitting model indicated that twin resemblance was due solely to genetic factors, with an estimated heritability of 35%.
In the only prospective twin study of premenstrual symptoms, Dalton et al. (13) examined 31 twin pairs ascertained through a premenstrual syndrome clinic and found the concordance rate to be considerably greater in the monozygotic pairs (93%, 14 of 15) than the dizygotic pairs (44%, seven of 16). In 300 pairs of volunteer Australian twins, Condon (16) found the correlation in “global PMS scores” to be nearly twice as great for monozygotic (r=0.55) as for dizygotic (r=0.28) pairs.
With the exception of the findings on the small Birmingham subsample reported by van den Akker et al. (14), the results from other population-based studies are consistent with the conclusion that premenstrual symptoms are modestly heritable and influenced little if at all by the familial environment.
Our results add to this previous literature by addressing the role of measurement error in the assessment of premenstrual symptoms. Retrospectively reported premenstrual symptoms are only moderately stable over time, thereby attenuating estimates of the genetic and environmental contributions to the true, or stable, liability to premenstrual symptoms. When this unreliability was incorporated into twin modeling, the heritability of premenstrual symptoms rose substantially. Our results suggest that in a standard twin model of premenstrual symptoms based on a single occasion of measurement, roughly one-half of what is assumed to be individual-specific environment (E) is actually error.
Consistent with the findings from nearly all prior studies, the estimates of common environment were zero in our full model, and we could confidently reject the hypothesis that twin resemblance for premenstrual symptoms is due entirely to family-environmental factors. These results are inconsistent with the hypothesis that premenstrual symptoms are strongly influenced by attitudes toward “the feminine role” consistently learned by daughters from parents, cultural/social background, and religious milieu (39).
Premenstrual Symptoms and Major Depression
Since affective changes are among those most commonly experienced in the premenstrual phase (1, 10), it is logical to examine the degree to which mood disorders and premenstrual symptoms are etiologically interrelated (23, 24). Epidemiologically based twin designs can be particularly powerful in addressing such questions. Our results suggest that, while premenstrual symptoms and major depression do share both genetic and environmental risk factors, the degree of sharing is modest. In particular, the genetically influenced biological processes that influence the vulnerability to premenstrual symptoms are only weakly related to those that affect the risk for major depression. Evidence that premenstrual dysphoria responds to serotonin reuptake inhibitors (23, 40) may not result from a close etiologic relationship between premenstrual syndromes and major depression.
We also collected self-reports of symptoms of depression (41) in our twin sample at the same two time points as we had assessed premenstrual symptoms. We applied a bivariate measurement model to premenstrual and depressive symptoms. The best-fitting model was quite similar to that obtained by using lifetime major depression with one exception. Genetic risk factors shared with depressive symptoms accounted for 22% of the total and 37% of the genetic variance in premenstrual symptoms. While nearly two-thirds of the genetic risk factors for premenstrual symptoms were independent of those for depressive symptoms, premenstrual symptoms do appear to have a stronger etiologic relationship with the temporally stable component of depressive symptoms than with lifetime major depression.
Limitations
These results should be interpreted in the context of four methodologic limitations. First, premenstrual symptoms were assessed retrospectively. The relative validity of retrospective and prospective reports of premenstrual symptoms has been extensively discussed, and prospective approaches are probably superior (1, 17-21). However, the logistics of obtaining such data in large epidemiologic samples are formidable. Our approach, assessing premenstrual symptoms twice 6 years apart, reduces the possible bias of transient symptoms or stressors, thereby increasing the validity of the measure over that obtained with a single retrospective report.
Second, both of our twin-measurement models were based on the assumption that the changes in premenstrual symptoms or lifetime history of major depression between the two occasions of measurement were uncorrelated in twins. To test this assumption, paths were added to the models to correlate the error terms for members of a twin pair within an occasion of measurement. In neither the univariate analysis of premenstrual symptoms nor the bivariate analysis of premenstrual symptoms and major depression did the addition of these paths result in an improvement in the AIC score.
Third, over 100 different psychological and physical symptoms have been associated with the premenstrual syndrome (42). We have assessed only three specific symptoms (tiredness, sadness, and irritability), which, although among the most commonly reported premenstrual phenomena (6, 7, 10), are all psychological in nature. We assessed neither the range of physical symptoms common in the premenstrual phase, such as bloating or pain, nor other psychological symptoms, such as impaired concentration, increased energy, or altered libido. Clearly, we have information on only a modest part of the complex premenstrual syndrome and hope that this work will stimulate further, more extensive evaluations of premenstrual symptoms in genetically informative populations.
