Hippocampal Changes in Patients With a First Episode of Major Depression
Abstract
OBJECTIVE: Previous work suggests that patients with unipolar depression may have structural as well as functional abnormalities in limbic-thalamic-cortical networks, which are hypothesized to modulate human mood states. A core area in these networks is the hippocampus. In the present study, differences in volumes of hippocampal gray and white matter between patients with a first episode of major depression and healthy comparison subjects were examined. METHOD: Thirty patients with a first episode of major depression and 30 healthy comparison subjects who were matched for age, gender, handedness, and education were examined with high-resolution magnetic resonance imaging. RESULTS: Male patients with a first episode of major depression had significantly smaller hippocampal total and gray matter volumes than healthy male comparison subjects. Both male and female patients showed significant alterations of left-right asymmetry and significant reductions of left and right hippocampal white matter fibers in relation to healthy comparison subjects. Hippocampal measurements were not significantly correlated with clinical variables, such as age at onset of illness, illness duration, or severity of depression. CONCLUSIONS: These results are consistent with findings of structural abnormalities of the hippocampal formation in patients with major depression that were more pronounced in male patients. The authors’ findings support the hypothesis that the hippocampus and its connections within limbic-cortical networks may play a crucial role in the pathogenesis of major depression.
Lesion studies in animals and neuropathological reports in humans have shown that defects in the medial temporal lobe region, including the hippocampal formation, are associated with a severe and global amnesia (1–3). Aside from its well-documented contribution to learning and memory, the hippocampal formation plays a critical role in the regulation of motivation and emotion (4). The hippocampus is a core region in the limbic system and has widespread connections to diverse cortical areas, like the prefrontal cortex, anterior thalamic nuclei, amygdala, basal ganglia, and hypothalamus (5), all of which are regions known to constitute the neuroanatomical network of mood regulation (6–8). Therefore, the hippocampus might be involved in the pathogenesis of major depression.
Until now, structural magnetic resonance imaging (MRI) studies of the hippocampal formation in patients with unipolar major depression have revealed conflicting results, which may be explained partly by differences in clinical and demographic variables of the various study designs. For example, some studies were conducted among elderly patients with depression and others among younger patients with depression.
Investigations of patients with geriatric depression or of elderly patients with recurrent episodes have consistently indicated that there are structural changes in the hippocampal formation. A finding of decreased T1 relaxation time in the hippocampus of elderly patients with unipolar depression has supported this finding (9). Moreover, Sheline et al. (10) reported smaller hippocampal gray matter volumes in patients with recurrent depression and described a negative correlation with cumulative illness duration. This finding was recently replicated in a larger sample of patients (11). Furthermore, patients with geriatric depression have shown tendencies to have smaller right and left hippocampal volumes (12). We know of only one study (13) that did not find significantly different hippocampal volumes in patients with geriatric depression. However, this study did not match patients for age and education.
With respect to samples of younger patients with recurrent depressive episodes, the literature is inconsistent. Vakili et al. (14) did not find significant differences in hippocampal volumes in younger patients (<60 years old) with major depression. However, other investigations have shown significantly smaller left hippocampal volumes in patients with unipolar major depression (15) or drug-resistant episodes of major depression (16).
Taken together, studies of geriatric depression or elderly patients with recurrent episodes seem to find smaller hippocampal volumes, whereas results of studies among younger patients are inconsistent. Until now, studies of patients with first-episode depression have not been available, to our knowledge.
Another explanation for the conflicting results in the literature might be differences in methods concerning the anatomical definitions within the limbic region. For example, earlier studies that did not separate the hippocampus and the amygdala did not find significant differences between patients with depression and age-matched comparison subjects (17–20). It seems to be of interest in this context that amygdala volumes were found to be enlarged in patients with major depression (15, 21) as well as in patients with temporal lobe epilepsy and dysthymia (22). This fact indicates that the overall volume of the amygdala-hippocampal formation might not be altered in depression but that the volume of the amygdala is larger, while that of the hippocampus is smaller.
The aim of the present study was to compare the hippocampal formation in patients at the time of their first depressive episode to that in matched healthy comparison subjects. We know of no studies to date that have investigated first-episode depression. It is important to determine whether a smaller hippocampal volume predisposes an individual to the development of depression.
