Risks and Benefits of Cannabis and Cannabinoids in Psychiatry
Abstract
Objective:
The United States is in the midst of rapidly changing laws regarding cannabis. The increasing availability of cannabis for recreational and medical use requires that mental health clinicians be knowledgeable about evidence to be considered when counseling both patients and colleagues. In this review, the authors outline the evidence from randomized double-blind placebo-controlled trials for therapeutic use of cannabinoids for specific medical conditions and the potential side effects associated with acute and chronic cannabis use.
Methods:
Searches of PubMed and PsycInfo were conducted for articles published through July 2021 reporting on “cannabis” or “cannabinoids” or “medicinal cannabis.” Additional articles were identified from the reference lists of published reviews. Articles that did not contain the terms “clinical trial” or “therapy” in the title or abstract were not reviewed. A total of 4,431 articles were screened, and 841 articles that met criteria for inclusion were reviewed by two or more authors.
Results:
There are currently no psychiatric indications approved by the U.S. Food and Drug Administration (FDA) for cannabinoids, and there is limited evidence supporting the therapeutic use of cannabinoids for treatment of psychiatric disorders. To date, evidence supporting cannabinoid prescription beyond the FDA indications is strongest for the management of pain and spasticity.
Conclusions:
As cannabinoids become more available, the need for an evidence base adequately evaluating their safety and efficacy is increasingly important. There is considerable evidence that cannabinoids have a potential for harm in vulnerable populations such as adolescents and those with psychotic disorders. The current evidence base is insufficient to support the prescription of cannabinoids for the treatment of psychiatric disorders.
The United States is in the midst of a period of rapid change in cannabis policy. Current state laws range from those where cannabis has been legalized for both medical and recreational purposes to those where cannabis products remain illegal. In between those extremes are states where cannabis has been decriminalized or is available solely for medical use (with or without decriminalization). The legal status of cannabis in some states is starkly contrasted with its public perception as an illicit substance in other states. As of September 2021, 36 states and the District of Columbia have enacted medical cannabis laws, with markedly varying specific limits, and 16 states and the District of Columbia have legalized the recreational use of cannabis by persons age 21 and older.
The widespread accessibility and increasing recreational use of cannabis in recent decades has promulgated the notion that cannabis products are overwhelmingly benign. An estimated 43.5 million Americans age 12 years or older used cannabis in 2018 (1). Approximately 10% of cannabis users report cannabis consumption to treat specific medical disorders or symptoms (2), including stress management and relaxation, treatment of mood and anxiety symptoms, and pain, nausea, and vomiting (3).
Cannabis is a Schedule I substance according to the U.S. Drug Enforcement Agency (DEA), which, by definition, is a drug with no currently accepted medical use and a high potential for abuse. Other examples of Schedule I drugs include heroin and LSD. Cannabis is touted as a treatment for myriad medical conditions. However, in contrast to therapeutics approved by the U.S. Food and Drug Administration (FDA), cannabis has not been sufficiently studied to determine whether it meets safety and efficacy thresholds for approval as a therapeutic. Thus, it remains understudied, underregulated, and surrounded by controversy. Polarizing debates that have skirted the fundamental issues of safety and efficacy have played out in many states and resulted in cannabis policies that are inadequately supported by research and the dissemination of misinformation about the potential risks and benefits of cannabis use (4).
There are many competing interests that create pressure for increasing the availability of medicalized or legalized cannabis. For example, patients desperate for relief of psychiatric symptoms may be motivated to seek relief via cannabis even in the absence of formal evidence of efficacy. Similarly, individuals who enjoy recreational cannabis or who have cannabis use disorder may wish to have their cannabis use sanctioned by the medical establishment. Some advocates for cannabis legalization hope to profit from legalization, and state legislatures considering medicalization and legalization often hope to generate tax revenue. These conflicts of interest create strong pressure at the state and federal levels to find paths to legalize cannabis.
Medical cannabis has been proposed as appropriate for treatment of more than 50 medical conditions despite scant evidence to support its therapeutic use for many of them (5). In fact, all cannabinoid products except three FDA-approved drugs (dronabinol, nabilone, and cannabidiol) remain underregulated and have limited data supporting use in clinical populations.
It is critical that the field of psychiatry uphold its role in limiting its prescription of cannabis to clinical indications where there is a sufficiently strong evidence base. By implication, it is imperative that psychiatrists and other physicians not become gatekeepers for access to recreational cannabis under the guise of medicalization. To this end, it is important to review the evidence base to ascertain the strength of the evidence supporting the safety and efficacy of cannabis and cannabinoid treatments (6).
To support evidence-based care and inform discussions on this topic, we have reviewed the risks and benefits of cannabis and the well-known cannabinoids THC and CBD. Here, we provide an overview of the issues related to cannabis and cannabinoids facing psychiatrists, with a focus on studies, including randomized double-blind placebo-controlled trials, of the therapeutic use of cannabis and cannabinoids for medical and psychiatric conditions and the potential side effects associated with acute and chronic cannabis use.
We conducted searches of PubMed and PsycInfo for articles published through July 2021 reporting on “cannabis” or “cannabinoids” or “medicinal cannabis.” Additional articles were identified from the reference lists of published reviews. Articles that did not contain the terms “clinical trial” or “therapy” in the title or abstract were not reviewed. For PubMed, the following search strategy was used: (“cannabis”[mesh] OR “cannabis”[tiab] OR “cannabi*”[tiab] OR “cannabinoids”[mesh] OR “cannabinoids”[tiab] OR “cannabinoid”[tiab]) AND (“clinical trial”[pt] OR “clinical trial”[tiab] OR “therapeutics”[mesh] OR “therapy”[tiab]). For PsycInfo, the following search strategy was used: (cannabis OR cannabinoids OR cannabidiol) Subject AND (clinical trial OR therapy OR therapeutic) Subject. A total of 4,431 articles were screened, and 841 articles that met criteria for inclusion were reviewed by two or more authors.
CANNABINOID PHARMACOLOGY
The cannabis plant contains hundreds of chemicals, including over 140 cannabinoids (6). The term “cannabis” refers to plant material in the form it is found on the cannabis plant. “Cannabinoids” are compounds that are either found only in the cannabis plant or synthesized from components of the cannabis plant. The term “medical cannabis” refers to cannabis products recommended by a clinician for the treatment of a medical condition. Medical cannabis is often not chemically distinct from cannabis intended for recreational purposes—that is, both are often plant material products. However, there are FDA-approved cannabinoids for specific clinical indications. In some states, the cannabis plant is not permitted for therapeutic use, and medical cannabis is restricted to use of specific, controlled extracts.