Fourth, in our general population sample, women with severe premenstrual symptoms were rare. Could the familial factors that influence severe premenstrual symptoms differ from those responsible for variation in the less deviant range? We evaluated this hypothesis by using a multiple threshold model (43). We set two thresholds dividing our sample, on the basis of premenstrual symptoms, into the top 5%, the next 10%, and the lowest 85%. The model fit well for dizygotic twins at time 1 (χ2=4.54, df=3, p=0.21) and time 2 (χ2=2.50, df=3, p=0.48) and for monozygotic twins at time 1 (χ2=3.36, df=3, p=0.34) but marginally failed for monozygotic twins at time 2 (χ2=8.20, df=3, p=0.04), a set of results not different from chance expectation (44). We found no evidence to reject the hypothesis that the varying levels of premenstrual symptoms reported in our sample reflect different degrees of a single symptomatic continuum.
Received May 7, 1997; revision received Dec. 11, 1997; accepted Feb. 26, 1998. From the Department of Psychiatry and the Department of Human Genetics, Medical College of Virginia, Virginia Commonwealth University.. Address reprint requests to Dr. Kendler, Department of Psychiatry, Medical College of Virginia, P.O. Box 980126, Richmond, VA 23298-0126; [email protected]. edu (e-mail). Supported by grants MH-40828, MH-49492, MH-01277, and MH-54150 from NIMH. The Virginia Twin Registry, established and maintained by W. Nance, M.D., Ph.D., and L. Corey, Ph.D., is supported by grant HD-26746 from the National Institute of Child Health and Human Development and grant NS-31564 from the National Institute of Neurological and Communicative Disorders and Stroke.The authors thank Dr. Carol Prescott for her contribution to this project.
 |
 |
1. Moos RH: Perimenstrual Symptoms: A Manual and Overview of Research With the Menstrual Distress Questionnaire. Palo Alto, Calif, Stanford University School of Medicine, Social Ecology Laboratory, 1985Google Scholar
2. Logue CM, Moos RH: Perimenstrual symptoms: prevalence and risk factors. Psychosom Med 1986; 48:388–414Crossref, Medline, Google Scholar
3. Severino SK, Moline ML: Premenstrual Syndrome: A Clinician’s Guide. New York, Guilford Press, 1989Google Scholar
4. Blumenthal SJ, Nadelson CC: Late luteal phase dysphoric disorder (premenstrual syndromes): clinical implications. J Clin Psychiatry 1988; 49:469–474Medline, Google Scholar
5. Rivera-Tovar AD, Frank E: Late luteal phase dysphoric disorder in young women. Am J Psychiatry 1990; 147:1634–1636Link, Google Scholar
6. Van Keep PA, Lehert P: The premenstrual syndrome: an epidemiological and statistical exercise, in The Premenstrual Syndrome. Edited by Van Keep PA, Utian WH. Lancaster, England, MTP Press, 1981, pp 31–42Google Scholar
7. Woods N, Most A, Dery GK: Prevalence of perimenstrual symptoms. Am J Public Health 1982; 72:1257–1264Crossref, Medline, Google Scholar
8. Gath D, Osborn M, Bungay G, Iles S, Day A, Bond A, Passingham C: Psychiatric disorder and gynaecological symptoms in middle aged women: a community survey. Br Med J 1987; 294:213–218Crossref, Medline, Google Scholar
9. Johnson SR, McChesney C, Bean JA: Epidemiology of premenstrual symptoms in a nonclinical sample, I: prevalence, natural history and help-seeking behavior. J Reproduct Med 1988; 33:340–346Medline, Google Scholar
10. Merikangas KR, Foeldenyi M, Angst J: The Zurich Study, XIX: patterns of menstrual disturbances in the community: results of the Zurich Cohort Study. Eur Arch Psychiatry Clin Neurosci 1993; 243:23–32Crossref, Medline, Google Scholar
11. Kantero RL, Widholm O: Correlations of menstrual traits between adolescent girls and their mothers. Acta Obs Gynaecol Scand (Suppl) 1971; 14:30–36Crossref, Google Scholar
12. Glick H, Endicott J, Nee J: Premenstrual changes: are they familial? Acta Psychiatr Scand 1993; 88:149–155Google Scholar
13. Dalton K, Dalton ME, Guthrie K: Incidence of the premenstrual syndrome in twins. Br Med J 1987; 295:1027–1028Crossref, Medline, Google Scholar
14. van den Akker OB, Stein GS, Neale MC, Murray RM: Genetic and environmental variation in menstrual cycle: histories of two British twin samples. Acta Genet Med Gemellol (Roma) 1987; 36:541–548Crossref, Medline, Google Scholar
15. Kendler KS, Silberg JL, Neale MC, Kessler RC, Heath AC, Eaves LJ: Genetic and environmental factors in the aetiology of menstrual, premenstrual and neurotic symptoms: a population-based twin study. Psychol Med 1992; 22:85–100Crossref, Medline, Google Scholar
16. Condon JT: The premenstrual syndrome: a twin study. Br J Psychiatry 1993; 162:481–486Crossref, Medline, Google Scholar