Method
Subjects
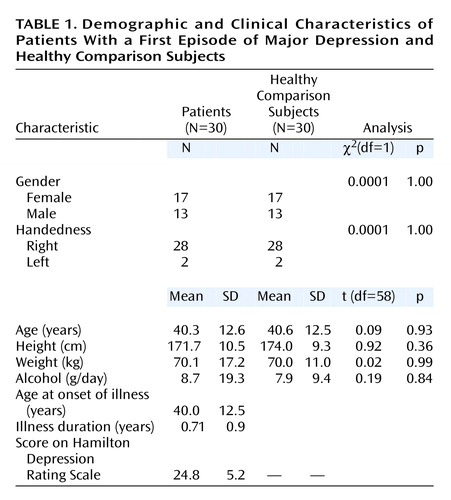
Thirty inpatients with a first episode of major depression seen in the Department of Psychiatry in Ludwig-Maximilians University in Munich were recruited (mean age=40.3 years, SD=12.6, range=18–59) (Table 1). Psychiatric diagnoses based on DSM-IV criteria were determined by a consensus of at least two psychiatrists. Mean duration of illness was 0.71 years (SD=0.9). All subjects were examined by an experienced psychiatrist using a standardized questionnaire. Clinical variables were documented by using the Clinical Global Impression (CGI) scale and the 21-item Hamilton Depression Rating Scale.
For comparison, 30 healthy subjects were matched in a one-to-one fashion with respect to age (mean=40.6 years, SD=12.5, range=19–58), gender, handedness, and educational level with the patients. Moreover, the groups did not differ with regard to height and daily alcohol consumption. Individual age pairings were such that the widest age difference in a pair was 4 years (two pairs). Neither the healthy comparison subjects nor their first-degree relatives had a history of neurological or mental illness. Exclusion criteria for patients and comparison subjects were previous head injury, cortisol or benzodiazepine medication in the previous 3 months, neurological diseases, and comorbidity with other mental illnesses. Handedness was determined by the Edinburgh Inventory (23).
After a complete description of the study was given to the patients with first-episode major depression and the healthy comparison subjects, written informed consent was obtained. The study design was approved by the local ethics committee and was prepared in accordance with the ethical standards laid down in the Declaration of Helsinki.
MRI Procedures
MRI images were obtained (1.5-T Magnetom Vision, Siemens, Erlangen, Germany) by using a coronal T2 proton-density-weighted dual-echo sequence (TR=3710 msec, TE=22/90 msec, total acquisition time=9 minutes, number of acquisitions=1, field of view=230 mm, matrix=240×256, slice thickness=3 mm) and a three-dimensional magnetization-prepared rapid gradient echo sequence (TR=11.6 msec, TE=4.9 msec, total acquisition time=9 minutes, number of acquisitions=1, field of view=230 mm, matrix=512×512, slice thickness=1.5 mm). The commercial software package Analyze (ANALYZE, Biomedical Imaging Resource, Mayo Foundation, Rochester, Minn.) was used for further image processing, with size reduction from 16 to 8 bits and transformation to a uniform matrix of 256×256 on 192 slices of 1.0-mm slice thickness. All data sets were realigned and resampled three dimensionally on the anterior commissure-posterior commissure line according to the coordinates of Talairach with the software program BRAINS (Brain Research: Analysis of Images, Networks, and Systems, developed by Andreasen et al. [24]). Regions of interest were marked with the aid of an interactive cursor-guided system on a computer display of coronal MRI images. BRAINS allowed the regions of interest to be controlled on sagittal and transverse sections simultaneously and allowed segmentation for calculation of the intracranial content and the gray and white matter volumes (cm3) within the defined region of interest.
Definition of the Hippocampal Formation
We used the definition of the hippocampus (Figure 1, A–F) according to Niemann et al. (25) and the detection of the hippocampal-amygdala border from the description of Convit et al. (26). On each scan we began with the most posterior coronal slice in which the hippocampus was clearly detectable (Figure 1, B). Both the fimbria and the subiculum were included in the measurement. More anterior, the hippocampal body and the intralimbic sulcus can be seen (Figure 1, C, D). The shape of the hippocampus can be compared with a rabbit with the head directed vertically, separating the medial ambient cistern from the temporal horn of the lateral ventricles (Figure 1, E). The uncal sulcus then separates the uncus from the underlying parahippocampal gyrus and forms the basal border of the hippocampus. The amygdala-hippocampal transition zone (5) appears as a diffuse area of gray matter between the anterior portion of the hippocampus and the posterior portion of the amygdala (Figure 1, F). This structure can be identified most reliably in the axial plane. This area was transected at its narrowest point. The frontal cleft was used to differentiate the anterior hippocampal head and the amygdala. If the frontal cleft was not visible, the myelin layer of the alveus was used for differentiation from the amygdala. This boundary between hippocampus and amygdala is clearly detectable in the sagittal plane (Figure 1, G). The anterior part of the hippocampus ends where the cornu inferius of the lateral ventricle becomes vertically oriented.