Like many pharmaceuticals, plant-derived or synthetic cannabinoids mimic human neurotransmitters. Humans and many other mammals produce endogenous cannabinoids, which bind to the G-protein-coupled endocannabinoid receptors, cannabinoid receptor 1 (CB1) and cannabinoid receptor 2 (CB2), the predominant endocannabinoid receptors. CB1 receptors are located throughout the brain and CB2 receptors are found on immune cells most often located in the periphery. CB2 receptors are also located on dopaminergic terminals in the striatum. When muscarinic acetylcholine M4 receptors are stimulated, they cause the release of 2-arachidonoylglycerol, which stimulates CB2 and suppresses dopamine release (7). Delta-9-tetrahydrocannabinol (THC), the plant’s psychoactive constituent, is a partial agonist at both CB1 and CB2 and can cause euphoria, psychosis, and cognitive dysfunction and has analgesic and anti-inflammatory effects (8). The THC content in available cannabis preparations varies widely.
Cannabidiol (CBD) is a CB1 antagonist, a negative allosteric modulator at CB2, and an agonist at the transient receptor potential cation channel subfamily V member 1 (TRPV1) and serotonin 1A (5-HT1A) receptors, with anxiolytic, antipsychotic, anticonvulsant, antioxidant, analgesic, and immunomodulatory functions, some of which may buffer the harmful effects of THC, such as psychosis (9). In particular, CB1, TRPV1, and 5-HT1A are receptors that have been linked to psychosis, anxiety, and pain, respectively.
CBD also functions as a G protein-coupled receptor (GPR55) antagonist and suppresses GPR55’s activities. The GPR55-dependent mechanism is thought to play a major role in CBD’s antipsychotic and antiepileptic activities (10) (Figure 1).
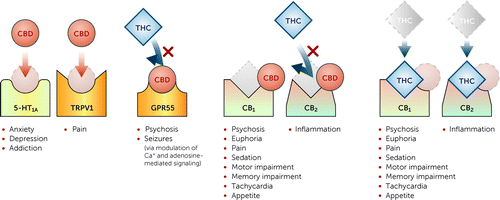
FIGURE 1. Cannabinoid interactions with receptors and neurotransmitter systems in human brain and proposed target symptoms and conditionsa
aCBD=cannabidiol; THC=delta-9-tetrahydrocannabinol.
The binding affinity of CBD for these receptors varies significantly, and these relative affinities become important when considering dosing of CBD for various medical indications (Table 1). CBD’s diverse receptor targets and the varying affinities for these receptors imply that dose-response studies are needed to establish the safety and efficacy of CBD. CBD is widely available commercially and, while not without risk, appears to be safer and better tolerated than THC.
| Receptor | Amount Required to Induce Action by CBD |
|---|---|
| CB1 | 4.3–27.5 µM |
| CB2 | 1–10 µM |
| 5-HT1A | >10 µM |
| TRPV1 | 1–10 µM |
| GPR55 | <1 µM |
TABLE 1. Relative affinities of CBD for various receptorsa
CONTEMPORARY CANNABIS ISSUES
With the legalization of cannabinoids for recreational and medical use over the past decade, consumers face a growing number of outlets and options for obtaining cannabinoids. Both synthetic and natural cannabinoid products have greater potency and cannabinoid content than those available in the past. Averaging 1%−4% from the 1960s through the 1980s, THC concentrations in cannabis have increased over the past two decades to more than 19% on average (11). The harmful effects of cannabis, such as development of psychosis, appear to be THC dose dependent (12). Increased potency of cannabis products, combined with advanced delivery systems such as vaping devices (for use with cannabis flower—dried buds of the cannabis plant—or concentrates, such as “wax” and “shatter”) have contributed to increasing risk for adverse outcomes (13).
Epidemiology
Changing policy has led to potential problems for cannabis users across demographic groups. Although adult use has increased and youth use has remained flat in recent years, the perception of risk has dropped among both of these groups (14). Decreases in perception of risk are associated with increased use and with regular use in a wide range of users, from adolescents to pregnant women (15). The number of individuals meeting criteria for cannabis use disorder is on the rise; past-year prevalence of DSM-IV cannabis use disorder was 1.5% in 2001–2002 and 2.9% in 2012–2013 (16). Similarly, the number of women using cannabis during pregnancy is also increasing; one study showed a significant increase from 2.85% in 2002 to 4.98% in 2016 (17). Use among the elderly remains low. Recent data from the University of Michigan’s Monitoring the Future study show that vaping of cannabis more than doubled from 2017 to 2019, reflecting one of the largest increases that researchers have seen since the study started tracking vaping (18).
Psychiatrists must also pay special attention to cannabis use and its relationship to COVID-19. Both smoking and vaping may increase risk for medical conditions associated with higher risk of COVID-19 complications (19). Cannabis smokers are susceptible to chronic obstructive pulmonary disease, which may cause severe complications of COVID-19 and lead to a higher fatality rate (20). Patients with chronic respiratory disease in China had a COVID-19 case fatality rate of 6.3%, compared with 2.3% overall (21). Similarly, cannabis use meeting criteria for cannabis use disorder is associated with an increase in incidence of COVID-19 and worse outcomes. A recent retrospective case-control study of electronic health records data showed that patients with a diagnosis of a substance use disorder within the past year were at significantly increased risk for COVID-19 (adjusted odds ratio=8.699, 95% CI=8.411, 8.997, p<10−30), and COVID-19 patients with substance use disorders had significantly worse outcomes (death: 9.6%; hospitalization: 41.0%) than COVID-19 patients overall (death: 6.6%; hospitalization: 30.1%) (22).
Acute Effects
Acute cannabis use is associated with impaired learning, memory, attention, and motor coordination. These acute effects are often related to route of ingestion (23). The wide-ranging effects of cannabis can be attributed in part to the presence of CB1 receptors in the prefrontal cortex, globus pallidus, substantia nigra, hippocampus, striatum, and cerebellum (24). Acute cannabis use can also affect executive functioning, including the ability to plan, organize, solve problems, and make decisions (25). This may potentially result in users making risky decisions that they would not otherwise make (26). Cannabis intoxication can be associated with marked feelings of anxiety and paranoia as well as possible cannabis-induced psychosis. “Overdoses” (i.e., cases of subjective intoxication beyond the desired recreational effect or resulting in undesirable or unanticipated acute effects) are often the result of inadequate education about serving sizes and onset of action of edible cannabis products, but they can also be the result of product adulteration and lack of consistency of cannabis product contents.
