Sensitivity of Schizophrenia Endophenotype Biomarkers to Anticholinergic Medication Burden
While schizophrenia is characterized by generalized cognitive deficits across the course of illness (1–3), emerging evidence suggests many medications can also negatively impact cognitive performance (4). We recently reported that anticholinergic medication burden (ACMB) accumulated from multiple medication classes—antipsychotics, antidepressants, and traditional anticholinergics (e.g., diphenhydramine, benztropine, trihexyphenidyl)—is associated with deficits across nearly all cognitive measures in individuals with schizophrenia in the cross-sectional, multi-site Consortium on the Genetics of Schizophrenia study (COGS-2) (5). These data align with other studies describing the impact of ACMB on outcomes in schizophrenia (6). While these findings are also consistent with results from studies of healthy older adults reporting increased cognitive impairment and elevated dementia risk with greater ACMB exposure, it is difficult to disambiguate the degree to which cognitive impairment results from residual symptoms or is driven by factors like cumulative ACMB in cross-sectional studies of medicated schizophrenia patients (7–10). Indeed, pharmacotherapy, in addition to psychotherapy and psychosocial rehabilitation, is an essential component of comprehensive schizophrenia treatment and can help enhance cognitive health through symptom reduction. However, identifying objective measures of ACMB-associated cognitive impairment would significantly enhance treatment selection in routine clinical care, and ultimately improve outcomes for those living with schizophrenia.
Mismatch negativity (MMN) and P3a are sequentially evoked neurophysiological biomarkers of early auditory information processing (EAIP) that are widely studied in schizophrenia research and therapeutic development (11–16). MMN is believed to reflect automatic sensory discrimination, while P3a is thought to index the rapid involuntary redirection of attention and subsequent transitions to higher order attention-dependent cognitive processing (17, 18). These measures are considered “pre-attentive” since they are reliably elicited in the absence of directed attention and require no overt behavioral responses from the participant; MMN responses are heritable, and able to be assessed in fetuses, newborn babies and even individuals who are comatose or in a persistent vegetative state prior to regaining consciousness (19–23).
While studies have shown that MMN/P3a are resistant to fluctuations in clinical state and symptoms, and are largely unaffected by individual antipsychotic medications or when switching between antipsychotics, the emphasis of those reports was on individual antipsychotic medications rather than total ACMB (24–28). Here, we directly evaluated whether these neurophysiological biomarkers of early auditory information processing are associated with degree of ACMB in a large schizophrenia cohort.
Methods
Participants in the COGS-2 study have previously been described, including inclusion and exclusion criteria (29). Participants were either healthy control subjects (HCS, N=1,062) or had a diagnosis of schizophrenia (N=1,415; N=60 had schizoaffective disorder, depressed type and are included in schizophrenia analyses). From the initial schizophrenia cohort, N=265 were excluded due to missing/incomplete medication data. Those who were taking prescribed stimulants, opiates, steroids, or benzodiazepines (N=197) were excluded to reduce the potential for confounding effects on MMN/P3a biomarker response and cognition. A total of N=555 schizophrenia patients had complete demographic, medication, clinical, cognitive, and MMN/P3a data available for analysis.
MMN and P3a have been reported in this cohort, including procedures related to EEG recording and data analysis (15). Briefly, an auditory oddball paradigm consisting of frequently presented tone “standards” interspersed with infrequent duration-increment “deviants,” was used. Standard and deviant waveforms were calculated by averaging EEG responses to each stimulus type. Deviant minus standard difference waveforms were calculated for the MMN (125–210 msec) and P3a (250–300 msec) time windows. MMN/P3a data from HCS are visualized for reference in Figure 1 but were not included in subsequent analyses. ACMB was scored as previously described via a modified Anticholinergic Cognitive Burden (ACB) scale which rates medications on 0 to 3 scale (5). Total ACB scores were generated by summing the individual ACB values from all medications for each participant, consistent with established methods. Participants were grouped as follows: no (ACB score=0), low (ACB score=1 or 2), moderate (ACB score=3 or 4), high (ACB score=5 or 6) or very high ACMB (ACB score>6). For reference, a single strong anticholinergic medication has an ACB score of 3 (e.g., diphenhydramine). Relative frequencies and specific ACB ratings for each medication have been previously described in this dataset (5).