For determination of interrater reliability, 10 brains were randomly chosen, and regions of interest were determined by two raters (T.F., T.Z.) independently (intraclass correlation for interrater reliability of hippocampal gray matter: r=0.97, df=9, p<0.0001; hippocampal white matter: r=0.82, df=9, p=0.008). For intrarater reliability, 10 brains were selected 4 weeks apart by one rater (T.F.) (intraclass correlation for intrarater reliability of hippocampal gray matter: r=0.96, df=9, p<0.0001; hippocampal white matter: r=0.93, df=9, p=0.0002).
Statistical Analyses
Morphometric data were normally distributed. They were subjected to a repeated measurement analysis of covariance (ANCOVA) assessing the main and interaction effects of the within-subjects factors hemisphere (left, right) and the between-subjects factors diagnosis (depression, comparison) and gender (female, male) by using total cranial volume as the cofactor. Significant interactions were resolved by univariate ANCOVAs on the hippocampal volumes for each region and diagnostic group, whereby total cranial volume was controlled. Dependent Student’s t tests were used for post hoc analysis of left-right hemispheric differences. Pearson’s product-moment correlations were used to explore the relationship between hippocampal volumes and age, age at onset of illness, as well as illness duration.
Results
An overview of the demographic characteristics is given in Table 1. Patients with a first episode of major depression did not differ significantly from healthy comparison subjects with respect to age, gender, handedness, education, height, weight, and alcohol consumption. Furthermore, these variables also did not significantly differ between both male and female patients with first-episode depression and their matched healthy comparison subjects. Male and female patients did not differ according to age, age at onset of illness, illness duration, or severity of depression, as measured by the CGI and the Hamilton depression scale. Additionally, there was no significant difference in total brain volumes between patients and comparison subjects.
Gray Matter Hippocampal Volume
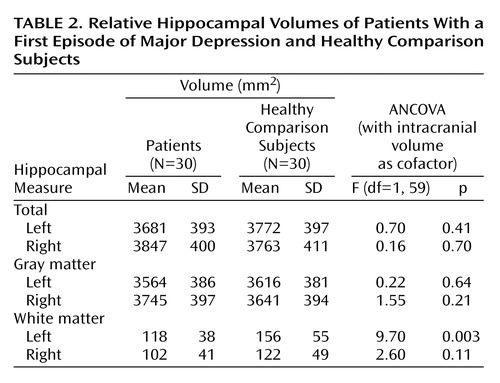
The brain volumetric data are shown in Table 2. No significant main effects on hippocampal gray matter volume were found for diagnosis (F=0.001, df=1, 55, p=0.99). The interaction between diagnosis and gender was significant (F=5.25, df=1, 55, p<0.03). Figure 2 depicts the individual values for men and women. Post hoc univariate ANCOVA revealed that male patients with a first episode of depression had a significantly smaller left hippocampal volume than the healthy male comparison subjects (F=6.36, df=1, 25, p<0.02), whereas the right hippocampal volume was not significantly different (F=0.12, df=1, 25, p=0.73). On the other hand, female patients with a first episode showed a tendency to have a significantly larger right hippocampal volume than female healthy comparison subjects (F=3.81, df=1, 33, p=0.06), whereas no significant differences were detected for the left hemisphere (F=1.66, df=1, 33, p=0.22). The main gender effect was significant, with male subjects having greater hippocampal gray matter volume than female subjects (F=8.34, df=1, 55, p=0.006). The hemisphere effect (F=5.58, df=1, 55, p=0.02) and the interaction of diagnosis and hemisphere (F=8.13, df=1, 55, p=0.006) were significant. Post hoc analysis revealed a significant left-right asymmetry in the first-episode patients (t=3.72, df=29, p=0.001), whereas the healthy comparison subjects did not show significant hemispheric differences (t=0.65, df=29, p=0.52) (Figure 2). The interactions of hemisphere and gender (F=0.91, df=1, 55, p=0.34) and of diagnosis, hemisphere, and gender (F=1.10, df=1, 55, p=0.30) did not reveal significant effects. The statistical analysis for total hippocampal volumes showed nearly similar results, in comparison to hippocampal gray matter volumes.
