Randomized Controlled Trial of Motivational Interviewing, Cognitive Behavior Therapy, and Family Intervention for Patients With Comorbid Schizophrenia and Substance Use Disorders
Abstract
OBJECTIVE: Comorbidity of substance abuse disorders with schizophrenia is associated with a greater risk for serious illness complications and poorer outcome. Methodologically sound studies investigating treatment approaches for patients with these disorders are rare, although recommendations for integrated and comprehensive treatment programs abound. This study investigates the relative benefit of adding an integrated psychological and psychosocial treatment program to routine psychiatric care for patients with schizophrenia and substance use disorders. METHOD: The authors conducted a randomized, single-blind controlled comparison of routine care with a program of routine care integrated with motivational interviewing, cognitive behavior therapy, and family or caregiver intervention. RESULTS: The integrated treatment program resulted in significantly greater improvement in patients’ general functioning than routine care alone at the end of treatment and 12 months after the beginning of the study. Other benefits of the program included a reduction in positive symptoms and in symptom exacerbations and an increase in the percent of days of abstinence from drugs or alcohol over the 12-month period from baseline to follow-up. CONCLUSIONS: These findings demonstrate the effectiveness of a program of routine care integrated with motivational interviewing, cognitive behavior therapy, and family intervention over routine psychiatric care alone for patients with comorbid schizophrenia and alcohol or drug abuse or dependence.
Many studies have shown that the rate of substance use in subjects with severe mental illness is high; estimates of recent or current abuse for community samples range from 20% to 40% (1). These rates are higher than those for the general population (2), and patients with comorbid mental illness and substance abuse disorders (“dually diagnosed” patients) have been a cause for concern because even low levels of substance abuse or dependence represent a risk factor for serious complications, including suicide, poor compliance with treatment, more inpatient stays, violence, and a poor overall prognosis (3, 4). In the United States, difficulties arising from treating individuals with dual diagnoses in either the substance use system or the mental health system or from excluding such patients from both systems have been described (5). This has led to recommendations for the integration of treatments for substance abuse or dependence and mental illness. However, reviews of integrated programs for patients with dual diagnoses (6, 7) suggest that the methodological weaknesses of studies to date prevent drawing any conclusions about the efficacy of treatments.
With these issues in mind, we designed a randomized controlled trial to evaluate the effectiveness of a treatment program for patients with schizophrenia and either drug or alcohol use problems. The program integrated three intervention approaches with routine care: 1) motivational interviewing, 2) individual cognitive behavior therapy, and 3) family or caregiver intervention. The value of the latter two approaches has been evaluated for patients with schizophrenia and no identified substance use problems (8–11). In our current study, these approaches were integrated with an intervention designed to enhance motivation to reduce substance use (12, 13). The rationale for this treatment synthesis has been detailed elsewhere (14). Briefly, the expectations were 1) that the majority of patients would be unmotivated to change their substance use at the outset, 2) that patients’ symptoms might be a factor in the maintenance of substance use but that the drug and alcohol use might exacerbate symptoms, and 3) that family stress might have a particularly detrimental effect on outcomes of patients with dual diagnoses. The aim of this study was to investigate whether the program of interventions had a beneficial effect on illness and substance use outcomes over and above that achieved by routine care.
Method
Design
This was a randomized, controlled, single-blind clinical trial. Patient-caregiver dyads were allocated to either the experimental intervention program plus routine care or routine care alone.
Patient and Caregiver Selection and Allocation
Subjects were entered into the trial as patient-caregiver dyads (for patients with more than one relative or caregiver, the person with the major care role was selected). Inclusion criteria for patients were as follows: 1) a nonaffective psychotic disorder (schizophrenia or schizoaffective disorder according to ICD-10 and DSM-IV criteria), 2) meeting DSM-IV criteria for substance abuse or dependence, 3) in current contact with mental health services, 4) age=18–65 years, 5) a minimum of 10 hours of face-to-face contact with the caregiver per week, and 6) no evidence of organic brain disease, clinically significant concurrent medical illness, or learning disability. Diagnoses were established by an experienced diagnostician (S.W.L.) on the basis of chart review and, when indicated, consensus discussion. No systematic assessment was made of axis II disorders.
