Higher Prevalence of Antibodies to Borrelia Burgdorferi in Psychiatric Patients Than in Healthy Subjects
Abstract
OBJECTIVE: Borrelia burgdorferi infection can affect the CNS and mimic psychiatric disorders. It is not known whether Borrelia burgdorferi contributes to overall psychiatric morbidity. The authors compared the prevalence of antibodies to Borrelia burgdorferi in groups of psychiatric patients and healthy subjects to find out whether there is an association between this infection and psychiatric morbidity. METHOD: Between 1995 and 1999 the authors screened for antibodies to Borrelia burgdorferi in 926 psychiatric patients consecutively admitted to Prague Psychiatric Center. They compared the results of this screening with findings from 884 consecutive healthy subjects who took part in an epidemiological survey of antibodies to Borrelia burgdorferi in the general population of the Czech Republic. Sera were tested by means of enzyme-linked immunosorbent assay. Circulating immune complexes were isolated by polyethylene glycol precipitation. To control for potential confounders, the two groups of patients and healthy subjects were matched according to gender and age. Results were obtained in a sample of 499 matched pairs. RESULTS: Among the matched pairs, 166 (33%) of the psychiatric patients and 94 (19%) of the healthy comparison subjects were seropositive in at least one of the four assays. CONCLUSIONS: These findings support the hypothesis that there is an association between Borrelia burgdorferi infection and psychiatric morbidity. In countries where this infection is endemic, a proportion of psychiatric inpatients may be suffering from neuropathogenic effects of Borrelia burgdorferi.
Lyme disease is a multisystem inflammatory disease that may affect the skin, joints, heart, and nervous system. The etiologic agent of Lyme disease is the gram-negative spirochete Borrelia burgdorferi(1). Spirochetes belong to Treponemataceae family. The other notorious member of this family is Treponema pallidum, the etiologic agent of neurosyphilis—the well-known infectious disorder associated with neuropsychiatric disturbances. Around 1900, neurosyphilis accounted for 10%–15% of psychiatric hospital admissions, but today, because of penicillin treatment, it has become an uncommon disorder.
Borrelia burgdorferi is transmitted mainly by tick bites. It is currently the most frequently recognized arthropod-borne infection of the CNS in Europe and the United States (2, 3). Lyme disease, like neurosyphilis, has distinctive stages and a wide range of neurological manifestations. Its relationship to psychiatric illness has been suggested primarily by case studies (4–8). However, such reports cannot exclude the possibility of coincidental comorbidity. To our knowledge, there is only one epidemiologic survey of the prevalence of Borrelia burgdorferi infection in a psychiatric population. Nadelman et al. (9) found only one subject with antibodies to Borrelia burgdorferi among 517 psychiatric patients from an area in the United States where the prevalence of antibodies to Borrelia burgdorferi among the healthy population was less than 1%.
The aim of the current study was to compare the seroprevalence of antibodies to Borrelia burgdorferi in representative samples of psychiatric patients and healthy comparison subjects. A higher percentage of antibody-positive subjects among psychiatric patients would suggest that there is an association between Borrelia burgdorferi and psychiatric morbidity. The extent of the difference, if any, would help to ascertain the potential relevance of Borrelia burgdorferi in psychiatric epidemiology.
Method
Subjects
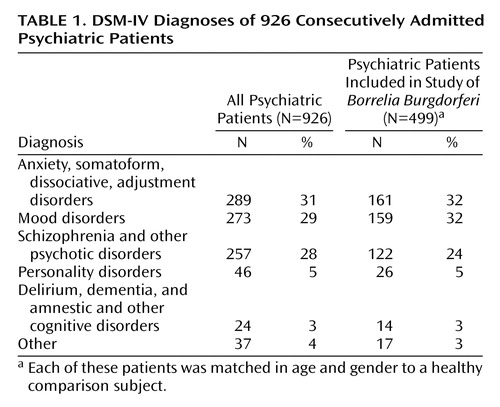
A total of 926 psychiatric patients consecutively admitted to Prague Psychiatric Center between 1995 and 1999 participated in the study. Prague Psychiatric Center is a teaching psychiatric hospital of the School of Medicine, Charles University, Prague. The DSM-IV diagnoses of all 926 patients as well as of the smaller group of 499 patients included in the study are shown in Table 1. The comparison group consisted of 884 consecutive healthy subjects who were recruited during the same period for an epidemiological survey of antibodies to Borrelia burgdorferi in the general healthy population of the Czech Republic. Exclusion criteria for the comparison subjects were acute illness, hospitalization, or immunodeficiency.