White Matter Hippocampal Volume
Diagnosis had a significant effect on hippocampal white matter volumes (F=7.54, df=1, 55, p=0.008). First-episode patients with major depression exhibited a significantly smaller hippocampal white matter volume than healthy comparison subjects (Figure 3). Furthermore, a significant main effect was found for hemisphere (F=6.11, df=1, 55, p<0.02), whereas the interactions of hemisphere and diagnosis (F=2.88, df=1, 55, p<0.10) and of hemisphere and gender (F=1.91, df=1, 55, p=0.17) were not significant. No significant main gender effect was found (F=0.54, df=1, 55, p=0.47).
Clinical Variables
Pearson’s correlations between age and hippocampal volumes were not significant for either healthy comparison subjects or patients with depression. Furthermore, age at onset of illness and illness duration were not significantly correlated with hippocampal volumes. Inclusion of the cofactor age in a partial correlation also did not produce a significant relationship between age at onset of illness or duration of illness and hippocampal volume.
Discussion
The present study investigated structural abnormalities of the hippocampus in patients during their first episode of major depression in order to determine whether structural changes in this core region precede the first manifestation of depression. To our knowledge, this is the first study to examine with high-resolution in vivo MRI hippocampal volumes in male and female patients affected by a first episode of major depression.
Our data suggest that male patients with a first episode of major depression have significantly smaller left hippocampal total and gray matter volumes than healthy male comparison subjects. This seems to conflict with epidemiologic studies that have consistently shown that the prevalence of major depression is greater in female than in male patients (27, 28). The greater prevalence in female patients does not seem to be due either to differences in rates of reported stressful life events or to differential sensitivity to their pathogenetic effects (29). However, some studies have indicated gender differences in the development of the brain and its reaction to stress and neurotoxic substances.
Gender differences in the development and functional organization of the brain are well known (30, 31). Moreover, a substantial number of brain diseases seem to affect male patients more severely than female patients. For example, previous studies have shown that male patients with temporal lobe epilepsy have more pronounced brain atrophy than female patients and may be more vulnerable to seizure-associated brain abnormalities (32). One possible explanation for gender-specific findings might be the neuroprotective effects of estrogens, which were shown in animal studies (33, 34) and in investigations of stroke patients (35) and by the fact that testosterone exacerbates vulnerability to neurotoxic processes (36).
Experimental studies have revealed decisive gender differences concerning the variety of stress conditions that lead to experimental depression. Of interest, while defeat stress leads to reduced weight gain and increased adrenal and plasma corticosterone levels only in male rats, social instability stress reduces weight gain and induces thymus involution, adrenal hypertrophy, and elevated plasma corticosterone levels only in female rats (37).
In contrast to our findings, Sheline and colleagues (11) demonstrated a direct relationship between both age and hippocampal changes and cumulative illness duration and hippocampal changes in recurrent depression. However, as cumulative illness duration was very low in our first-episode patients who had a small range of duration, we could not necessarily expect to detect a significant correlation of duration and hippocampal volume.
The finding that there is no asymmetry in volumes of the normal human hippocampus is confirmed by anatomical investigations (38). Of interest, left-right asymmetry effects were detected in both male and female patients with a first depressive episode. This left-right asymmetry may be supported by MRI investigations that found—although not statistically tested—19% volume reductions in the left in comparison to 12% in the right hippocampus (15). It is tentative to speculate that the left-right asymmetry found in our first-episode patients might indicate the beginning of volume loss in the area of the left hippocampus.