Chronic Effects
While studies of chronic cannabis use may be characterized by inherent sampling biases, findings show it to be associated with an increased risk of cognitive difficulties, psychiatric illness, addiction, and other systemic effects. Sampling bias is a potential confounder because individuals who chronically use cannabis may be characterized by other disorders or behaviors that cause or are associated with poor health and functioning. That limitation notwithstanding, several studies have described the adverse effects of regular cannabis use on the frontal lobe and executive function. These effects are more pronounced in young regular cannabis users. For example, Gruber et al. (27) showed that those who begin using cannabis regularly before age 16 had deficits on standard neurocognitive tests. A longitudinal study of 799 adolescents revealed a dose-dependent association between cannabis use from baseline to 5-year follow-up and neurodevelopmental abnormalities, including accelerated cortical thinning, primarily in prefrontal regions of the brain (28). Additionally, adolescents use cannabis in greater amounts and at greater frequency than those who initiate use later in life (29), underscoring the importance of prevention efforts to discourage cannabis use among the young. While older users have been studied less, one study in a nonclinical sample of users age 35 or older demonstrated significantly poorer performance than nonusers across cognitive domains of attention/working memory (information processing speed and executive functioning) (30). The differential consequences of cannabis use by age are relevant for regulatory requirements.
There is additional evidence that cannabis exposure is detrimental to the developing brain (31) while also making individuals more susceptible to other addictive substances. Exposure to THC in the prenatal and adolescent periods can lead to impaired neural connectivity, particularly in the hippocampus, which may contribute to the association between early and regular cannabis use and decreased IQ (32, 33). Of note, residual cognitive effects persisting after acute intoxication are still debated, especially in individuals who used cannabis regularly as adolescents (34).
Other studies have shown that adolescent rats exposed to cannabis are cross-sensitized to cocaine, changing the initial behavioral, molecular, and epigenetic responses to cocaine compared with rats who were not exposed to cannabis (35, 36). Cannabis use is not a definite “gateway drug” to use of other substances, but lifetime cumulative probability estimates indicate that 44.7% of individuals with lifetime cannabis use progressed to other illicit drug use at some time in their lives (37). Cannabis use is also associated with an increased risk of alcohol use disorder onset and persistence of alcohol use disorder (38).
Cannabis and the cannabinoid THC are potentially addictive substances that may be associated with cannabis use disorder; tolerance and/or withdrawal develops in 10%−30% of cases of cannabis use disorder (16, 39–41). Cannabis use disorder is associated with worsening functional status, including lower income, greater need for socioeconomic assistance, criminal behavior, unemployment, and decreased life satisfaction (14). Beyond the cannabis use disorder syndrome itself, cannabis use has been reported to worsen existing anxiety, depression, and bipolar disorder symptoms and to increase the likelihood of developing a depressive disorder (41–45). A recent analysis of survey data from 281,650 young adults ages 18–34 showed that cannabis use was associated with increased risk of suicidal ideation, suicidal plan, and suicide attempts (46).
A recent multicenter study in psychiatric centers evaluated the relationship between first-episode psychosis and cannabis use (47). Compared with control subjects who never used cannabis, daily users had a 3.2-fold increased risk (95% CI=2.2, 4.1) for developing psychosis, and use of high-potency types of cannabis brought the odds ratio up to 4.8 (95% CI=2.5, 6.3). A nationwide register-based historical prospective cohort study from Denmark showed an increase in the population-attributable risk fraction of cases of schizophrenia attributable to cannabis use disorder from about 2.0% in 1995 to 6.0%–8.0% since 2010 (48). This three- to fourfold increase in population-attributable risk fraction during the past two decades is expected given the increased use and potency of cannabis. These studies reinforce a significant association between daily cannabis use and the development of psychotic disorders, particularly with exposure to high-potency marijuana (49) and in individuals with genetic vulnerability (14).
A recent genome-wide association study of people with cannabis use disorder identified two genome-wide significant loci on chromosomes 7 and 8 (50). In addition to conferring genetic vulnerability for cannabis use disorder, these loci are associated with attention deficit hyperactivity disorder (ADHD), major depressive disorder, and schizophrenia. Individual susceptibility to developing a psychiatric disorder after exposure to cannabis is highly variable. Some patients and clinicians report amelioration of anxiety and low mood, while others report the opposite (51).
While researchers have begun to elucidate the effects of cannabis on the brain, cannabis also has wide-ranging effects on multiple physiological processes and organ systems. Chronic cannabis users spend less time in rapid eye movement (REM) sleep, the restorative sleep stage associated with learning, memory, and mood (52). Cannabis users may also experience cannabinoid hyperemesis syndrome, a syndrome marked by cyclic vomiting and abdominal pain that can lead to dehydration and anorexia (53). As many as 2.75 million Americans may experience cannabinoid hyperemesis syndrome, making it an important syndrome for clinicians to watch for, and one that is especially challenging to discern in patients who initially turned to cannabis to treat nausea (54). Apparently as a result of various diluents in THC products purchased outside of regulated markets, a recent spike in severe lung injury was recently observed among individuals vaping cannabinoids, representing another type of new and potentially lethal risk associated with cannabis use (55).
While multiple case reports describe acute coronary syndrome after cannabis use, there have been no prospective studies that have shown a strong association between cannabis and long-term cardiovascular outcomes (56). Desai et al. showed that the all-cause hospital mortality of cannabis users with arrhythmias increased from 3.7% to 4.4% from 2010 to 2014, respectively (p<0.001) (57). Cannabis’s effect on testosterone is not yet established, but one study suggested it reduces sperm count and concentration (58).
Indirect Effects
In addition to direct effects of cannabis use, there are indirect, or secondhand effects resulting from cannabis use. Secondhand cannabis smoke produces the same cannabis-related changes in brain and behavior as first-hand exposure (59, 60). For example, cannabis withdrawal syndrome can occur in patients with exposure to cannabis smoke following administration of cannabinoid antagonists (59). Cannabis use by a pregnant woman is also associated with indirect effects on the fetus; for example, a recent study of perinatal outcomes showed that the crude rate of preterm birth was 12.0% among cannabis users, compared with 6.1% among nonusers (risk difference=5.88%, 95% CI=5.22, 6.54) (60). Additionally, cannabis smoking may make cigarette smoking more likely (61).
Indirect effects extend to the impact of cannabis use on broader society. The impact of cannabis policies on cannabis-related traffic accidents have been mixed. The first three states to legalize recreational cannabis saw a combined 5.2% increase in police-reported traffic crashes as well as a 6% increase in auto insurance collision claims since legalization compared with neighboring states where cannabis is illegal (62). From 2012 to 2017, the number of drivers in fatal traffic crashes with THC their blood more than doubled in Washington State (63), although it is not possible to conclude that impairment by cannabis caused some or all of those collisions. Conversely, Colorado recently had a decline in cannabis-impaired traffic fatalities, from 12% in 2016 to 8% in 2017 (64). More data are needed to fully characterize potential indirect harms or benefits associated with cannabis use.