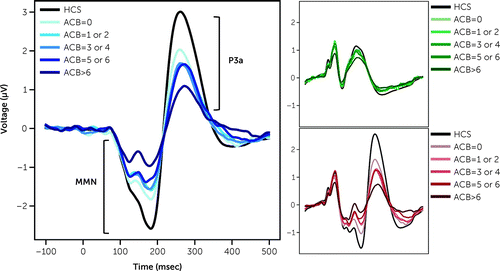
FIGURE 1. Effect of ACB group on mismatch negativity (MMN) and P3a responsea
aLarge panel shows MMN and P3a in blue traces, with black as reference from healthy control subjects (HCS). MMN and P3a generated by subtracting the evoked response of deviant stimuli (inset, red traces) from standard stimuli (inset, green traces).
The Scale for the Assessment of Positive Symptoms (SAPS), Scale for the Assessment of Negative Symptoms (SANS), Scale of Function (SOF), and chlorpromazine equivalents (CPZ) were assessed or calculated as previously described (30–33). The Penn Computerized Neurocognitive Battery (PCNB) was used as the primary outcome measure for cognitive functioning. The PCNB includes accuracy and speed measures of eight domains: abstraction and mental flexibility, attention, working memory, face memory, verbal memory, spatial memory, spatial ability, and emotion processing, reported as age-and gender-corrected z-scores (34). Efficiency scores for these eight domains were obtained as average of accuracy and speed scores; PCNB Global Cognition was derived by averaging individual efficiency z-scores scores as previously described (5, 34).
Statistical Analysis
The effect of ACB (none, low, moderate, high, or very high ACMB) on EAIP biomarkers, SAPS, SANS, PCNB Global Composite, chlorpromazine equivalents, and SOF were examined by one-way ANOVA with the significance value set to 0.05 with nominal p values without multiple comparison correction reported. Simple linear regression was used to measure the impact of ACB score on MMN and P3a. Subsequently, stepwise multiple regression models were applied using MMN and P3a, separately, as dependent variables, with ACB score as independent variable, followed by the addition of age, SAPS, SANS, PCNB Global Composite, CPZ, and SOF to the model to determine changes in fit with an α set to 0.05.
Results
Higher ACB score was associated with reduction in magnitude of both MMN and P3a, driven by ACB-associated effects on deviant stimuli, but not standards (Table 1, Figure 1). Post-hoc ANOVA difference contrasts revealed that the very high ACB subgroup (ACB>6) had attenuated MMN (p<0.001) and P3a (p=0.007) response compared to other groups. Follow-up linear regression revealed that ACB score predicted MMN (R2=0.03, F1,553=17.03, p<0.001; ACB score β=0.057), and P3a response (R2=0.014, F1,553=8.110, p=0.005; ACB score β=–0.052) implying that a change of ACB score of only three (e.g., comparable to the addition or subtraction of olanzapine or diphenhydramine) predicts a change in MMN by 0.17μV, and in P3a by 0.16μV. Stepwise multiple regression analyses indicated ACB score remained a significant predictor of MMN when cognitive, clinical, and functional measures were included, with age and global neurocognitive composite contributing to the model (R2=0.15; ACB score β=0.037, p=0.016; age β=0.023, p<0.001; PCNB Global Composite z-score β=–0.121, p=0.008). By contrast, adding other measures eliminated the significant relationship between ACB score and P3a (R2=0.107; ACB score β=–0.024, p=0.266; age β=–0.019, p<0.001; PCNB Global Composite z-score β=0.191, p=0.002; SANS score β=0.03, p=0.006, SOF total score β=0.02, p=0.043; SAPS and CPZ non-significant).