Because there were significant differences in age and gender between the two groups and because both age and gender are significantly related to at least some Borrelia burgdorferi markers, we analyzed a subgroup of matched pairs to control for these confounders. The matching was done according to the following rules: each psychiatric patient was matched with a healthy subject of the same gender and age (within 1 year); patients with no matching comparison subject were excluded from the analysis; if there was more than one match, one was chosen randomly.
The project was approved by the hospital’s ethics committee. The comparison group consented specifically to screening for antibodies to Borrelia burgdorferi, and the patient group provided written consent for routine screening and diagnostic assessments. The requirement to collect a separate written informed consent for inclusion of both data sets in this study was waived because the study consisted of a retrospective data analysis. Contacting the subjects would be difficult and was considered not essential because of the anonymous nature of the study. All subject data were anonymous.
Borrelia Burgdorferi Antibody Detection
Samples of sera were analyzed by the National Reference Laboratory for Lyme Disease of the Czech Republic. Coded samples from both groups arrived at the laboratory during the same period of time. The laboratory technicians were not aware of the samples’ origin. Whole cell sonicate enzyme-linked immunosorbent assay (ELISA) with Borrelia afzelii Kc90 Cz was applied (10, 11). Serum immunoglobulin (Ig) G extinction values greater than 900 and serum IgM values greater than 1000 were defined as positive.
Circulating immune complexes were isolated by polyethylene glycol precipitation (12, 13). Dissociated serum immune complexes were analyzed by ELISA for anti-Borrelia IgM and IgG antibody simultaneously with patients’ serum samples and with seven negative and eight positive comparison samples. Each test was run in duplicate. Serum circulating immune complex IgG values greater than 900 and serum circulating immune complex IgM values greater than 1200 were defined as positive. We also performed recombinant immunoblot IgM and IgG (BAG Borrelia Blot) to confirm the reliability of ELISA (14).
Statistical Analysis
Tests for equality of two proportions (by means of U statistics) were used to compare the percentage of antibody-positive subjects in the two groups for each of the four assays and for having a positive finding in at least one of them (15). As a control, McNemar chi-square tests were also used to compare these proportions in matched groups. To assess the effect of age and gender on the immunological findings, we used t tests for independent samples and Yates-corrected chi-square tests, as appropriate. All tests were two-tailed.
Results
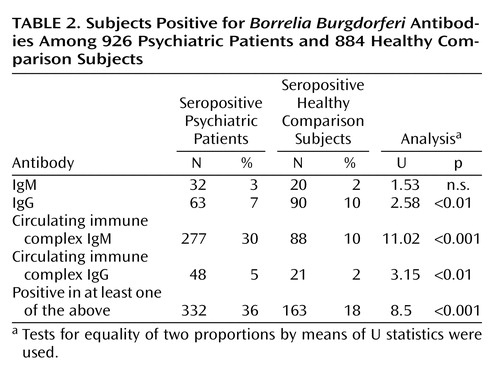
In the comparison of all patients and comparison subjects, 332 (36%) of 926 psychiatric patients and 163 (18%) of 884 comparison subjects were seropositive in at least one antibody class (Table 2).
The mean age of the psychiatric patients was 36 years (SD=14, range=16–99), and 377 (41%) were men. The mean age of the healthy subjects was 27 years (SD=20, range=1–82), and 422 (48%) were men (t=–10.13, df=1808, p<0.001 for age and Yates-corrected χ2=8.77, df=1, p<0.01 for gender). Both age (t=–2.27, df=1808, p<0.05) and gender (Yates-corrected χ2=7.63, df=1, p<0.01) were associated with seropositivity.