APPROVED AND POTENTIAL THERAPEUTIC USES
Two cannabinoids related to THC, dronabinol and nabilone, were approved by the FDA for chemotherapy-induced nausea and vomiting in 1985. Dronabinol received an additional indication for appetite stimulation in wasting conditions, such as AIDS, in 1992.
A third cannabinoid, CBD, was approved by the FDA in 2018 for the treatment of two forms of pediatric epilepsy, Dravet syndrome and Lennox-Gastaut syndrome, and in 2020 it gained an indication for seizures associated with tuberous sclerosis complex (Table 2).
| Cannabinoid and Indication | Year Approved |
|---|---|
| Dronabinol | |
| Chemotherapy-induced nausea and vomiting | 1985 |
| Appetite stimulation in wasting conditions (e.g., HIV infection) | 1992 |
| Nabilone | |
| Chemotherapy-induced nausea and vomiting | 1985 |
| Cannabidiol | |
| Seizures associated with Dravet syndrome and Lennox-Gastaut syndrome | 2018 |
| Seizures associated with tuberous sclerosis complex | 2020 |
TABLE 2. FDA-approved cannabinoids
The FDA approval of CBD for these medical conditions was based on the strength of safety and efficacy results from rigorous randomized controlled trials. A similar regulatory approach is needed for developing other cannabinoid drugs that have potential for treating mood and anxiety disorders, but currently there are no FDA-approved cannabinoids for psychiatric indications (65, 66).
Beyond the FDA-approved indications for cannabinoids, the best evidence for the medical use of cannabinoids is in chronic pain (including neuropathic pain) and muscle spasticity associated with multiple sclerosis, for which multiple randomized controlled trials and systematic reviews support their efficacy (67, 68). The National Academies Committee on the Health Effects of Marijuana concluded that there is “conclusive or substantial evidence” that cannabis is effective for the treatment of chronic pain in adults. This position is based on their expert committee’s assessment of the positive findings from multiple good-quality studies in individuals with chronic pain (68). An earlier meta-analysis of 28 such studies by Whiting et al. (69) determined that there was “moderate-quality evidence” and found that the published data supported the use of cannabinoids in the treatment of chronic pain.
Other reviews, however, describe the evidence for cannabinoids in chronic pain as weaker. For example, a 2017 meta-analysis of 27 studies examining the effectiveness of cannabis in chronic pain (70) found the quality of evidence for cannabis in alleviating neuropathic pain to be weak overall; notably, they also found no evidence supporting the use of cannabis in other types of pain. A subsequent meta-analysis of 91 publications specifically examining the use of cannabis to treat non-cancer-related chronic pain found cannabinoids to reduce pain 30% more than placebo (odds ratio=1.46, 95% CI=1.16, 1.84), but the number needed to treat to achieve meaningful pain reduction was 24 (95% CI=15, 61), whereas the number needed to harm for any adverse effect of cannabis was 6 (95% CI=5, 8) (71), suggesting that analgesic effects for individuals without cancer who have chronic pain is modest and side effects are common.
Another area of accumulating clinical trial data provides some support for the use of cannabis and cannabinoids to treat spasticity associated with multiple sclerosis. A recent meta-analysis evaluating 17 randomized controlled trials of cannabis and cannabinoids included over 3,000 patients with aggregate data showing modest, but statistically significant, positive effects on spasticity, pain, and bladder dysfunction in this population (72). The American Academy of Neurology published guidelines in 2014 that specified that nabiximols, a medication not available in the United States that includes THC and CBD in a ratio close to 1:1, carries the highest level of empirical evidence supporting its use as pharmacotherapy for spasticity and pain associated with multiple sclerosis (73).
While evidence supporting the therapeutic use of cannabis and cannabinoids for chronic pain, neuropathic pain, and spasticity is growing, the quality of the overall evidence base remains suboptimal in part because of heterogeneity of outcome measures, small sample sizes, and lack of long-term follow-up. Additionally, safety data regarding tolerance, withdrawal, and potential for drug-drug interactions are not well established for unregulated cannabinoid therapies (74).
Cannabinoids for the Treatment of Psychiatric Disorders
Aside from chronic noncancer pain, psychiatric disorders are among the most common reasons that people use cannabis and cannabinoids medicinally (75). A recent systematic review and meta-analysis by Black et al. (51) evaluated 83 studies of cannabinoids for symptoms of mental disorders, including anxiety, depression, posttraumatic stress disorder (PTSD), psychosis, ADHD, and Tourette’s syndrome. The available evidence base was limited by lack of high-quality randomized controlled trials, small sample sizes, and lack of standardization across trials. However, the authors found limited efficacy of cannabinoids in treating anxiety disorders in patients with co-occurring medical conditions. They did not find evidence supporting cannabinoid pharmacotherapy for the other psychiatric indications they assessed.
Multiple states have specified PTSD as a condition that may be treated with medical cannabis. In a double-blind crossover randomized controlled trial of 10 male soldiers with PTSD, 7 weeks of nabilone pharmacotherapy led to a significant reduction in nightmares compared with placebo as measured by mean reduction in the Clinician-Administered PTSD Scale (CAPS) but no differences in the CAPS items “difficulty falling asleep” or “staying asleep” (76). However, in an observational study of 2,276 veterans with PTSD, cannabis use was significantly associated with worse outcomes in PTSD symptom severity, violent behavior, and measures of alcohol and drug use when compared with veterans who never used cannabis and those who stopped cannabis use during the period of observation (77). Finally, a recent double-blind crossover randomized controlled trial of three concentrations of smoked cannabis in 80 participants with PTSD did not find a significant difference on the CAPS in change in PTSD symptom severity between cannabis and placebo (78).
Cannabinoids for the Treatment of Substance Use Disorders
Multiple cannabinoids have been studied as potential treatments for cannabis use disorder, a syndrome for which there is currently no FDA-approved pharmacotherapy. In a 12-week cannabis use disorder trial, dronabinol led to significantly better treatment retention and less withdrawal symptoms than placebo, but it did not separate from placebo on the primary outcome measure: a 2-week abstinence from cannabis (79). Nabilone appears to be safe and well tolerated in patients with cannabis use disorder, but its efficacy for the disorder has yet to be evaluated in adequately powered trials (80). Several other novel agents have undergone preliminary investigation, including CB1 receptor antagonist/inverse agonists and inhibitors of α-7 nicotinic acetylcholine receptors (81, 82). Considering the decriminalization of cannabis and the increasing incidence of cannabis use disorder, identifying effective pharmacotherapies for cannabis use disorder is a priority for the national research agenda.