To our knowledge, the present study also demonstrated for the first time a significantly smaller hippocampal white matter volume in both male and female patients with a first depressive episode in relation to comparison subjects. White matter of the hippocampal formation constitutes only 3% of the total hippocampal formation. The white matter of the hippocampal formation reflects the outgoing fibers of the fimbria and intrahippocampal white matter connections. The outgoing fimbria fibers connect the hippocampal formation with additional structures of the limbic system, which are known as the circuit of Papez (39). Alterations of these connections might favor cognitive as well as emotional disturbances during depression, although there might not be a direct relationship to severity of depression and hippocampal volume in our study. These white matter changes may be the result of an axonal reduction due to cell loss or to primary dystrophic changes of the myelin sheets. Studies of white matter volume reduction may be of special interest because alterations of neurons may influence white matter volume to a greater extent than gray matter volume. In this respect, white matter atrophy could be an indirect indicator of nerve cell loss, since the volume of a nerve cell is much smaller than its myelinated fiber (40).
Two major causal relationships between depression and hippocampal changes have to be considered. Firstly, hippocampal volume loss could result during the acute depressive episode from dynamic state factors, like vascular hypoperfusion, or trait factors, like cell loss or atrophy due to toxic substances. The numbers of apical dendritic branch points and the length of apical dendrites in hippocampal neurons, particularly in laminar CA3, have been shown to decrease during prolonged stress in animals studied by means of glutamatergic toxic effects. This effect is glucocorticoid dependent and can emerge after a few weeks of overexposure in rodents (41–43). However, the finding that the primate hippocampus, in contrast to the rat brain, has a high density of mineralocorticoid receptors (44) might indicate that this glucocorticoid theory be considered with caution, because mineralocorticoid receptors may serve a neuroprotective role in response to excitotoxic challenges (45). Another possible mechanism might be that the down-regulated expression of brain-derived neurotrophic factor in response to acute and repeated immobilization stress could contribute to atrophy and, in extreme cases, death of stress-sensitive CA3 pyramidal neurons in the hippocampus (46). Furthermore, increased expression of the receptor TrkB, which mediates the action of brain-derived neurotrophic factor, seems to serve to protect hippocampal neurons from damage (47).
Second, it cannot be excluded, although it is highly speculative with regard to the present level of our knowledge, that stressful life events or other biologically effective factors that influence neuronal development (like pre-, peri-, or postnatal infections) may change hippocampal structures in a way that would render confounded subjects more vulnerable to the development of depressive diseases.
We found reductions of hippocampal volumes in male patients and hippocampal left-right asymmetry as well as reduced white matter hippocampal fibers in male and female patients that are in line with the neuroanatomical model of mood regulation, which supposes a role for the interaction of the hippocampus and other brain areas in the pathogenesis of depression. However, it is unclear to what extent the duration of the first episode already had influenced the hippocampus. Thus, longitudinal studies should investigate if or to what extent structural alterations in major depression predispose subjects to depression or are the result of the ongoing disease due to stress-related toxicity.
 |
 |
Received June 13, 2001; revision received Feb. 7, 2002; accepted Feb. 25, 2002. From the Department of Psychiatry and the Department of Radiology (Klinkum Innenstadt), Ludwig-Maximilians University of Munich. Address reprint requests to Dr. Meisenzahl, Department of Psychiatry, Nussbaumstr. 7, 80336 Munich, Germany; [email protected] (e-mail). Supported by the German Federal Research Ministry within the German Research Networks in Medicine promotion as part of the German Research Network on Depression project. The authors thank Nancy C. Andreasen and her staff for their help with the segmentation program BRAINS and Dr. Strauss and B. Burgermeister, who provided technical support.
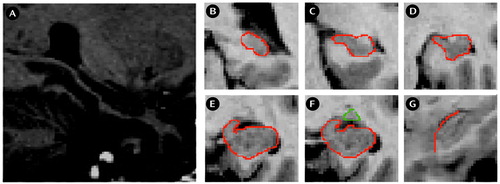
Figure 1. Magnetic Resonance Imaging (MRI) Brain Slices of a Patient With a First Episode of Major Depressiona
aImage A shows a sagittal slice through the temporal lobe and hippocampal formation. Images B through F are coronal MRI slices that run in the occipitorostral direction; areas of interest are outlined in red. Image B is the most posterior coronal slice in which the hippocampus was clearly detectable. Images C and D show the hippocampal body. In image E, the shape of the hippocampus may be compared to a rabbit with the head directed vertically. Image F shows the amygdala-hippocampal transition area. Image G is a sagittal MRI slice for differentiation of the amygdala from the hippocampus (in red).