Potential subjects were identified by first screening the hospital admission records from the mental health units of three National Health Service hospital trusts in the northwest of England (Tameside & Glossop, Stockport, and Oldham). Patients were approached first for consent, then caregivers of consenting patients were approached for consent. Only when both patient and caregiver provided written informed consent were patients accepted into the study. Patients and caregivers were assessed by using multiple measures before random assignment to one of the two arms in the controlled trial: 1) motivational interviewing, cognitive behavior intervention, and family intervention in addition to routine care, and 2) routine care alone. Individual patients were allocated to each condition by a third party with no affiliation to the study who used a computer-generated randomization list stratified for sex and three types of substance use (alcohol alone, drugs alone, or drugs and alcohol) to ensure equal male-female and substance use representation in each arm of the trial.
Interventions
Integrated intervention program
The planned intervention period was 9 months; sessions took place in the caregivers’ and patients’ homes, except when patients or caregivers expressed a preference for a clinic-based appointment (one individual in the integrated care group expressed this preference). All patients in the study were allocated a family support worker from the voluntary organization Making Space. The services of this support worker included providing information, giving advice on benefits, advocacy, emotional support, and practical help. The frequency and nature of contact with the support worker was decided by mutual agreement between caregiver and support worker. The integrated treatment program attempted to combine three treatment approaches: motivational interviewing, individual cognitive behavior therapy, and family or caregiver intervention. The interventions are described elsewhere (14), and only brief details will be given here.
Motivational interviewing (12) was used to increase motivation for change in those patients who were ambivalent. Key concepts fostered in this style of interviewing are that responsibility for problems and their consequences is left with the patient, and efforts to change are not started before the patient has committed himself or herself to particular goals and strategies. The individual cognitive behavior therapy was modified from approaches used to ameliorate delusions and hallucinations in patients with chronic psychosis (15). The general approach to family intervention was as described elsewhere (16). For the purposes of this study, an emphasis was placed on promoting a family response that was consistent with the motivational interviewing style.
The interventions began with the motivational interviewing phase and five initial weekly sessions designed to assess and then enhance the patient’s motivation to change. If the patient’s commitment was obtained, changes in substance use were negotiated on an individual basis. With the introduction of the individual cognitive behavior therapy at week 6 (or earlier if appropriate), the motivational interviewing style was integrated into subsequent cognitive behavior therapy sessions. The individual cognitive behavior therapy took place over approximately 18 weekly sessions, followed by six biweekly sessions (a total of 29 individual sessions, including the motivational interviewing).
Following assessment of both patients and caregivers, shared goals were generated that became the focus of conjoint patient/family sessions. The family intervention consisted of 10–16 sessions, some of which took the form of integrated family/patient sessions, some of which involved family members alone.
All of the clinicians involved in the trial received training in motivational interviewing style from an experienced interviewer with extensive training in the techniques (J.M.). Six clinicians (five clinical psychologists [C.B., G.H., J.McG., N.T., and Ian Lowens] and one nurse therapist [R.O.]) conducted the cognitive behavior therapies (individual and family). All had experience in cognitive behavior therapy work with psychotic patients and were eligible for accreditation as cognitive behavior therapists with the British Association for Behavioural and Cognitive Psychotherapy. Therapy was detailed in a comprehensive treatment manual (available from C.B.), and the therapists received weekly supervision based on audiotaped sessions to ensure treatment fidelity.
Routine care
Routine care in the context of the National Health Service of Great Britain consists of psychiatric management by the clinical team, coordinated through case management and including maintenance neuroleptic medication, monitoring through outpatient and community follow-up, and access to community-based rehabilitative activities, such as day centers and drop-in clinics. All of the patients in the integrated treatment program also received routine care.
Assessment Procedures and Instruments
Primary and secondary outcomes
Selection of the primary outcome was influenced by the fact that no single measure encompasses both substance use and symptom outcomes for patients with dual diagnoses. To reflect the multicomponent nature of the interventions, the primary outcome selected was change in the Global Assessment of Functioning Scale (DSM-IV, p. 32), a measure that assesses the individual’s overall functioning on a rating scale that ranges from 0 to 100. Multiple secondary outcomes were also employed, including measures of patient symptoms and patient substance use. Caregiver outcomes were also assessed but will not be reported on here. Demographic details of patients and caregivers were collected by using a short checklist at the first interview.
The outcome assessment measures were administered at three time points: before random assignment to the two treatment conditions, immediately after treatment (9 months after the beginning of the study), and 3 months after the end of treatment (12 months after the beginning of the study). Additionally, detailed patient interviews to assess substance use were conducted every 3 months throughout the intervention. The assessments were conducted by independent assessors (two psychology graduate research assistants [N.S. and Joanne Quinn]). The assessors were blind to treatment allocation; attempts to maintain their blindness included use of separate rooms and administrative procedures for project staff, multiple coding of treatment allocations, and requesting subjects not to disclose information about the treatment.