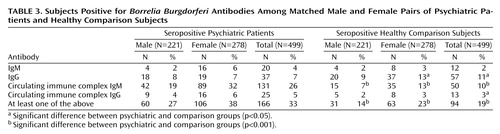
Matched groups consisted of 499 subjects each; 221 (44%) of the pairs were male. The mean age of the psychiatric patients was 36.2 years (SD=15.3, range=16–82), and the mean age of the comparison subjects was 36.1 (SD=15.3, range=16–82). Matching did not affect the proportion of subjects positive for each antibody type significantly and caused only a small reduction in the difference between the groups (Table 3). Among the matched subjects, 166 (33%) of the psychiatric patients and 94 (19%) of the healthy subjects were positive in at least one antibody class (test for equality of two proportions, U=5.3, p<0.001, McNemar χ2=25.21, df=1, p<0.001).
More women than men were seropositive in the comparison group (23% versus 14%) (U=2.52, p<0.001) and in the psychiatric patient group (38% versus 27%) (U=2.63, p<0.001). Seropositivity was more frequent in the psychiatric group in both women (U=4.02, p<0.001, McNemar χ2=14.34, df=1, p<0.001) and men (U=3.46, p=0.001, McNemar χ2=10.18, df=1, p<0.001) (total McNemar χ2=25.21, df=1, p<0.001) (Table 3).
There were no significant differences between the groups in number of IgM-positive subjects (women U=1.67, p=0.1, McNemar χ2=2.04, df=1, p=0.15; men, U=0.000, p=1.0, McNemar χ2=0.13, df=1, p=0.72; total, U=1.43, p=0.15, McNemar χ2=1.53, df=1, p=0.22). There were more IgG-positive healthy women (U=2.55, p=0.01, McNemar χ2=6.02, df=1, p=0.01) and more IgG-positive healthy subjects overall (U=2.17, p<0.05, McNemar χ2=4.4, df=1, p<0.05) and no significant differences in IgG in men (U=0.34, p=0.73, McNemar χ2=0.029, df=1, p=0.86). There were more circulating immune complex IgM-positive psychiatric patients (women, U=5.66, p<0.001, McNemar χ2=27.01, df=1, p<0.001; men, U=3.9, p=0.0001, McNemar χ2=13.26, df=1, p<0.001; total, U=6.8, p<0.001, McNemar χ2=41.29, df=1, p<0.001). There were more circulating immune complex IgG-positive psychiatric patients overall (U=1.99, p=0.05, McNemar χ2=3.18, df=1, p=0.07), although the differences were not significant for each gender separately (women, U=1.67, p=0.09, McNemar χ2=2.04, df=1, p=0.15; men, U=1.09, p=0.28, McNemar χ2=0.64, df=1, p=0.42) (Table 3).
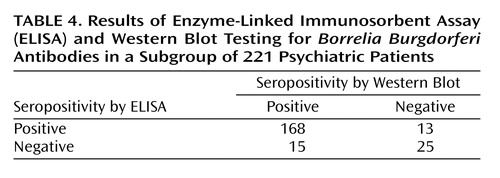
To verify the reliability of ELISA, samples from 221 psychiatric patients were examined by Western blot test. Agreement between the two tests was 87% overall, with 92% sensitivity and 66% specificity of ELISA as assessed by Western blot (Table 4).
Discussion
One-third of the psychiatric patients had serological signs of past Borrelia burgdorferi infection. This rate is 1.7 times higher than the rate in matched comparison subjects.
Our results contrast with those of Nadelman et al. (9), who reported only 0.2% Borrelia burgdorferi seropositive subjects among psychiatric patients in a region of the United States where prevalence of Borrelia burgdorferi is below 1%. The much higher prevalence of antibodies to Borrelia burgdorferi in our study is typical for several areas of Europe (16). The U.S. study used an older method (fluorescent immunoassay) in 90% of cases and ELISA in only 10%. It did not include antibodies bound in circulating immune complexes, where we found the highest number of positive cases. It is also possible that different genospecies of Borrelia burgdorferi may be associated with different clinical manifestations (17). In the United States, Borrelia burgdorferi predominates, but in Europe, Borrelia burgdorferi,Borrelia garinii, and Borrelia afzelii are all detected.