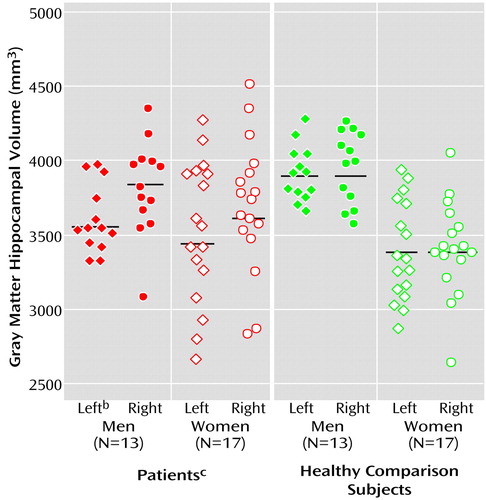
Figure 2. Volumes of Left and Right Hippocampal Gray Matter for Patients With a First Episode of Major Depression and Healthy Comparison Subjects, by Sexa
aThe means are signified by the black lines.
bSignificantly smaller left hippocampal volume in male patients than in male comparison subjects (F=6.36, df=1, 25, p<0.02).
cSignificantly smaller left than right hippocampal volume in all patients (t=3.72, df=29, p=0.001).
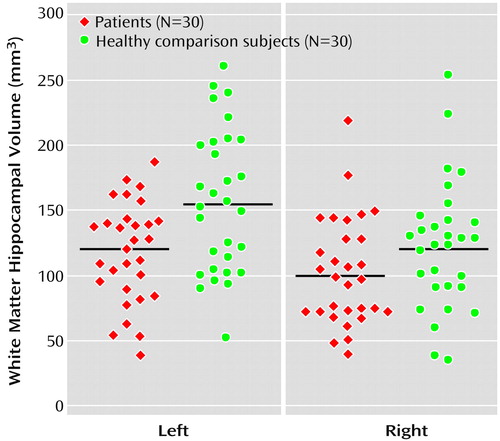
Figure 3. Volumes of Left and Right Hippocampal White Matter for Patients With a First Episode of Major Depression and Healthy Comparison Subjectsa,b
aThe means are signified by the black lines.
bSignificantly smaller overall hippocampal volume in patients than in comparison subjects (F=7.54, df=1, 55, p=0.008).
1. Scoville WB, Millner B: Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry 1957; 20:11-21Crossref, Medline, Google Scholar
2. Damasio AR, Eslinger PJ, Damasio H, Van Hoessen GW, Cornell S: Multinodal amnestic syndrome following bilateral temporal and basal forebrain damage. Arch Neurol 1985; 42:252-259Crossref, Medline, Google Scholar
3. Zola-Morgan SM, Squire LR: The primate hippocampal formation: evidence for a time limited role in memory storage. Science 1990; 250:288-300Crossref, Medline, Google Scholar
4. Gray JA, McNaughton N: Comparison between the behavioural effects of septal and hippocampal lesions: a review. Neurosci Biobehav Rev 1983; 7:119-188Crossref, Medline, Google Scholar
5. Rosene DL, Van Hoesen GW: The hippocampal formation of the primate brain: a review of some comparative aspects of cytoarchitecture and connections, in Cerebral Cortex, vol 6. Edited by Jones EG, Peters A. New York, Plenum, 1987, pp 345-456Google Scholar
6. Salloway S, Cummings J: Subcortical disease and neuropsychiatric illness. J Neuropsychiatry Clin Neurosci 1994; 6:93-99Crossref, Medline, Google Scholar
7. Soares JC, Mann JJ: The anatomy of mood disorders: review of structural neuroimaging studies. Biol Psychiatry 1997; 41:86-106Crossref, Medline, Google Scholar
8. Drevets WC: Functional anatomical abnormalities in limbic and prefrontal cortical structures in major depression. Prog Brain Res 2000; 126:413-431Crossref, Medline, Google Scholar
9. Krishnan KR, Doraiswamy PM, Figiel GS, Husain MM, Shah SA, Na C, Boyko OB, McDonald WM, Nemeroff CB, Ellinwood EH: Hippocampal abnormalities in depression. J Neuropsychiatry Clin Neurosci 1991; 3:387-391Crossref, Medline, Google Scholar
10. Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW: Hippocampal atrophy in recurrent major depression. Proc Natl Acad Sci USA 1996; 93:3908-3913Crossref, Medline, Google Scholar
11. Sheline YI, Sanghavi M, Mintun MA, Gado MH: Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. J Neurosci 1999; 19:5034-5043Crossref, Medline, Google Scholar
12. Steffens DC, Byrum CE, McQuoid DR, Greenberg DL, Payne ME, Blitchington TF, MacFall JR, Krishnan KR: Hippocampal volume in geriatric depression. Biol Psychiatry 2000; 48:301-309Crossref, Medline, Google Scholar
13. Ashtari M, Greenwald BS, Kramer-Ginsberg E, Hu J, Wu H, Patel M, Aupperle P, Pollack S: Hippocampal/amygdala volumes in geriatric depression. Psychol Med 1999; 29:629-638Crossref, Medline, Google Scholar