Assessment of patients’ symptoms and functioning
The Global Assessment of Functioning Scale (DSM-IV, p. 32), Positive and Negative Syndrome Scale (17), and Social Functioning Scale (18) were used to assess patients’ symptoms and functioning. Interrater reliability was assessed for the clinician-rated assessments. The two research assistants independently rated a set of 10 randomly selected patients on the Global Assessment of Functioning Scale. Good reliability was found; the intraclass correlation coefficient (ICC) was 0.93. The interrater reliability of the assessors on the Positive and Negative Syndrome Scale was established before the study by computing ICCs for the ratings of 14 videotaped interviews by the assessors and an experienced research psychiatrist external to the study. For the Positive and Negative Syndrome Scale positive subscale, ICC=0.83; for the negative subscale, ICC=0.88; and for total score, ICC=0.95.
Medication compliance
The Drugs Attitude Inventory (19), a self-report scale shown to be highly predictive of compliance, was used to measure medication compliance.
Patient relapse outcomes
Two methods of assessing the frequency and duration of relapse were used for relapses in the 2 years before the study and during the study period: 1) number and duration of hospital admissions, identified from hospital record systems, and 2) number and duration of exacerbations of symptoms lasting longer than 2 weeks and requiring a change in patient management (increased observation and/or medication change by the clinical team), assessed from hospital case notes. When symptom exacerbation preceded hospitalization, only one relapse was recorded. Reliability for number and duration of exacerbations was checked by comparing ratings for 10 randomly selected patients. No differences were found between the two independent assessors for these variables.
Patients’ substance use
“Timeline follow-back” interviewing techniques (20) were used to collect data on substance use behavior. Briefly, the technique involves asking individuals to reconstruct their drug use/drinking behavior over a specified interval. For the purposes of the current study 3-month intervals were used: 1) patient timeline follow-back interviews were conducted before patients were randomly assigned to the two treatments and collected data for the 3 months before the start of the study, 2) they were conducted at 3 and 6 months during treatment, and 3) they were conducted 9 months and 12 months after the beginning of treatment. At all assessment points, details of behavior for all substance use (alcohol and nonprescribed drugs) were sought, irrespective of specific use at baseline. Two variables were computed for evaluating outcomes: percent of days of abstinence from the most frequently used substance (identified from the Addiction Severity Index [21]) and percent of days of abstinence from all substances.
The drug and alcohol subscales of the Addiction Severity Index (21) were used. The scores used for analyses were the composite scores for responses to a set of items based on the last 30 days (thus capable of showing change). Composite scale scores range from 0 to 1. Adequate psychometric properties for use with patients with dual diagnoses have been published (22, 23). In order to compare scores between individuals who differed in type of substance use, the two composite scores (drug and alcohol) were summed.
The Leeds Dependence Questionnaire (24) is a 10-item questionnaire used to measure psychological dependence across a wide range of substances. In order to compare individuals using different substances, Leeds Dependence Questionnaires completed for the patient’s most frequently used substance were selected for outcome assessments. The alpha coefficient for patients completing the most frequently used substance scale at baseline was 0.85, showing that the scale has good internal consistency.
The Alcohol Use Scale and the Drug Use Scale of the Clinician Rating Scales (25) were employed at the start of the study to permit comparison between patient and clinician reports of substance use. These scales were completed by the patient’s key worker. Each scale consists of five points (1=abstinence to 5=dependence with institutionalization), and the rater is encouraged to use all available information sources in making a judgment. In comparing the Clinician Rating Scales with self-report measures, the scale appropriate to the patients’ most frequently used substance was selected (the Alcohol Use Scale or the Drug Use Scale).
Concurrent Validity of Substance Use Measures
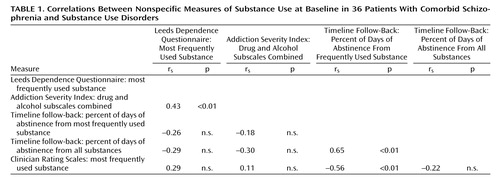
Spearman’s correlation coefficient (rs) (26) was used to create a correlation matrix of self-report and clinician-rated substance use measures taken at baseline (Table 1). There was a significant correlation between the two questionnaire measures of substance use (the Addiction Severity Index drug and alcohol subscales combined and the Leeds Dependence Questionnaire). Scores on the Clinician Rating Scales showed good association with the data from the timeline follow-back interviews; greater substance use rated by clinicians was associated with a smaller percent of days of abstinence for the most frequently used substances. As would be expected, there was greater association between self-report measures that examined all substance use. In summary, there was substantial concurrent validity for the measures employed in the study.