The evidence supporting the use of cannabinoids as pharmacotherapy for other substance use disorders is mixed. Some patients report that cannabis use has helped limit their use of opioids or alcohol (75). While data from randomized controlled trials evaluating cannabinoids as pharmacotherapy for substance use disorders is lacking, 400 mg or 800 mg of CBD once daily for 3 days was found to significantly reduce craving, anxiety, and physiological responses associated with drug cues in patient with opioid use disorder (83). By contrast, cannabis use has been associated with a significantly lower percentage of days abstinent from alcohol in patients with alcohol use disorder (84).
Population-level evidence evaluating the impact of cannabis policies on opioid use is also equivocal (85, 86). It is therefore important to draw a distinction between the patient utilizing cannabinoids to reduce prescribed opioids for chronic pain and the patient with primary opioid use disorder. A physician may consider recommending cannabinoid therapy in the former situation after a careful risk-benefit assessment and provision of ample education to the patient. Use of cannabinoids as primary pharmacotherapy for opioid use disorder in lieu of the effective FDA-approved medications for opioid use disorder—buprenorphine, methadone, and naltrexone—is not evidence based.
CBD
CBD is sold over the counter in a variety of formulations for psychiatric and medical purposes, and it is estimated that up to 14% of Americans used CBD in the past year (87). Unlike cannabis, CBD use does not produce the psychoactive “high” experience that characterizes cannabinoids containing THC. And, when present in combination with THC, CBD may mitigate the potentially harmful effects of THC (88).
Interest in CBD has increased as a result of several key developments in the past few years, including the FDA approval of a CBD formulation for three forms of epilepsy. The Agriculture Improvement Act of 2018 allowed for the legal cultivation of CBD from hemp and distinguished it from illegal cultivation of cannabis plants because hemp contains no more than 0.3% THC. The result of this bill has been an increasing supply of CBD for companies to market a broad range of indications for CBD, including anxiety, inflammation, and opioid craving (88, 89).
Most CBD is obtained without a prescription, in products that are unregulated with regard to purity, potency, and accuracy of labeling (90). As with other over-the-counter supplements, patients may receive flawed or incomplete education about CBD (91). Few randomized controlled trials have systematically investigated the feasibility, safety, and efficacy of CBD in psychiatric patients (92–96). Randomized controlled trials examining the effectiveness of CBD in psychosis have produced mixed results. One trial showed that 800 mg/day of CBD for 4 weeks was as effective as amisulpride in treating both positive and negative symptoms of schizophrenia and had fewer associated side effects (94). Another trial, comparing 1000 mg/day of CBD for 6 weeks to placebo as adjunctive pharmacotherapy for schizophrenia, demonstrated a significant improvement in positive symptoms, without any difference in adverse effect reporting compared with placebo (95). However, in another recent trial comparing 600 mg/day of CBD for 6 weeks to placebo as adjunctive pharmacotherapy for schizophrenia, CBD did not produce any significant change in positive symptoms or cognition (96).
The literature on CBD is nascent; drug-drug interactions and long-term effects continue to be identified but poorly understood. CBD has a number of interactions with medications commonly prescribed to psychiatric patients. It has been shown to interact with antiepileptic drugs, antidepressants, opioid analgesics, acetaminophen, and alcohol (97). For example, CBD will increase the level or effect of lorazepam, and clinicians should consider lowering a patient’s lorazepam dosage if they are also taking CBD. In a recent study, several healthy adults who received high daily doses of CBD (1500 mg/day) experienced transaminase elevations that exceeded five times the upper limit of normal, raising the question of possible liver injury with chronic CBD administration (98). While CBD appears to have a more favorable risk profile than THC, it is critical that clinicians be familiar with the limitations of the available evidence base for CBD as a primary therapy for psychiatric disorders and be comfortable routinely discussing concurrent cannabinoid use with their patients.
GUIDANCE FOR PSYCHIATRIC CLINICIANS
As the evidence develops on the risks and benefits of cannabinoids, psychiatric clinicians need guidance now on whether cannabinoids should be a part of their patients’ treatment plans. There are some clinical circumstances in which cannabinoid pharmacotherapy may be helpful. Cannabinoids may be reasonable as a third-line pharmacotherapy for chronic pain for patients whose psychiatric presentation is heavily influenced by comorbid chronic pain and who show no evidence of behaviors indicative of substance use disorders. In such cases, collaboration should be ongoing between the psychiatric clinician and other medical specialists or primary care providers involved in diagnosing and treating the pain syndrome.
The level of evidence supporting cannabinoids for treating anxiety is low, despite the anecdotal reports describing its efficacy. When patients report that cannabinoids provide subjective relief of anxiety, psychiatric clinicians have an opportunity to provide education about the current state of the evidence. Since the number needed to harm is likely to be much lower for THC-containing compounds than those with pure CBD, clinicians whose anxious patients are reporting benefits from THC may consider recommending a monitored trial of CBD instead.
In general, however, prescribing clinicians should avoid initiating or recommending cannabinoid pharmacotherapy for most psychiatric patients. There are no clinical trials that support the use of cannabinoids as pharmacotherapy for mood disorders, and there is limited evidence supporting their use in PTSD. Converging lines of evidence suggest that THC-containing compounds are likely to cause harm in patients with existing psychosis or at high risk for psychosis. While CBD may have some promise in these patients, evidence from randomized controlled trials has been equivocal (94–96). Overall, there is little data indicating that cannabinoids are helpful in treating psychiatric illness, while there is considerable evidence that there is potential for harm in vulnerable populations such as adolescents and those with psychotic disorders.
Communication with patients about cannabinoids is crucial yet complicated. These discussions must consider that many patients may feel that cannabis preparations have been helpful to them or people they know, reflecting the overall polarized debate. Clinicians should always ask patients about their use of licit or illicit cannabinoid products. In clinical scenarios where there is a potential for harm to psychiatric patients using cannabinoids, a strategy of gently providing evidence and asking permission to continue exploring a topic (similar to a motivational interviewing approach) is likely necessary.
FUTURE RESEARCH DIRECTIONS
Advances continue in multiple areas of cannabinoid research despite considerable barriers to progress. Cannabis’s status as a Schedule I substance according to the DEA and a lack of research funding from states and companies profiting from sales of cannabis have made it difficult for cannabis science to keep pace with policy. Cannabinoids approved by the federal government for federally funded studies may not closely approximate cannabinoids that are publicly available. Basic science aimed at improved understanding the mechanism of action of cannabinoids, clinical research investigating the risks and potential therapeutic benefits in psychiatric samples, and policy research with the goal of allowing access to cannabinoids where the clinical indications are supported by research while limiting risk in vulnerable populations are all essential areas for future research. A major area of research will involve developing strategies for reducing cannabis use in patients with chronic psychosis, where a motivational interviewing approach is often more difficult because of the presence of negative symptoms that decrease the appreciation of long-term consequences.