17. Rubinow DR, Roy-Byrne P: Premenstrual syndromes: overview from a methodologic perspective. Am J Psychiatry 1984; 141:163–172Link, Google Scholar
18. Christensen AP, Oei TPS: Correlates of confirmed premenstrual dysphoria. J Psychosom Res 1989; 33:307–313Crossref, Medline, Google Scholar
19. McFarland C, Ross M, DeCourville N: Women’s theories of menstruation and biases in recall of menstrual symptoms. J Pers Soc Psychol 1989; 57:522–531Crossref, Medline, Google Scholar
20. Hart WG, Coleman GJ, Russell JW: Assessment of premenstrual symptomatology: a re-evaluation of the predictive validity of self report. J Psychosom Res 1987; 31:185–190Crossref, Medline, Google Scholar
21. Schilling KM: What is a real difference? content or method in menstrual findings, in The Menstrual Cycle: Research and Implications for Women’s Health. Edited by Komnenich P, McSweeney M, Joack JA. New York, Springer, 1981Google Scholar
22. Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ: The lifetime history of major depression in women: reliability of diagnosis and heritability. Arch Gen Psychiatry 1993; 50:863–870Crossref, Medline, Google Scholar
23. Halbreich U: Premenstrual dysphoric disorders: a diversified cluster of vulnerability traits to depression. Acta Psychiatr Scand 1997; 95:169–176Crossref, Medline, Google Scholar
24. Hallman J: The premenstrual syndrome—an equivalent of depression? Acta Psychiatr Scand 1986; 73:403–411Google Scholar
25. Hurt SW, Schnurr PP, Severino SK, Freeman EW, Gise LH, Rivera-Tovar A, Steege JF: Late luteal phase dysphoric disorder in 670 women evaluated for premenstrual complaints. Am J Psychiatry 1992; 149:525–530Link, Google Scholar
26. Tsuang MT, Faraone SV: The Genetics of Mood Disorders. Baltimore, Johns Hopkins University Press, 1990Google Scholar
27. Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ: A population-based twin study of major depression in women: the impact of varying definitions of illness. Arch Gen Psychiatry 1992; 49:257–266Crossref, Medline, Google Scholar
28. Eaves LJ, Eysenck HJ, Martin NG, Jardine R, Heath AC, Feingold L, Young PA, Kendler KS: Genes, Culture and Personality: An Empirical Approach. London, Oxford University Press, 1989Google Scholar
29. Spence JE, Corey LA, Nance WE, Marazita ML, Kendler KS, Schieken RM: Molecular analysis of twin zygosity using VNTR DNA probes (abstract). Am J Hum Genet 1988; 43(3):A159Google Scholar
30. Spitzer RL, Williams JBW: Structured Clinical Interview for DSM-III-R (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1985Google Scholar
31. Gorsuch RL: Factor Analysis, 2nd ed. Hillsdale, NJ, Lawrence Erlbaum Associates, 1983Google Scholar
32. Kendler KS: Social support: a genetic-epidemiologic analysis. Am J Psychiatry 1997; 154:1398–1404Link, Google Scholar
33. Kendler KS: Twin studies of psychiatric illness: current status and future directions. Arch Gen Psychiatry 1993; 50:905–915Crossref, Medline, Google Scholar
34. Neale MC, Cardon LR: Methodology for Genetic Studies of Twins and Families. Dordrecht, The Netherlands, Kluwer Academic, 1992Google Scholar
35. Neale MC: Mx: Statistical Modeling, 2nd ed. Richmond, Medical College of Virginia, Department of Psychiatry, 1994Google Scholar
36. Joreskog KG, Sorbom D: PRELIS 2: User’s Reference Guide. Chicago, Scientific Software International, 1996Google Scholar
37. Akaike H: Factor analysis and AIC. Psychometrika 1987; 52:317–332Crossref, Google Scholar
38. Derogatis LR, Serio JC, Cleary PA: An empirical comparison of three indices of factorial similarity. Psychol Rep 1972; 30:791–804Crossref, Google Scholar
39. Berry C, McQuirre F: Menstrual distress and acceptance of sexual role. Am J Obstet Gynecol 1972; 114:83–86Crossref, Medline, Google Scholar
40. Yonkers KA, Halbreich U, Freeman E, Brown C, Endicott J, Frank E, Parry B, Pearlstein T, Severino S, Stout A, Stone A, Harrison W: Symptomatic improvement of premenstrual dysphoric disorder with sertraline treatment: a randomized controlled trial: Sertraline Premenstrual Dysphoric Collaborative Study Group. JAMA 1997; 278:983–988Crossref, Medline, Google Scholar
41. Derogatis LR, Lipman RS, Covi L: SCL-90: an outpatient psychiatric rating scale—preliminary report. Psychopharmacol Bull 1973; 9:13–28Medline, Google Scholar
42. Smith S, Schiff I: The premenstrual syndrome—diagnosis and management. Fertil Steril 1989; 52:527–543Crossref, Medline, Google Scholar
43. Reich T, James JW, Morris CA: The use of multiple thresholds in determining the mode of transmission of semi-continuous traits. Ann Hum Genet 1972; 36:163–184Crossref, Medline, Google Scholar
44. Feild HS, Armenakis AA: On use of multiple tests of significance in psychological research. Psychol Rep 1974; 35:427–431Crossref, Google Scholar