Analyses
All analyses were conducted on an intent-to-treat basis. Patient deaths were treated as relapses, and subject attrition did not affect the analyses of relapse outcomes because relapse was assessed from service records. When scores from assessment measures deviated significantly from a normal distribution, log-transformed scores were used, and when distributions remained skewed or there was significant kurtosis, nonparametric statistics were used. Nonparametric analyses used included the Mann-Whitney test (U), the Wilcoxon matched-pairs signed-ranks test (z), and Spearman’s correlation coefficient for ranked data (rs) (26). Two-tailed tests of significance were used in all analyses.
Results
Participant Flow and Follow-Up
From the 66 eligible patient-caregiver pairs invited to participate in the study, 23 (35%) patients and a further seven (11%) caregivers refused, making a total 30 (45%) refusers.
Using information from hospital records, we compared refusers and participants on a number of patient variables, including household composition, age, sex, employment, type of substance, and illness history characteristics. Patients who refused were significantly older (median=35.0 years, range=21–57, versus median=30.50 years, range=17–62) (U=299.0, p<0.03); had a longer duration of illness dated from their first admission (median=7.50 years, range=1–23, versus median=4.00 years, range=1–19) (U=273.0, p<0.05); and had fewer admissions in the last 3 years (median=1, range=0–4, versus median=2, range=0–7) (U=286.5, p<0.05). No other differences were found between the two groups.
The final study group consisted of 36 patient-caregiver dyads. Thirty-three (92%) of the patients were male; the mean age of the patients was 31.1 years (SD=9.69); mean illness duration was 8.4 years (SD=8.44); mean number of hospitalizations was 4.9 (SD=4.08); and all were of white European ethnic origin. Eighteen patients lived with their caregiver. There were no differences between the patients who participated in the integrated program and those given routine care on any of the demographic or illness history variables.
Nineteen of the patients used both drugs and alcohol, 11 used alcohol only, and six used drugs only. There was no difference between groups in the distribution of drug and alcohol use. Fifteen patients used only one substance: 11 used alcohol only, three cannabis only, and one amphetamines only. Ten patients used only cannabis with alcohol, and one used only heroin and alcohol. Ten patients used multiple drugs. The drug used by most patients was cannabis (22 patients), followed by amphetamines (N=10), cocaine (N=4), and heroin (N=4).
At baseline, all patients scored above the cutoff score of 5 for a clinically significant substance use problem in psychiatric populations on either the Drug Abuse Screening Test (27) or the Michigan Alcoholism Screening Test (28). From the timeline follow-back data, the mean number of days per week of use (for all substances) was 5.2 (SD=1.8). There was no difference in Drugs Attitude Inventory scores between patients participating in the integrated program (median=15.0, range=–22 to 24) and those given routine care (median=11, range=–10 to 26) (U=157.0, n.s.).
Of the caregivers, 27 were women and nine were men; their mean age was 51 (SD=12.12). In terms of relationships, the majority (N=24, 66.7%) were parents, six (16.7%) were partners, and the remainder were one sibling, one grandparent, two landladies, and two ex-partners. For all caregiver variables assessed, including expressed emotion status (29), there were no statistical or clinical differences between the groups at baseline.
There were three deaths during the 9-month intervention period: one in the integrated care group (heart attack) and two in the routine care group (one drug overdose and one fall from a high bridge). One additional patient in the routine care group refused to complete assessments at the end of 9 months of treatment and at the 3-month follow-up, and one patient in the integrated care group refused to complete the assessment at the end of 9 months of treatment. Thus the final numbers of patients after treatment were 17 in the integrated care group and 15 in the routine care group at 9 months and 17 in the integrated care group and 15 in the routine care group at 12 months.
Participation in the Integrated Program
The median number of family intervention sessions was 11 (range=1–20). For individual cognitive behavior therapy intervention, the median number of sessions was 22 (range=0–29). The number of support worker contacts with patients was significantly higher for the patients receiving routine care (median=7.5, range=0–21) than for those in the integrated program (median=4, range=0–10) (U=78.5, N=36, p<0.008); however, there were no differences in number of contacts with caregivers in the two groups (routine care group median=4.5, range=0–22, integrated care group median=5.5, range=1–17).