We controlled for between-group differences in age and gender in the analysis. However, there are other potential confounding factors that need to be clarified and considered in future research. These include false positive results, reliability of the laboratory tests, hospitalization in psychiatric hospitals, and subjects’ lifestyles.
Sonicate ELISA IgM antibodies can show false positive results in patients with acute Epstein Barr virus, Varicella zoster virus, and cytomegalovirus infections owing to polyclonal B lymphocyte stimulation (3). These infectious agents have been reported to be occasionally associated with psychiatric symptoms (18). To influence the results of this study, the prevalence of these acute viral infections among psychiatric patients would have to be high and the acute illnesses would have to remain undetected. Also, patients exposed to Treponema pallidum, or other spirochete infections (the prevalence of which is rather small in comparison to prevalence of Lyme borreliosis, let alone of Borrelia burgdorferi antibodies), and patients suffering from rheumatic illnesses (systemic sclerosis or systemic lupus erythematosus) could show false positive results. It is unlikely that these patients would remain undiagnosed and treated in our clinic. To make sure that we were not dealing with false positive results, we used Western blot to test samples from a subgroup of 221 patients. In 93% the Western blot confirmed the ELISA positive results.
In regard to the reliability of the laboratory tests, samples from both groups were analyzed in the same laboratory, and the technicians performing the analyses were blind to sample allocation.
At least some patients might have encountered Borrelia burgdorferi in psychiatric hospitals, which are often situated in parks with mature trees and bushes. However, IgM antibodies to Borrelia burgdorferi appear only days to weeks after the infection. In our patients, the blood samples for the serological assessment were taken immediately after their admission. There is still a possibility, however, that some patients might have been infected during previous stays in other psychiatric hospitals.
Psychiatric patients may differ from healthy subjects in variables related to lifestyle that could increase the risk of Borrelia burgdorferi infection. Exposure to ticks in the urban population is usually associated with outdoor activities. Such activities are not likely to be particularly more common in psychiatric patients. However, in rural areas, the lifestyle variables may be relevant. Also, some psychiatric patients could have experienced transient homeless situations that could have increased the risk of exposure to infection. This would affect only a small proportion of subjects and for only short periods of time, however. Homelessness is rare in the Czech Republic, and all the patients in this study had a permanent home address.
There are additional limitations to the generalizability of our data. The psychiatric group was recruited in an academic psychiatric facility, which admits patients with unclear symptoms. Psychopathology associated with Borrelia burgdorferi infection may be atypical and thus found more often in this setting than in the general psychiatric population.
In one of the markers, IgG, there was a higher proportion of seropositive healthy women than of women with psychiatric illness. There is no obvious explanation of this finding. One can speculate that active outdoor leisure activities that raise the risk of infection may be less common among female psychiatric patients. However, the possibility cannot be excluded that this is a chance result. Future studies should revisit this issue.
The finding of a high rate of psychiatric patients who are positive for circulating immune complex IgM might suggest that circulating immune complexes are involved in the pathogenesis of psychiatric symptoms associated with Lyme disease. Comparisons of antibody profiles in different manifestations of Lyme disease (e.g., cardiological, rheumatic, neurological, etc.) are warranted.
It is difficult to make any conclusions from our data about how the course of Lyme disease is related to psychiatric symptoms. The first antibodies to be produced early in the infection are IgM, followed by IgG. IgM response peaks 4–6 weeks after the infection but may persist for month to years regardless of the activity of the disease. IgG response peaks 6 weeks after the infection and may also persist for years (19–23). Early after the infection or later in its course, the antibodies are not in excess of antigens and appear in circulating immune complexes only. Complexed antibodies are likely to signify disease activity (24).