14. Vakili K, Pillay SS, Lafer B, Fava M, Renshaw PF, Bonello-Cintron CM, Yurgelun-Todd DA: Hippocampal volume in primary unipolar major depression: a magnetic resonance imaging study. Biol Psychiatry 2000; 47:1087-1090Crossref, Medline, Google Scholar
15. Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS: Hippocampal volume reduction in major depression. Am J Psychiatry 2000; 157:115-117Link, Google Scholar
16. Mervaala E, Fohr J, Kononen M, Valkonen-Korhonen M, Vainio P, Partanen K, Partanen J, Tiihonen J, Viinamaki H, Karjalainen AK, Lehtonen J: Quantitative MRI of the hippocampus and amygdala in severe depression. Psychol Med 2000; 30:117-125Crossref, Medline, Google Scholar
17. Swayze VW II, Andreasen NC, Alliger RJ, Yuh WT, Ehrhardt JC: Subcortical and temporal structures in affective disorder and schizophrenia: a magnetic resonance imaging study. Biol Psychiatry 1992; 31:221-240Crossref, Medline, Google Scholar
18. Coffey CE, Wilkinson WE, Weiner RD, Parashos IA, Djang WT, Webb MC, Figiel GS, Spritzer CE: Quantitative cerebral anatomy in depression: a controlled magnetic resonance imaging study. Arch Gen Psychiatry 1993; 50:7-16Crossref, Medline, Google Scholar
19. Axelson DA, Doraiswamy PM, McDonald WM, Boyko OB, Tupler LA, Patterson LJ, Nemeroff CB, Ellinwood EH, Krishnan KR: Hypercortisolemia and hippocampal changes in depression. Psychiatry Res 1993; 47:163-173Crossref, Medline, Google Scholar
20. Pantel J, Schroder J, Essig M, Popp D, Dech H, Knopp MV, Schad LR, Eysenbach K, Backenstrass M, Friedlinger M: Quantitative magnetic resonance imaging in geriatric depression and primary degenerative dementia. J Affect Disord 1997; 42:69-83Crossref, Medline, Google Scholar
21. Frodl T, Meisenzahl EM, Zetzsche T, Bottlender R, Born C, Groll C, Jäger M, Leinsinger G, Hahn K, Möller H-J: Enlargement of the amygdala volume in patients with a first episode of major depression. Biol Psychiatry 2002; 51:708-714Crossref, Medline, Google Scholar
22. Tebartz van Elst L, Woermann FG, Lemieux L, Trimble MR: Amygdala enlargement in dysthymia: a volumetric study of patients with temporal lobe epilepsy. Biol Psychiatry 1999; 46:1614-1623Crossref, Medline, Google Scholar
23. Oldfield RC: The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 1971; 9:97-113Crossref, Medline, Google Scholar
24. Andreasen NC, Cohen G, Harris G, Cizadlo T, Parkkinen J, Rezai K, Swayze VW II: Image processing for the study of brain structure and function: problems and programs. J Neuropsychiatry Clin Neurosci 1992; 4:125-133Crossref, Medline, Google Scholar
25. Niemann K, Hammers A, Coenen VA, Thron A, Klosterkotter J: Evidence of a smaller left hippocampus and left temporal horn in both patients with first episode schizophrenia and normal control subjects. Psychiatry Res 2000; 99:93-110Crossref, Medline, Google Scholar
26. Convit A, McHugh P, Wolf OT, de Leon MJ, Bobinski M, De Santi S, Roche A, Tsui W: MRI volume of the amygdala: a reliable method allowing separation from the hippocampal formation. Psychiatry Res 1999; 90:113-123Crossref, Medline, Google Scholar
27. Boyd JH, Weissmann MM: Epidemiology of affective disorders: a reexamination and future directions. Arch Gen Psychiatry 1981; 38:1039-1046Crossref, Medline, Google Scholar
28. Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB: Sex and depression in the National Comorbidity Survey, I: lifetime prevalence, chronicity and recurrence. J Affect Disord 1993; 29:85-96Crossref, Medline, Google Scholar