Patient Outcomes
Symptoms and functioning
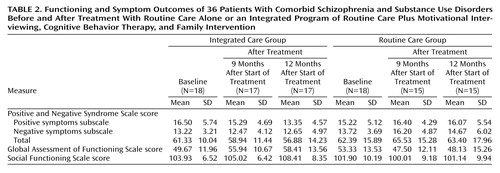
Table 2 gives the scores for the two groups of patients on measures of global functioning, symptoms, and social functioning. Actual means and standard deviations are given in this table, but in the text we report adjusted means and standard errors. To compare the effects between the groups on the outcome measures from baseline to just after the 9-month treatment period and from baseline to 12 months after the beginning of treatment, analyses of covariance were used with the baseline scores entered as the covariate.
For the primary outcome of interest, patients’ level of functioning, there was a superior result for the group given integrated treatment according to Global Assessment of Functioning Scale scores at both 9 months (adjusted mean=57.2, SE=2.11, versus adjusted mean=46.16, SE=2.17) (F=13.11, df=1, 30, p<0.001) and 12 months (adjusted mean=60.14, SE=2.47, versus adjusted mean=46.28, SE=2.54) (F=15.06, df=1, 30, p=0.001).
For the Positive and Negative Syndrome Scale, the integrated care group had lower scores on the positive symptoms subscale over time, and the routine care group had slightly higher scores. This difference was not significant at 9 months (adjusted mean=14.8, SE=0.8, versus adjusted mean=17.0, SE=0.8) (F=3.43, df=1, 29, p<0.07) but was significant at 12 months (adjusted mean=12.85, SE=0.94, versus adjusted mean=16.63, SE=1.00) (F=7.43, df=1, 29, p<0.01). Although there was a statistical difference in the negative symptoms subscale of the Positive and Negative Syndrome Scale in favor of the integrated care group at 9 months (adjusted mean=12.50, SE=0.97, versus adjusted mean=16.17, SE=1.04) (F=6.67, df=1, 29, p<0.02), this difference was not maintained at 12 months (adjusted mean=12.68, SE=1.19, versus adjusted mean=14.63, SE=1.27) (F=1.25, df=1, 29, n.s.). There was no difference between the two groups for Positive and Negative Syndrome Scale total scores at 9 months (integrated care group adjusted mean=58.97, SE=2.69, versus routine care group adjusted mean=65.50, SE=2.88) (F=2.76, df=1, 29, n.s.) or 12 months (integrated care group adjusted mean=56.91, SE=3.21, versus routine care group adjusted mean=63.36, SE=3.42) (F=1.89, df=1, 29, n.s.).
Regarding social functioning, there was no difference between the groups in Social Functioning Scale total scores at 9 months (integrated care group adjusted mean=104.20, SE=1.51, versus routine care group adjusted mean=100.94, SE=1.60) (F=2.15, df=1, 29, n.s.) or at 12 months (integrated care group adjusted mean=107.06, SE=1.53, versus routine care group adjusted mean=102.78, SE=1.68) (F=3.42, df=1, 28, p<0.08).
Relapse
At the end of the 9-month treatment, 10 (55.5%) of 18 patients in the routine care group had at least one relapse, compared with five (27.8%) of 18 patients in the integrated care group (χ2=2.86, df=1, p<0.09). At 12 months the difference in relapse rate was significant: six patients (33.3%) in the integrated care group relapsed, compared with 12 patients (66.7%) in the routine care group (χ2=4.00, df=1, p<0.05). The difference between groups in the total number of days spent in relapse was not significant at 9 months (integrated care group median=0, range=0–79, versus routine care group median=13, range=0–98) (U=117.0, N=33, n.s.) or at 12 months (integrated care group median=0, range=0–112, routine care group median=26, range=0–106) (U=98.0, N=33, p<0.06). Looking at the “mirror images” of days spent in relapse before and after treatment according to the relapse history obtained from case notes, we found that the number of days was significantly smaller for the integrated care group at 9 months but not for the routine care group (z=–2.19, N=17, p<0.03, versus z=–1.02, N=16, n.s.). This advantage for the integrated care group was maintained at 12 months (z=–1.99, N=17, p<0.05, versus z=–0.53, N=15, n.s.).