THE FUTURE OF CANNABINOIDS: CAUTIOUS OPTIMISM
Many states have implemented policies that decriminalize cannabis, and multicenter controlled research trials recently led to a third cannabinoid drug earning FDA approval (a CBD formulation, for seizures). Nevertheless, research has not kept pace with the public interest in cannabis, and much work remains to determine whether cannabis as a plant can be a useful therapeutic and the appropriate medical applications for cannabinoids. This public enthusiasm mirrors an enthusiasm for psychedelic compounds, with a similar lack of evidence (99).
Widely held assumptions about the safety of today’s cannabinoids may arise from past decades of experience with cannabis products, such as cannabis from the 1970s with 3%–4% THC, that are far less relevant today. It is important to realize that cannabis, THC, and other cannabinoids are often sought as treatments for medical conditions that cannot be successfully treated by the current standard-of-care approaches. However, the fact remains that there are no psychiatric disorders for which cannabinoids have been FDA approved.
The success of cannabinoids in rare pediatric epilepsies has stimulated use in Angelman’s syndrome and autism spectrum disorder. Adverse consequences will likely emerge and be defined by these patient populations as field-testing of cannabinoids, rather than prospective investigation in controlled scientific studies, continues to generate most of the available safety data. In this context, understanding the importance of route of administration, purity, bioavailability, and dose is critical (100), but the Schedule I status of cannabis and a lack of funding have proven to be barriers to advancing cannabinoid science.
Clinicians will best serve their patients and the field by following the model unfolding with CBD for pediatric conditions, where rigorous science in a targeted clinical population precedes medical use, and regulated products are used under medical supervision. Meanwhile, we urge caution about the use of cannabis in medical settings.
Trends in cannabis laws and patterns of use in the United States suggest that mental health clinicians will continue to treat patients who are either interested in cannabinoids or are already taking them. Psychiatric clinicians should be educated and prepared for sensible, evidence-based discussions with their patients. The ability to summarize the published data and relevant science is especially important given the polarizing nature of the cannabis debate. Reviewing the risks and benefits of cannabinoids with patients in a thoughtful manner will provide patients with information about harms previously unfamiliar to them, enhance adherence to treatments that are grounded in evidence-based medicine, and allow for side effect monitoring and reporting when a decision is made to proceed with the use of CBD under medical supervision. While recognizing that cannabis-related products may become legal recreational drugs, like tobacco and alcohol, it is important for clinicians to consider the definition of a medication and the level of evidence required to prove a medication safe and effective. As in the hydroxychloroquine for COVID-19 debacle, elected officials often are at odds with the FDA, FDA-quality evidence, and the physicians’ oath to first do no harm (101).
1
2 : Use of marijuana for medical purposes among adults in the United States. JAMA 2017; 317:209–211Crossref, Medline, Google Scholar
3 : Relationship of cannabis use disorder and irritable bowel syndrome (IBS): an analysis of 6.8 million hospitalizations in the United States. Subst Use Misuse 2020; 55:281–290Crossref, Medline, Google Scholar
4 : Marijuana use and use disorders in adults in the USA, 2002–14: analysis of annual cross-sectional surveys. Lancet Psychiatry 2016; 3:954–964Crossref, Medline, Google Scholar
5 : Medical use of cannabis in 2019. JAMA 2019; 322:974–975Crossref, Medline, Google Scholar
6 : Clinical uses of cannabis and cannabinoids in the United States. J Neurol Sci 2020; 411:116717Crossref, Medline, Google Scholar
7 : Roles of the M4 acetylcholine receptor in the basal ganglia and the treatment of movement disorders. Mov Disord 2019; 34:1089–1099Crossref, Medline, Google Scholar
8 : The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology 2004; 29:1558–1572Crossref, Medline, Google Scholar
9 : An update on safety and side effects of cannabidiol: a review of clinical data and relevant animal studies. Cannabis Cannabinoid Res 2017; 2:139–154Crossref, Medline, Google Scholar
10 : Cannabinoid- and lysophosphatidylinositol-sensitive receptor GPR55 boosts neurotransmitter release at central synapses. Proc Natl Acad Sci USA 2013; 110:5193–5198Crossref, Medline, Google Scholar
11 : New trends in cannabis potency in USA and Europe during the last decade (2008–2017). Eur Arch Psychiatry Clin Neurosci 2019; 269:5–15Crossref, Medline, Google Scholar
12 : Cannabis use and the risk of developing a psychotic disorder. World Psychiatry 2008; 7:68–71Crossref, Medline, Google Scholar
13 : Medical marijuana, recreational cannabis, and cardiovascular health: a scientific statement from the American Heart Association. Circulation 2020; 142:e131–e152Crossref, Medline, Google Scholar
14 : Adverse health effects of marijuana use. N Engl J Med 2014; 370:2219–2227Crossref, Medline, Google Scholar
15 : Self-reported medical and nonmedical cannabis use among pregnant women in the United States. JAMA 2019; 322:167–169Crossref, Medline, Google Scholar
16 : Prevalence of cannabis use disorders in the United States between 2001–2002 and 2012–2013. JAMA Psychiatry 2015; 72:1235–1242Crossref, Medline, Google Scholar
17 : Alcohol, cigarette, and cannabis use between 2002 and 2016 in pregnant women from a nationally representative sample. JAMA Pediatr 2019; 173:95–96Crossref, Medline, Google Scholar
18 : Monitoring the Future: National Survey Results on Drug Use, 1975–2019: Overview, Key Findings on Adolescent Drug Use. Ann Arbor, University of Michigan, Institute for Social Research, 2020Crossref, Google Scholar
19 : Collision of the COVID-19 and addiction epidemics. Ann Intern Med 2020; 173:61–62Crossref, Medline, Google Scholar
20 : Prevalence, severity, and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One 2020; 15:e0233147Crossref, Medline, Google Scholar
21 : Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control And Prevention. JAMA 2020; 323:1239–1242Crossref, Medline, Google Scholar
22 : COVID-19 risk and outcomes in patients with substance use disorders: analyses from electronic health records in the United States. Mol Psychiatry 2021; 26:30–39Crossref, Medline, Google Scholar