If Borrelia burgdorferi is related to psychiatric morbidity, at least two main mechanisms could be responsible. First, patients vulnerable to psychiatric disease may be also more susceptible to Borrelia burgdorferi infection or perhaps to its neurotoxic effects because of genetic or intrauterine factors. Second, Borrelia burgdorferi infection may cause psychiatric symptoms. If the latter, a question arises as to how the same infectious agent can cause different psychiatric disorders. In theory, symptoms may vary according to the location and type of Borrelia burgdorferi-related damage. Alternatively, the infection may lower the host’s resistance, and the symptoms may reflect patient’s specific vulnerability (diathesis).
One approach to clarifying the relationship between Borrelia burgdorferi and psychiatric morbidity would be to examine differences in psychiatric epidemiology across countries, regions, and possibly time periods with different rates of Borrelia burgdorferi. To our knowledge, no such studies exist. Future research should address this issue.
Overall, the most parsimonious explanation seems to be that Borrelia burgdorferi infection can cause psychiatric symptoms, leading to psychiatric diagnosis and treatment, and that perhaps about 10% of psychiatric inpatients may be suffering from neuropathogenic effects of Borrelia burgdorferi. The potential implications of this are considerable. However, this is the first study reporting such a finding, there exist some alternative explanations of our results, and replications are needed. More advanced methods such as polymerase chain reaction analysis will likely improve the accuracy of the diagnosis in the future. To contribute to the clarification of some of these issues, we are currently comparing seropositive and seronegative psychiatric patients with regard to diagnosis, length of hospitalization, family history, and other factors.
 |
 |
 |
 |
Received Dec. 7, 2000; revision received Aug. 14, 2001; accepted Sept. 13, 2001. From Prague Psychiatric Center and the Department of Epidemiology, Charles University, Third Faculty of Medicine, Prague, Czech Republic; and Barts and The London School of Medicine and Dentistry, University of London, U.K. Address reprint requests to Dr. Hájek, Psychiatric Center Prague, Ústavní 91, 181 03, Praha 8, Bohnice, Czech Republic; [email protected] (e-mail). Supported by grant 2934-5 from the Internal Grant Agency of the Czech Republic.
1. Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP: Lyme disease—a tick-borne spirochetosis? Science 1982; 216:1317-1319Crossref, Medline, Google Scholar
2. Mrazek V, Bartunek P: [Lyme borreliosis]. Cas Lek Cesk 1999; 138:329-332 (Czech)Medline, Google Scholar
3. Kaiser R: Neuroborreliosis. J Neurol 1998; 245:247-255Crossref, Medline, Google Scholar
4. Fallon BA, Nields JA: Lyme disease: a neuropsychiatric illness. Am J Psychiatry 1994; 151:1571-1583Link, Google Scholar
5. Fallon BA, Nields JA, Parsons B, Liebowitz MR, Klein DF: Psychiatric manifestations of Lyme borreliosis. J Clin Psychiatry 1993; 54:263-268Medline, Google Scholar
6. Roelcke U, Barnett W, Wilder-Smith E, Sigmund D, Hacke W: Untreated neuroborreliosis: Bannwarth’s syndrome evolving into acute schizophrenia-like psychosis: a case report. J Neurol 1992; 239:129-131Crossref, Medline, Google Scholar
7. Halperin JJ, Luft BJ, Anand AK, Roque CT, Alvarez O, Volkman DJ, Dattwyler RJ: Lyme neuroborreliosis: central nervous system manifestations. Neurology 1989; 39:753-759Crossref, Medline, Google Scholar