Substance use disorders
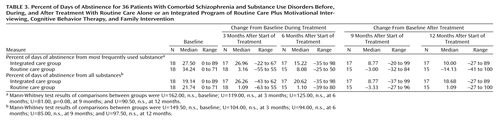
Table 3 gives the baseline values and the change in these values at the four assessment points (3, 6, 9, and 12 months after the beginning of the study) on the two outcomes of interest for the timeline follow-back: percent of days of abstinence for the most frequently used substance and percent of days of abstinence from all substances. Table 3 shows that the integrated care group had a greater increase in percent of days of abstinence over baseline values than the routine care group at all assessment points during and after treatment, although the differences were not significant at any single time point. When the mean change in percent of days of abstinence relative to baseline values over the four time points during and after treatment were compared between the two groups (sum of the percent of days of abstinence at 3, 6, 9, and 12 months subtracted from the baseline value), the percent of days of abstinence from all substances was greater for the integrated care group (median=19.99, range=–25.6 to 83.4, versus median=–6.52, range=–67.9 to 53.2) (U=86.5, p<0.03). However, the difference between groups in the mean percent of days of abstinence relative to baseline for the most frequently used substance was not significant (integrated care group median=17.76, range=–25.6 to 83.4, versus routine care group median=–3.11, range=–46.2 to 54.6) (U=103.0, n.s.).
Leeds Dependence Questionnaire and Addiction Severity Index scores
For the integrated care group, the median baseline score on the Leeds Dependence Questionnaire was 4.5 (range=0–15); for the routine care group, it was 6.0 (range=0–13). The median baseline score of the integrated care group on the Addiction Severity Index was 0.37 (range=0.18–0.60); for the routine care group it was 0.34 (range=0.12–0.77). There were no significant differences in change scores between groups at the posttreatment assessments.
Discussion
This study demonstrates that an intensive treatment program integrating routine care with motivational interviewing, cognitive behavior therapy, and family intervention resulted in significant improvement in the main outcome of patients’ general functioning when compared with routine care alone. There were also significant benefits to patients in terms of some secondary outcomes, including a significant reduction in positive symptoms, a reduction in symptom exacerbations, and an increase in percent of days of abstinence from drugs and alcohol averaged over the 12-month period. Thus, the advantage of the integrated treatment was evident in terms of both symptom improvement and reduction in substance use. The acceptability of treatment to patients was also good, demonstrated by the finding that 94% (N=17) of the 18 patients completed the program. Other studies have found the rate of treatment completion to be low (30, 31), and it has been suggested (7) that treatments taking into account a person’s readiness to change may be more effective in engaging people in treatment.
The relatively small number of subjects in this study is a limitation, and a key issue concerns the potential generalizability of the findings to other patients with comorbid schizophrenia and substance use disorders. Certainly, the demographic characteristics of our study group would seem to be in accord with sex and age biases found in larger studies: substance use in schizophrenia (as in the general population) is more likely to be found in young men (e.g., reference 32). Similarly, the substance use profile of the study group matches the type of substance use most prominent in patients with schizophrenia. A recent review of prevalence studies for substance use in schizophrenia (33) reported that cannabis is the most frequently used drug, that alcohol use frequently occurs with drug use; and that multiple substance use is common. Alcohol is also the most frequently found substance of abuse in this patient population (3).
Little information is available to indicate what percent of patients with comorbid schizophrenia and substance use disorders have contact with their families, or whether patients with family contacts have a different profile of substance use from those without such contacts. However, the poor outcomes for the routine care patients in our study are consistent with reports in the literature for those who have comorbid substance use disorders and severe mental illness. Two-thirds of the patients receiving routine psychiatric care relapsed within 12 months. These patients also experienced a deterioration in general functioning and an increase in positive symptoms of schizophrenia, and there is some indication that they increased their substance use. Therefore, there is some evidence that our patient group was representative of other patients with comorbid schizophrenia and substance use disorders.
Bellack and DiClemente (34) noted that despite the absence of definitive data on specific intervention techniques, researchers appear to have broad agreement about some general requirements for treatment. First, they agree that patients with dual diagnoses need a special program that integrates and coordinates elements of both psychiatric treatment and substance abuse treatment. Second, they agree that treatment needs to match the patient’s stage of change and that the person’s motivation to change is likely to wax and wane. We took these factors into account in designing the treatments used in the study reported here, which gives some empirical support to the efficacy of such integrated treatments for patients with dual diagnoses. However, studies with larger numbers of subjects are required before definitive conclusions can be made about treatment options for this patient group. Additionally, since the integrated care group received considerably more therapy time than did the routine care group, a further limitation of the study design is that we are unable to rule out the possibility that the superior outcomes for the integrated care group were due to the additional attention rather than the specific cognitive behavior therapy interventions that they received.