29. Kendler KS, Thornton LM, Prescott CA: Gender differences in the rates of exposure to stressful life events and sensitivity to their depressogenic effects. Am J Psychiatry 2001; 158:587-593Link, Google Scholar
30. MacLusky NJ, Naftolin F: Sexual differentiation of the central nervous system. Science 1981; 211:1294-1303Crossref, Medline, Google Scholar
31. Benes FM, Turtle M, Farol P: Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry 1994; 51:477-484Crossref, Medline, Google Scholar
32. Briellmann RS, Berkovic SF, Jackson GD: Men may be more vulnerable to seizure-associated brain damage. Neurology 2000; 55:1479-1485Crossref, Medline, Google Scholar
33. Miller DB, Ali SF, O'Callaghan JP, Laws SC: The impact of gender and estrogen on striatal dopaminergic neurotoxicity. Ann NY Acad Sci 1998; 844:153-165Crossref, Medline, Google Scholar
34. Singer CA, Rogers KL, Dorsa DM: Modulation of Bcl-2 expression: a potential component of estrogen protection in NT2 neurons. Neuroreport 1998; 9:2565-2568Crossref, Medline, Google Scholar
35. Alkayed NJ, Harukuni I, Kimes AS, London ED, Traystman RJ, Hurn PD: Gender-linked brain injury in experimental stroke. Stroke 1998; 29:159-166Crossref, Medline, Google Scholar
36. Nishino H, Nakajima K, Kumazaki M, Fukuda A, Muramatsu K, Deshpande SB, Inubushi T, Morikawa S, Borlongan CV, Sanberg PR: Estrogen protects against while testosterone exacerbates vulnerability of the lateral striatal artery to chemical hypoxia by 3-nitropropionic acid. Neurosci Res 1998; 30:303-312Crossref, Medline, Google Scholar
37. Haller J, Fuchs E, Halsz J, Makara GB: Defeat is a major stressor in males while social instability is stressful mainly in females: towards the development of social stress model in female rats. Brain Res Bull 1999; 1:33-39Crossref, Google Scholar
38. Davidson R, Hugdahi K: Brain Asymmetry. Cambridge, Mass, MIT Press, 1995Google Scholar
39. Papez JW: A proposed mechanism of emotion. Arch Neurol Psychiatry (Chicago) 1937; 38:725-743Crossref, Google Scholar
40. Meier-Ruge W, Ulrich J, Brühlmann M, Meier E: Age-related white matter atrophy in the human brain. Ann NY Acad Sci 1992; 673:260-269Crossref, Medline, Google Scholar
41. Sapolsky RM: Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 2000; 57:925-935Crossref, Medline, Google Scholar
42. Woolley CS, Gould E, McEwen BS: Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res 1990; 531:225-231Crossref, Medline, Google Scholar
43. Reagen L, McEwen B: Controversies surrounding glucocorticoid-mediated cell death in the hippocampus. J Chem Neuroanat 1997; 13:149-167Crossref, Medline, Google Scholar
44. Sanchez MM, Young LJ, Plozky PM, Insel TR: Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. J Neurosci 2000; 20:4657-4668Crossref, Medline, Google Scholar
45. McCullers DL, Herman JP: Blockade of mineralocorticoid receptors increases hippocampal cell death following kainic acid treatment. Abstracts of the Society for Neuroscience 1998; 24:1380Google Scholar
46. Smith MA, Makino S, Kvetnansky R, Post RM: Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci 1995; 15:1768-1777Crossref, Medline, Google Scholar
47. Nibuya M, Morinobu S, Duman RS: Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J Neurosci 1995; 15:7539-7547Crossref, Medline, Google Scholar