8. Fallon BA, Schwartzberg M, Bransfield R, Zimmerman B, Scotti A, Weber CA, Liebowitz MR: Late-stage neuropsychiatric Lyme borreliosis: differential diagnosis and treatment. Psychosomatics 1995; 36:295-300Crossref, Medline, Google Scholar
9. Nadelman RB, Herman E, Wormser GP: Screening for Lyme disease in hospitalized psychiatric patients: prospective serosurvey in an endemic area. Mt Sinai J Med 1997; 64:409-412Medline, Google Scholar
10. Jirous J, Pokorny J, Zastera M, Doutlik S, Bojar M: First experience with ELISA serosurvey for tick-borne borreliosis (Lyme disease) in Czechoslovakia. J Hyg Epidemiol Microbiol Immunol 1988; 32:169-172Medline, Google Scholar
11. Stiernstedt G, Dattwyler R, Duray PH, Hansen K, Jirous J, Johnson RC, Karlsson M, Preac-Mursic V, Schwan TG: Diagnostic tests in Lyme borreliosis. Scand J Infect Dis Suppl 1991; 77:136-142Medline, Google Scholar
12. Digeon M, Laver M, Riza J, Bach JF: Detection of circulating immune complexes in human sera by simplified assays with polyethylene glycol. J Immunol Methods 1977; 16:165-183Crossref, Medline, Google Scholar
13. Schutzer SE, Coyle PK, Dunn JJ, Luft BJ, Brunner M: Early and specific antibody response to OspA in Lyme disease. J Clin Invest 1994; 94:454-457Crossref, Medline, Google Scholar
14. Ma B, Christen B, Leung D, Vigo-Pelfrey C: Serodiagnosis of Lyme borreliosis by Western immunoblot: reactivity of various significant antibodies against Borrelia burgdorferi. J Clin Microbiol 1992; 30:370-376Medline, Google Scholar
15. Eberhardt KR, Fligner MA: A comparison of two tests for equality of two proportions. Am Statistician 1997; 31:151-155Google Scholar
16. Gustafson R, Svenungsson B, Gardulf A, Stiernstedt G, Forsgren M: Prevalence of tick-borne encephalitis and Lyme borreliosis in a defined Swedish population. Scand J Infect Dis 1990; 22:297-306Crossref, Medline, Google Scholar
17. van Dam AP, Kuiper H, Vos K, Widjojokusumo A, de Jongh BM, Spanjaard L, Ramselaar AC, Kramer MD, Dankert J: Different genospecies of Borrelia burgdorferi are associated with distinct clinical manifestations of Lyme borreliosis. Clin Infect Dis 1993; 17:708-717Crossref, Medline, Google Scholar
18. Srikanth S, Ravi V, Poornima KS, Shetty KT, Gangadhar BN, Janakiramaiah N: Viral antibodies in recent onset, nonorganic psychoses: correspondence with symptomatic severity. Biol Psychiatry 1994; 36:517-521Crossref, Medline, Google Scholar
19. Feder HM, Gerber MA, Luger SW, Ryan RW: Persistence of serum antibodies to Borrelia burgdorferi in patients treated for Lyme disease. Clin Infect Dis 1992; 15:788-793Crossref, Medline, Google Scholar
20. Karlsson M, Mollegard I, Stiernstedt G, Wretlind B: Comparison of Western blot and enzyme-linked immunosorbent assay for diagnosis of Lyme borreliosis. Eur J Clin Microbiol Infect Dis 1989; 8:871-877Crossref, Medline, Google Scholar
21. Aguero-Rosenfeld ME, Nowakowski J, Bittker S, Cooper D, Nadelman RB, Wormser GP: Evolution of the serologic response to Borrelia burgdorferi in treated patients with culture-confirmed erythema migrans. J Clin Microbiol 1996; 34:1-9Medline, Google Scholar
22. Hammers-Berggren S, Lebech AM, Karlsson M, Svenungsson B, Hansen K, Stiernstedt G: Serological follow-up after treatment of patients with erythema migrans and neuroborreliosis. J Clin Microbiol 1994; 32:1519-1525Medline, Google Scholar
23. Cooke WD, Bartenhagen NH: Seroreactivity to Borrelia burgdorferi antigens in the absence of Lyme disease. J Rheumatol 1994; 21:126-131Medline, Google Scholar
24. Schutzer SE, Coyle PK, Belman AL, Golightly MG, Drulle J: Sequestration of antibody to Borrelia burgdorferi in immune complexes in seronegative Lyme disease. Lancet 1990; 335:312-315Crossref, Medline, Google Scholar