A related issue is that although the indications are that multifaceted treatments may be required to have an impact on the complex and challenging problems of this patient group, in the longer term, research is needed to examine the relative efficacy of different components of integrated interventions. More work is also required to examine the long-term outcomes and cost benefits of such treatment programs.
 |
 |
 |
Received April 25, 2000; revisions received Oct. 31, 2000, and Feb. 22, 2001; accepted March 6, 2001. From the Academic Division of Clinical Psychology and the Academic Division of Psychiatry, School of Psychiatry and Behavioural Sciences, University of Manchester, UK; and Tameside & Glossop Community & Priority National Health Service Trust, UK. Address reprint requests to Dr. Barrowclough, Academic Unit of Clinical Psychology, Mental Health Unit, Tameside General Hospital, Fountain Street, Ashton-under-Lyne, Lancashire, OL6 9RW, UK; [email protected] (e-mail). Supported by West Pennine, Manchester, and Stockport Health Authorities and Tameside & Glossop National Health Service Trust Research and Development Support funds and by Making Space, the organization for supporting caregivers and sufferers of mental illness. The authors thank Joanne Quinn, Julie Morris, Gina Evans, and Ian Lowens for their help.
1. Mueser KT, Yarnold PR, Levinson DF, Singh H, Bellack AS, Kee K, Morrison RL, Yadalam KG: Prevalence of substance use in schizophrenia: demographic and clinical correlates. Schizophr Bull 1992; 16:31-56Crossref, Google Scholar
2. Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK: Comorbidity of mental disorders with alcohol and other drug abuse: results from the Epidemiological Catchment Area (ECA) study. JAMA 1990; 264:2511-2518Crossref, Medline, Google Scholar
3. Smith J, Hucker S: Schizophrenia and substance abuse. Br J Psychiatry 1994; 165:13-21Crossref, Medline, Google Scholar
4. Lehman AF, Dixon LB: Double Jeopardy: Chronic Mental Illness and Substance Use Disorders. Chur, Switzerland, Harwood Academic, 1995Google Scholar
5. Ridgely MS, Goldman HH, Willenbring M: Barriers to the care of persons with dual diagnosis. Schizophr Bull 1990; 16:123-132Crossref, Medline, Google Scholar
6. Drake RE, Mercer-McFadden C, Mueser KT, McHugo GJ, Bond GR: Review of integrated mental health and substance abuse treatment for patients with dual disorders. Schizophr Bull 1998; 24:589-608Crossref, Medline, Google Scholar
7. Ley A, Jeffery DP, McLaren S, Siegfried N: Treatment programmes for people with both severe mental illness and substance misuse (Cochrane review), in The Cochrane Library, Issue 2. Oxford, UK, Update Software, 1999, pp 1-21Google Scholar
8. Mari J, Streiner DL: Family intervention for those with schizophrenia, in Schizophrenia Module of the Cochrane Database of Systematic Reviews (September 1996): The Cochrane Collaboration, Issue 3. Edited by Adams C, Anderson J, De Jesus Mari J. Oxford, UK, Update Software, 1996, pp 1-24Google Scholar
9. Tarrier N, Yusupoff L, Kinney C, McCarthy E, Gledhill A, Haddock G, Morris J: A randomised controlled trial of intensive cognitive behaviour therapy for chronic schizophrenia. Br Med J 1998; 317:303-307Crossref, Medline, Google Scholar
10. Kuipers E, Garety P, Fowler D, Dunn G, Bebbington P, Freeman D, Hadley C: The London-East Anglia randomised controlled trial of cognitive behaviour therapy for psychosis: effects of the treatment phase. Br J Psychiatry 1997; 171:319-325Crossref, Medline, Google Scholar
11. Sensky T, Turkington D. Kingdon D, Scott JL, Scott J, Siddle R, O’Carroll M, Barnes TRE: Cognitive-behavioral treatment for persistent symptoms in schizophrenia. Arch Gen Psychiatry 2000; 57:165-173Crossref, Medline, Google Scholar
12. Miller WR, Rollnick S: Motivational Interviewing: Preparing People to Change Addictive Behavior. New York, Guilford, 1991Google Scholar
13. Rollnick S, Miller WR: What is motivational interviewing? Behavioural and Cognitive Psychotherapy 1995; 23:325-334Crossref, Google Scholar