23 : Acute illness associated with cannabis use, by route of exposure: an observational study. Ann Intern Med 2019; 170:531–537Crossref, Medline, Google Scholar
24 : Brain regional differences in CB1 receptor adaptation and regulation of transcription. Life Sci 2013; 92:446–452Crossref, Medline, Google Scholar
25 : Effects of chronic, heavy cannabis use on executive functions. J Addict Med 2011; 5:9–15Crossref, Medline, Google Scholar
26 : Effects of frequent marijuana use on risky decision-making in young adult college students. Addict Behav Rep 2020; 11:199253Google Scholar
27 : Age of onset of marijuana use impacts inhibitory processing. Neurosci Lett 2012; 511:89–94Crossref, Medline, Google Scholar
28 : Association of cannabis use during adolescence with neurodevelopment. JAMA Psychiatry 2021; 78:1–11Crossref, Medline, Google Scholar
29 : Early-onset drug use and risk for drug dependence problems. Addict Behav 2009; 34:319–322Crossref, Medline, Google Scholar
30 : Cannabis use and neurocognitive functioning in a non-clinical sample of users. Addict Behav 2014; 39:994–999Crossref, Medline, Google Scholar
31 : Perinatal exposure to delta-9-tetrahydrocannabinol causes enduring cognitive deficits associated with alteration of cortical gene expression and neurotransmission in rats. Addict Biol 2007; 12:485–495Crossref, Medline, Google Scholar
32 : Effect of long-term cannabis use on axonal fibre connectivity. Brain 2012; 135:2245–2255Crossref, Medline, Google Scholar
33 : Persistent cannabis users show neuropsychological decline from childhood to mid-life. Proc Natl Acad Sci USA 2012; 17:642–649Google Scholar
34 : Association of cannabis with cognitive functioning in adolescents and young adults: a systematic review and meta-analysis. JAMA Psychiatry 2018; 75:585–595Crossref, Medline, Google Scholar
35 : Cannabinoid modulation of eukaryotic initiation factors (eIF2α and eIF2B1) and behavioral cross-sensitization to cocaine in adolescent rats. Cell Rep 2018; 22:2909–2923Crossref, Medline, Google Scholar
36 : Cannabinoid exposure in rat adolescence reprograms the initial behavioral, molecular, and epigenetic response to cocaine. Proc Natl Acad Sci USA 2020; 117:9991–10002Crossref, Medline, Google Scholar
37 : Probability and predictors of the cannabis gateway effect: a national study. Int J Drug Policy 2015; 26:135–142Crossref, Medline, Google Scholar
38 : Is cannabis use associated with an increased risk of onset and persistence of alcohol use disorders? A three-year prospective study among adults in the United States. Drug Alcohol Depend 2016; 161:363–367Crossref, Medline, Google Scholar
39 : Behavioral characterization of the effects of cannabis smoke and anandamide in rats. PLoS One 2016; 11:e0153327Crossref, Medline, Google Scholar
40 : Cannabis addiction and the brain: a review. Focus Am Psychiatr Publ 2019; 17:169–182Medline, Google Scholar
41 WHO Expert Committee on Drug Dependence, Fortieth Report (WHO Technical Report Series, No 1013). Geneva, World Health Organization, 2018Google Scholar
42 : Cannabis and anxiety: a critical review of the evidence. Hum Psychopharmacol 2009; 24:515–523Crossref, Medline, Google Scholar
43 : The association between cannabis use and mood disorders: a longitudinal study. J Affect Disord 2015; 172:211–218Crossref, Medline, Google Scholar
44 : The association between cannabis use and depression: a systematic review and meta-analysis of longitudinal studies. Psychol Med 2014; 44:797–810Crossref, Medline, Google Scholar
45 : Exploring the association between cannabis use and depression. Addiction 2003; 98:1493–1504Crossref, Medline, Google Scholar
46 : Associations of suicidality trends with cannabis use as a function of sex and depression status. JAMA Netw Open 2021; 4:e2113025Crossref, Medline, Google Scholar
47 : The contribution of cannabis use to variation in the incidence of psychotic disorder across Europe (EU-GEI): a multicentre case-control study. Lancet Psychiatry 2019; 6:427–436Crossref, Medline, Google Scholar
48 : Development over time of the population-attributable risk fraction for cannabis use disorder in schizophrenia in Denmark. JAMA Psychiatry 2021; 78:1013–1019Crossref, Medline, Google Scholar
49 : Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull 2014; 40:1509–1517Crossref, Medline, Google Scholar
50 : A large-scale genome-wide association study meta-analysis of cannabis use disorder. Lancet Psychiatry 2020; 7:1032–1045Crossref, Medline, Google Scholar
51 : Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis. Lancet Psychiatry 2019; 6:995–1010Crossref, Medline, Google Scholar
52 : Effect of illicit recreational drugs upon sleep: cocaine, ecstasy, and marijuana. Sleep Med Rev 2008; 12:381–389Crossref, Medline, Google Scholar
53 : Cannabinoid hyperemesis syndrome: diagnosis, pathophysiology, and treatment: a systematic review. J Med Toxicol 2017; 13:71–87Crossref, Medline, Google Scholar
54 : The prevalence of cannabinoid hyperemesis syndrome among regular marijuana smokers in an urban public hospital. Basic Clin Pharmacol Toxicol 2018; 122:660–662Crossref, Medline, Google Scholar
55
56 : Cannabis and cardiovascular disease. Curr Atheroscler Rep 2019; 21:21Crossref, Medline, Google Scholar
57 : Primary causes of hospitalizations and procedures, predictors of in-hospital mortality, and trends in cardiovascular and cerebrovascular events among recreational marijuana users: a five-year nationwide inpatient assessment in the United States. Cureus 2018; 10:e3195Medline, Google Scholar
58 : Cannabis and male fertility: a systematic review. J Urol 2019; 202:674–681Crossref, Medline, Google Scholar
59 : Associations between cigarette smoking and cannabis dependence: a longitudinal study of young cannabis users in the United Kingdom. Drug Alcohol Depend 2015; 148:165–171Crossref, Medline, Google Scholar
60 : Association between self-reported prenatal cannabis use and maternal, perinatal, and neonatal outcomes. JAMA 2019; 322:145–152Crossref, Medline, Google Scholar
61 : Marijuana and tobacco: a major connection? J Addict Dis 2003; 22:51–62Crossref, Medline, Google Scholar
62 : Effect of recreational marijuana sales on police-reported crashes in Colorado, Oregon, and Washington. Arlington, Va, Insurance Institute for Highway Safety, October 2018 (https://www.iihs.org/api/datastoredocument/bibliography/2173)Google Scholar