| ACB=0 (N=47) | ACB=1 or 2 (N=177) | ACB=3 or 4 (N=166) | ACB=5 or 6 (N=86) | ACB>6 (N=79) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measures | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | F | p |
| Age (years) | 42.00 | 12.69 | 46.41 | 12.18 | 46.67 | 10.46 | 44.69 | 11.21 | 47.15 | 9.76 | 2.191 | 0.069 |
| MMN (μV) | −1.56 | 0.81 | −1.28 | 0.85 | −1.30 | 0.90 | −1.30 | 0.87 | −0.80 | 0.84 | 7.031 | <0.001 |
| P3a (μV) | 1.87 | 1.35 | 1.48 | 1.15 | 1.60 | 1.19 | 1.61 | 1.14 | 1.05 | 0.86 | 4.883 | <0.001 |
| Standard stimuli 125–210 msec (μV) | 0.77 | 0.59 | 0.76 | 0.71 | 0.80 | 0.83 | 0.82 | 0.81 | 0.62 | 0.72 | 0.884 | 0.473 |
| Standard stimuli 250–300 msec (μV) | −0.35 | 0.67 | −0.37 | 0.49 | −0.47 | 0.60 | −0.44 | 0.58 | −0.31 | 0.50 | 1.699 | 0.149 |
| Deviant stimuli 125–210 msec (μV) | −0.79 | 1.05 | −0.52 | 0.98 | −0.50 | 1.10 | −0.48 | 0.98 | −0.18 | 1.04 | 2.847 | 0.023 |
| Deviant stimuli 250–300 msec (μV) | 1.52 | 1.23 | 1.11 | 1.22 | 1.13 | 1.09 | 1.17 | 1.29 | 0.74 | 0.89 | 3.536 | 0.007 |
| SANS score | 11.90 | 4.27 | 11.07 | 5.02 | 10.75 | 5.57 | 12.52 | 5.51 | 14.15 | 4.46 | 7.167 | <0.001 |
| SAPS score | 9.49 | 4.27 | 6.20 | 3.93 | 6.57 | 3.84 | 6.58 | 4.05 | 7.57 | 4.18 | 6.929 | <0.001 |
| PCNB global cognition (z-score) | −0.45 | 0.61 | −0.64 | 0.78 | −0.76 | 0.92 | −0.82 | 0.92 | −1.24 | 0.89 | 8.843 | <0.001 |
| Chlorpromazine equivalents (mg) | 0.00 | 0.00 | 218.83 | 170.98 | 436.56 | 319.85 | 489.46 | 396.16 | 934.04 | 760.12 | 63.580 | <0.001 |
| SOF total score | 44.96 | 5.21 | 48.60 | 5.73 | 48.27 | 5.84 | 46.70 | 6.04 | 45.06 | 5.33 | 8.539 | <0.001 |
TABLE 1. Description of demographic, clinical, cognitive, and neurophysiologic measures as a function of ACB scorea
Discussion
Our results show for the first time that the total anticholinergic medication burden aggregated from all medications is associated with diminished MMN and P3a response in schizophrenia and suggests that ACB>6 may uniquely attenuate EAIP biomarker relationships in schizophrenia, a degree of ACB seen in at least 25% of schizophrenia patients (5). While MMN and P3a both rely on NMDA receptor neurotransmission (35–37), this finding is in line with previous studies which describe the effects of nicotinic and muscarinic modulation on EAIP biomarker response (38–40); that the relationship between ACB and MMN, but not P3a, appears to persist even when accounting for several cognitive, clinical, and functional measures may potentially speak to mechanistic differences between cortical sources underlying MMN and P3a, and requires further investigation (41, 42).
Since MMN and P3a are regarded as important neurophysiological indices that probe the earliest stages of core information processing necessary for most higher-order cognitive functioning, these data have several important implications for the field. First, given the knowledge that ACB score affects MMN and P3a, genetic studies investigating relationships between and among EAIP biomarkers and cognitive measures would benefit from comprehensive accounting of anticholinergic medication burden across medication classes in analyses. Indeed, previous reports suggested there were differences in MMN (but not P3a) in schizophrenia patients who were taking at least one traditional anticholinergic medication compared to those who were not taking any (15), but the current approach allows for ACB correction in the same way that demographic adjustments can be made (i.e., like age correction) for neuropsychological tests. Second, results support the idea that in patient-oriented studies, accounting for ACB may help optimize bench to bedside translational pipelines and add important information to “go or no-go” decision making, particularly those using EAIP biomarkers to test central nervous system target engagement of novel pro-cognitive therapeutic molecules or other treatment approaches (43, 44). Lastly, this study provides justification to consider EAIP biomarkers as objective, preattentive proxies of ACB-associated cognitive impairment, not only in schizophrenia but also in other patient populations. While comprehensive longitudinal studies are needed in order to untangle ACB-EAIP-cognition cause-effect relationships in schizophrenia, these data suggest that neurophysiological biomarkers may supplement traditional methods (e.g., assays of biofluid, imaging approaches, medication rating scales, etc.) that are used to assess anticholinergic medication burden in research studies and in clinical practice.
1. : Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”. Schizophr Bull 2000; 26:119–136Crossref, Medline, Google Scholar
2. : Cognitive impairment in never-medicated individuals on the schizophrenia spectrum. JAMA Psychiatry 2020; 77:543–545Crossref, Medline, Google Scholar
3. : Neurocognition and duration of psychosis: a 10-year follow-up of first-episode patients. Schizophr Bull 2016; 42:87–95Medline, Google Scholar
4. : Two hypotheses on the high incidence of dementia in psychotic disorders. JAMA Psychiatry 2021; 78:1305–1306Crossref, Medline, Google Scholar
5. : Anticholinergic medication burden-associated cognitive impairment in schizophrenia. Am J Psychiatry 2021; 178:838–847Link, Google Scholar
6. : The impact of anticholinergic burden on functional capacity in persons with schizophrenia across the adult life span. Schizophr Bull 2021; 47:249–257Crossref, Medline, Google Scholar
7. : Anticholinergic drug exposure and the risk of dementia: a nested case-control study. JAMA Intern Med 2019; 179:1084–1093Crossref, Medline, Google Scholar
8. : Anticholinergic use trends in 14, 013 patients with schizophrenia from three national surveys on the use of psychotropic medications in China (2002–2012). Psychiatry Res 2017; 257:132–136Crossref, Medline, Google Scholar
9. : Cognitive burden of anticholinergic medications in psychotic disorders. Schizophr Res 2017; 190:129–135Crossref, Medline, Google Scholar
10. : Anticholinergic burden and cognition in older patients with schizophrenia. J Clin Psychiatry 2017; 78:e1284–e1290Crossref, Medline, Google Scholar
11. : Accelerating Medicines Partnership Program–Schizophrenia. https://www.nimh.nih.gov/research/research-funded-by-nimh/research-initiatives/accelerating-medicines-partnershipr-program-schizophrenia-ampr-sczGoogle Scholar
12. : Mismatch negativity in response to auditory deviance and risk for future psychosis in youth at clinical high risk for psychosis. JAMA Psychiatry 2022; 79:780—789Crossref, Medline, Google Scholar
13. : Gamma oscillations predict pro-cognitive and clinical response to auditory-based cognitive training in schizophrenia. Transl Psychiatry 2020; 10:405Crossref, Medline, Google Scholar
14. : Oscillatory biomarkers of early auditory information processing predict cognitive gains following targeted cognitive training in schizophrenia patients. Schizophr Res 2020; 215:97–104Crossref, Medline, Google Scholar
15. : Validation of mismatch negativity and P3a for use in multi-site studies of schizophrenia: characterization of demographic, clinical, cognitive, and functional correlates in COGS-2. Schizophr Res 2015; 163:63–72Crossref, Medline, Google Scholar
16. : Reduced mismatch negativity predates the onset of psychosis. Schizophr Res 2012; 134:42–48Crossref, Medline, Google Scholar
17. : Updating P300: an integrative theory of P3a and P3b. Clin Neurophysiol 2007; 118:2128–2148Crossref, Medline, Google Scholar
18. : P3a from white noise. Int J Psychophysiol 2012; 85:236–241Crossref, Medline, Google Scholar
19. : Event-related brain potential of human newborns to pitch change of an acoustic stimulus. Electroencephalogr Clin Neurophysiol 1990; 77:151–155Crossref, Medline, Google Scholar
20. : Automatic auditory information processing in sleep. Sleep 2000; 23:821–828Crossref, Medline, Google Scholar
21. : Auditory magnetic responses of healthy newborns. Neuroreport 2003; 14:1871–1875Crossref, Medline, Google Scholar
22. : Event-related potentials--neurophysiological tools for predicting emergence and early outcome from traumatic coma. Intensive Care Med 1996; 22:39–46Crossref, Medline, Google Scholar