14. Barrowclough C: Cognitive behavioural intervention for clients with severe mental illness who have a substance misuse problem. Psychiatr Rehabilitation Skills 2000; 42:216-233Crossref, Google Scholar
15. Haddock G, Tarrier N, Spaulding W, Yusupoff L, Kinney C, McCarthy E: Individual cognitive-behaviour therapy in the treatment of hallucinations and delusions. Clin Psychol Rev 1998; 18:821-838Crossref, Medline, Google Scholar
16. Barrowclough C, Tarrier N: Families of Schizophrenic Patients: A Cognitive-Behavioural Intervention. London, Chapman & Hall, 1992Google Scholar
17. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261-276Crossref, Medline, Google Scholar
18. Birchwood M, Smith J, Cochrane R, Wetton S, Copestake S: The Social Functioning Scale: the development and validation of a scale of social adjustment for use in family intervention programmes with schizophrenic patients. Br J Psychiatry 1990; 157:853-859Crossref, Medline, Google Scholar
19. Hogan TP, Awad AG, Eastwood R: A self-report scale predictive of drug compliance in schizophrenics: reliability and discriminative validity. Psychol Med 1983; 13:177-183Crossref, Medline, Google Scholar
20. Sobell LC, Sobell MB: Timeline follow-back: a technique for assessing self-reported alcohol consumption, in Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Edited by Litten RZ, Allen JP. Totowa, NJ, Humana Press, 1992, pp 41-72Google Scholar
21. McLellan AT, Luborsky L, Woody GE, O’Brien CP: An improved diagnostic evaluation instrument for substance abuse patients: the Addiction Severity Index. J Nerv Ment Dis 1980; 168:26-33Crossref, Medline, Google Scholar
22. Hodgins DC, El-Guebaly N: More data on the Addiction Severity Index. J Nerv Ment Dis 1992; 180:197-201Crossref, Medline, Google Scholar
23. Zanis DA, McLellan AT, Corse S: Is the Addiction Severity Index a reliable and valid assessment instrument among clients with severe and persistent mental illness and substance abuse disorders? Community Ment Health J 1997; 33:213-227Crossref, Medline, Google Scholar
24. Raistrick D, Bradshaw J, Tober G, Weiner J, Allison J, Healey C: Development of the Leeds Dependence Questionnaire (LDQ): a questionnaire to measure alcohol and opiate dependence in the context of a treatment evaluation package. Addiction 1994; 89:563-572Crossref, Medline, Google Scholar
25. Drake RE, Mueser KT, McHugo GJ: Clinician Rating Scales, in Outcomes Assessment in Clinical Practice. Edited by Sederer LI, Dickey B. Baltimore, Williams & Wilkins, 1996, pp 113-116Google Scholar
26. Howell DC: Statistical Methods for Psychology, 4th ed. Belmont, Calif, Wadsworth, 1996, pp 645-664Google Scholar
27. Staley D, el-Guebaly N: Psychometric properties of the Drug Abuse Screening Test in a psychiatric patient population. Addict Behav 1990; 15:257-264Crossref, Medline, Google Scholar
28. Searles JS, Alterman AI, Purtill JJ: The detection of alcoholism in hospitalized schizophrenics: a comparison of the MAST and the MAC. Alcohol Clin Exp Res 1990; 14:557-560Crossref, Medline, Google Scholar
29. Leff J, Vaughn C: Expressed Emotion in Families: Its Significance for Mental Illness. New York, Guilford, 1985Google Scholar
30. Lehman AF, Herron JD, Schwartz RP, Myers CP: Rehabilitation for adults with severe mental illness and substance use disorders: a clinical trial. J Nerv Ment Dis 1993; 181:86-90Crossref, Medline, Google Scholar
31. Burnham MA, Morton SC, McGlynn EA, Peterson LP, Stecher BM, Hayes C, Vaccaro JV: An experimental evaluation of residential and nonresidential treatment for dually diagnosed homeless adults. J Addict Dis 1995; 14:111-134Crossref, Medline, Google Scholar
32. Meuser KT, Yarnold PR, Levinson DF, Singh H, Bellack AS, Kee K, Morrison RL, Yadalam KG: Prevalence of substance abuse in schizophrenia: demographic and clinical correlates. Schizophr Bull 1990; 16:31-56Crossref, Medline, Google Scholar
33. Blanchard JJ, Brown SA, Horan WA, Sherwood AR: Substance use disorders in schizophrenia: review, integration and a proposed model. Clin Psychol Rev 2000; 20:207-234Crossref, Medline, Google Scholar
34. Bellack AS, DiClemente C: Treating substance abuse among patients with schizophrenia. Psychiatr Serv 1999; 50:75-80Link, Google Scholar