63 : Cannabis use among drivers in fatal crashes in Washington State before and after legalization (Research Brief). Washington, DC, AAA Foundation for Traffic Safety, 2020 (https://aaafoundation.org/wp-content/uploads/2020/01/19-0637_AAAFTS-WA-State-Cannabis-Use-Among-Drivers-in-Fatal-Crashes_r4.pdf)Google Scholar
64 Impacts of Marijuana Legalization in Colorado: A Report Pursuant to Senate Bill 13-283. Denver, Colorado Department of Public Safety, Division of Criminal Justice, Office of Research and Statistics, October 2018 (https://cdpsdocs.state.co.us/ors/docs/reports/2018-SB13-283_Rpt.pdf)Google Scholar
65 : Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med 2017; 376:2011–2020Crossref, Medline, Google Scholar
66 : Cannabidiol in patients with seizures associated with Lennox-Gastaut syndrome (GWPCARE4): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018; 391:1085–1096Crossref, Medline, Google Scholar
67 : Medical marijuana for treatment of chronic pain and other medical and psychiatric problems: a clinical review. JAMA 2015; 313:2474–2483Crossref, Medline, Google Scholar
68
69 : Cannabinoids for medical use: a systematic review and meta-analysis. JAMA 2015; 313:2456–2473Crossref, Medline, Google Scholar
70 : The effects of cannabis among adults with chronic pain and an overview of general harms: a systematic review. Ann Intern Med 2017; 167:319–331Crossref, Medline, Google Scholar
71 : Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain 2018; 159:1932–1954Crossref, Medline, Google Scholar
72 : Assessment of efficacy and tolerability of medicinal cannabinoids in patients with multiple sclerosis: a systematic review and meta-analysis. JAMA Netw Open 2018; 1:e183485Crossref, Medline, Google Scholar
73 : Summary of evidence-based guideline: complementary and alternative medicine in multiple sclerosis: report of the guideline development subcommittee of the American Academy of Neurology. Neurology 2014; 82:1083–1092Crossref, Medline, Google Scholar
74 : Association of cannabinoid administration with experimental pain in healthy adults: a systematic review and meta-analysis. JAMA Psychiatry 2018; 75:1118–1127Crossref, Medline, Google Scholar
75 : Medical cannabis patterns of use and substitution for opioids and other pharmaceutical drugs, alcohol, tobacco, and illicit substances: results from a cross-sectional survey of authorized patients. Harm Reduct J 2019; 16:9Crossref, Medline, Google Scholar
76 : The efficacy of nabilone, a synthetic cannabinoid, in the treatment of PTSD-associated nightmares: a preliminary randomized, double-blind, placebo-controlled cross-over design study. Psychoneuroendocrinology 2015; 51:585–588Crossref, Medline, Google Scholar
77 : Marijuana use is associated with worse outcomes in symptom severity and violent behavior in patients with posttraumatic stress disorder. J Clin Psychiatry 2015; 76:1174–1180Crossref, Medline, Google Scholar
78 : The short-term impact of 3 smoked cannabis preparations versus placebo on PTSD symptoms: a randomized cross-over clinical trial. PLoS One 2021; 16:e0246990Crossref, Medline, Google Scholar
79 : Dronabinol for the treatment of cannabis dependence: a randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend 2011; 116:142–150Crossref, Medline, Google Scholar
80 : Nabilone pharmacotherapy for cannabis dependence: a randomized, controlled pilot study. Am J Addict 2017; 26:795–801Crossref, Medline, Google Scholar
81 : The future of endocannabinoid-oriented clinical research after CB1 antagonists. Psychopharmacology (Berl) 2009; 205:171–174Crossref, Medline, Google Scholar
82 : Reducing cannabinoid abuse and preventing relapse by enhancing endogenous brain levels of kynurenic acid. Nat Neurosci 2013; 16:1652–1661Crossref, Medline, Google Scholar
83 : Cannabidiol for the reduction of cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder: a double-blind randomized placebo-controlled trial. Am J Psychiatry 2019; 176:911–922Link, Google Scholar
84 : Cannabis use during treatment for alcohol use disorders predicts alcohol treatment outcomes. Addiction 2017; 112:685–694Crossref, Medline, Google Scholar
85 : Medical cannabis laws and opioid analgesic overdose mortality in the United States, 1999–2010. JAMA Intern Med 2014; 174:1668–1673Crossref, Medline, Google Scholar
86 : Association between medical cannabis laws and opioid overdose mortality has reversed over time. Proc Natl Acad Sci USA 2019; 116:12624–12626Crossref, Medline, Google Scholar
87 14% of Americans say they use CBD products. Gallup, August 7, 2019. https://news.gallup.com/poll/263147/americans-say-cbd-products.aspxGoogle Scholar
88 : Cannabidiol: state of the art and new challenges for therapeutic applications. Pharmacol Ther 2017; 175:133–150Crossref, Medline, Google Scholar
89 : Leading the next CBD wave: safety and efficacy. JAMA Psychiatry 2020; 77:341–342Crossref, Medline, Google Scholar
90 : Labeling accuracy of cannabidiol extracts sold online. JAMA 2017; 318:1708–1709Crossref, Medline, Google Scholar
91 : The trouble with CBD oil. Med Cannabis Cannabinoids 2018; 1:65–72Crossref, Medline, Google Scholar
92 : Cannabidiol enhancement of exposure therapy in treatment refractory patients with phobias: study protocol of a randomized controlled trial. BMC Psychiatry 2019 ; 19:69Crossref, Medline, Google Scholar
93 : A pilot randomised placebo-controlled trial of cannabidiol to reduce severe behavioural problems in children and adolescents with intellectual disability. Br J Clin Pharmacol 2021; 87:436–446Crossref, Medline, Google Scholar
94 : Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl Psychiatry 2012; 2:e94Crossref, Medline, Google Scholar
95 : Cannabidiol (CBD) as an adjunctive therapy in schizophrenia: a multicenter randomized controlled trial. Am J Psychiatry 2018; 175:225–231Link, Google Scholar
96 : The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia: a randomized placebo controlled trial. Psychopharmacology (Berl) 2018; 235:1923–1932Crossref, Medline, Google Scholar
97 : Cannabidiol interactions with medications, illicit substances, and alcohol: a comprehensive review. J Gen Intern Med 2021; 36:2074–2084Crossref, Medline, Google Scholar
98 : Cannabidiol and abnormal liver chemistries in healthy adults: results of a phase I clinical trial. Clinical Pharm Ther 2021; 109:1224–1231Crossref, Medline, Google Scholar
99 : Psychedelics and psychedelic-assisted psychotherapy. Am J Psychiatry 2020; 177:391–410Link, Google Scholar
100 : Importance of a standard unit dose for cannabis research. Addiction 2020; 115:1219–1221Crossref, Medline, Google Scholar
101 : Medicinal marijuana, stress, anxiety, and depression: primum non nocere. Missouri Medicine 2020; 117:410–415Google Scholar