23. : Mismatch negativity predicts recovery from the vegetative state. Clin Neurophysiol 2007; 118:597–605Crossref, Medline, Google Scholar
24. : Mismatch negativity in schizophrenia: a meta-analysis. Schizophr Res 2005; 76:1–23Crossref, Medline, Google Scholar
25. : Automatic sensory information processing abnormalities across the illness course of schizophrenia. Psychol Med 2012; 42:85–97Crossref, Medline, Google Scholar
26. : The effect of clozapine therapy on frontal lobe dysfunction in schizophrenia: neuropsychology and event-related potential measures. Int J Neuropsychopharmacol 1998; 1:19–29Crossref, Medline, Google Scholar
27. : Effects of clozapine on auditory event-related potentials in schizophrenia. Biol Psychiatry 1998; 44:716–725Crossref, Medline, Google Scholar
28. : Effects of risperidone on auditory event-related potentials in schizophrenia. Int J Neuropsychopharmacol 1999; 2:299–304Crossref, Medline, Google Scholar
29. : Consortium on the Genetics of Schizophrenia (COGS) assessment of endophenotypes for schizophrenia: an introduction to this Special Issue of Schizophrenia Research. Schizophr Res 2015; 163:9–16Crossref, Medline, Google Scholar
30. : Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1984Google Scholar
31. : The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl 1989:49–58Crossref, Medline, Google Scholar
32. : Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 2003; 64:663–667Crossref, Medline, Google Scholar
33. : Validation of the Scale of Functioning in older outpatients with schizophrenia. Am J Geriatr Psychiatry 1996; 4:218–228Crossref, Medline, Google Scholar
34. : Psychometric properties of the penn computerized neurocognitive Battery. Neuropsychology 2015; 29:235–246Crossref, Medline, Google Scholar
35. : Role of cortical N-methyl-D-aspartate receptors in auditory sensory memory and mismatch negativity generation: implications for schizophrenia. Proc Natl Acad Sci U S A 1996; 93:11962–11967Crossref, Medline, Google Scholar
36. : Memantine effects on sensorimotor gating and mismatch negativity in patients with chronic psychosis. Neuropsychopharmacology 2016; 41:419–430Crossref, Medline, Google Scholar
37. : Single-dose memantine improves cortical oscillatory response dynamics in patients withschizophrenia. Neuropsychopharmacology 2017; 42:2633–2639Crossref, Medline, Google Scholar
38. : Axonal α7 nicotinic ACh receptors modulate presynaptic NMDA receptor expression and structural plasticity of glutamatergic presynaptic boutons. Proc Natl Acad Sci U S A 2010; 107:16661–16666Crossref, Medline, Google Scholar
39. : Nicotinic modulation of human auditory sensory memory: evidence from mismatch negativity potentials. Int J Psychophysiol 2006; 59:49–58Crossref, Medline, Google Scholar
40. : Auditory mismatch responses are differentially sensitive to changes in muscarinic acetylcholine versus dopamine receptor function. Elife 2022; 11:e74835Crossref, Medline, Google Scholar
41. : Sources of the frontocentral mismatch negativity and P3a responses in schizophrenia patients and healthy comparison subjects. Int J Psychophysiol 2021; 161:76–85Crossref, Medline, Google Scholar
42. : Cortical substrates and functional correlates of auditory deviance processing deficits in schizophrenia. Neuroimage Clin 2014; 6:424–437Crossref, Medline, Google Scholar
43. : Verbal learning deficits associated with increased anticholinergic burden are attenuated with targeted cognitive training in treatment refractory schizophrenia patients. Schizophr Res 2019; 208:384–389Crossref, Medline, Google Scholar
44. : The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. Am J Psychiatry 2009; 166:1055–1062Link, Google Scholar



