REL-1017 (Esmethadone) as Adjunctive Treatment in Patients With Major Depressive Disorder: A Phase 2a Randomized Double-Blind Trial
Abstract
Objective: The purpose of this study was to examine the effects of REL-1017 (esmethadone), a novel N-methyl-d-aspartate receptor (NMDAR) channel blocker, in patients with major depressive disorder who failed to benefit from one to three standard antidepressant treatments in their current major depressive episode.
Methods: A 7-day phase 2 multicenter randomized double-blind placebo-controlled trial, comprising three arms, was conducted to assess the safety, tolerability, pharmacokinetics, and efficacy of two dosages of REL-1017 (25 mg or 50 mg orally once a day). Patients were randomly assigned in a 1:1:1 ratio to placebo (N=22), REL-1017 25 mg/day (N=19), or REL-1017 50 mg/day (N=21). Safety scales included the 4-item Positive Symptom Rating Scale for psychotomimetic symptoms, the Clinician-Administered Dissociative States Scale for dissociative symptoms, the Clinical Opiate Withdrawal Scale for withdrawal signs and symptoms, and the Columbia-Suicide Severity Rating Scale for suicidality. The primary efficacy endpoint was the Montgomery‐Åsberg Depression Scale (MADRS) score. All 62 randomly assigned patients were included in the full analysis set population analysis.
Results: Patients experienced mild or moderate transient adverse events and no evidence of dissociative or psychotomimetic effects, opioid effects, or withdrawal signs and symptoms. The improvement in MADRS score shown on day 4 in both of the REL-1017 dosage groups was sustained through day 7 (last dose) and day 14 (7 days after the last dose), with effect sizes from 0.7 to 1.0.
Conclusions: This trial showed favorable safety, tolerability, and pharmacokinetic profiles and suggests that REL-1017 may have rapid and sustained antidepressant effects compared with placebo in patients with inadequate responses to antidepressant treatments. These results will need confirmation in larger and longer trials.
Major depressive disorder is the second leading cause of disability and chronic disease burden in the United States, among all medical conditions, as measured by disability-adjusted life years (1). Data from the National Epidemiologic Survey on Alcohol and Related Conditions–III show that the 12-month and lifetime prevalences of major depressive disorder are 10.4% and 20.6%, respectively (2). Some 50%–60% of patients with major depressive disorder do not obtain adequate response following the first standard antidepressant treatment (3). Response to treatment is generally delayed by 4–8 weeks after initiation. Therefore, there is a clear need to develop rapid-onset treatments with improved efficacy. N-methyl-d-aspartate receptor (NMDAR) channel blockers, such as ketamine and esketamine, are emerging as a new drug class with potentially rapid and effective antidepressant activity in patients with major depressive disorder with inadequate response to antidepressant treatments (4). However, the adoption of intravenous ketamine and intranasal esketamine has been limited by dissociative and psychotomimetic effects requiring clinical patient supervision during and after administration (5). Recent evidence indicates that the two ketamine isomers may differ in their pharmacologic profile. In particular, only esketamine may exert opioid effects, and therefore esketamine may contribute more than arketamine to the side effects and abuse risk of racemic ketamine (6). Chiral configuration is known to impart opioid activity to molecules: as a rule, for chiral molecules, only one of the two enantiomers is opioid active (7–9).
REL-1017 (esmethadone; dextromethadone) is the opioid inactive dextro-isomer of racemic methadone and is a low-affinity, low-potency NMDAR channel blocker that binds the MK-801 site of the NMDAR with low-micromolar half-maximal inhibitory concentration (IC50) value (9). Esmethadone has 20-fold lower affinity for mu opioid receptors compared with levomethadone (10) and does not appear to contribute in a meaningful way to the opioid effects of racemic methadone, which are instead a result of its enantiomer, levomethadone (7, 8, 11–16). Additionally, esmethadone inhibits serotonin and norepinephrine transporters, with affinities in the micromolar range that are approximately 500-fold (serotonin transporters) and 100-fold (norepinephrine transporters) lower than those seen for duloxetine (17). These 100-plus-fold differences in IC50 compared with duloxetine suggest that a primary monoaminergic antidepressant mechanism of action for esmethadone may be less likely. In addition, esmethadone appears to have favorable tolerability, safety, and pharmacokinetic profiles and does not produce clinically meaningful opioid or psychotomimetic effects (13). According to a recent U.S. Drug Enforcement Administration statement on racemic methadone, esmethadone “lacks significant respiratory depressant action and abuse liability” (14).
In preclinical studies, REL-1017 is devoid of opioid effects (15, 16) and improves the phenotype of rodents in all tested models of depressed behavior (18, 19), increasing levels of synaptic proteins in the medial prefrontal cortex of mice (19). Similar to ketamine, the antidepressant-like effects of REL-1017 were found to be mediated by mammalian target of rapamycin complex 1 and brain-derived neurotrophic factor (BDNF), signaling its potential for neural plasticity modulation via NMDAR channel block (19).
REL-1017 administered orally at a daily dose of 25 mg increased plasma BDNF levels in healthy volunteers undergoing a 14-day inpatient phase 1 study (20).
In this study, we hypothesized that REL-1017 would confirm, in patients with major depressive disorder, the favorable tolerability, safety, and pharmacokinetic profiles observed in phase 1 trials (12). Additionally, based on available preclinical and clinical data (9, 18–20), we hypothesized that REL-1017 would provide rapid-onset and effective treatment for patients with major depressive disorder.
METHODS
Study Design
This was a 7-day double-blind placebo-controlled randomized phase 2a trial with three arms (1:1:1 ratio): two drug treatment groups (two dosages) and one placebo group. The study was conducted in inpatients at 10 centers in the United States (a list of sites and principal investigators is provided in the online supplement) from May 2018 to August 2019. Essential trial documents were reviewed and approved by an institutional review board at each trial site, and written informed consent was obtained from all participants after they had received a complete description of the study and prior to any study procedures. The patient screening process and rater review process for patient eligibility are presented in the online supplement. Raters were required to demonstrate sufficient assessment experience and experience in treating patients with major depressive disorder and required to obtain certification and/or training prior to rating participants entering into the study. The overall duration of the trial, including the screening period, was 51 days (Figure 1).
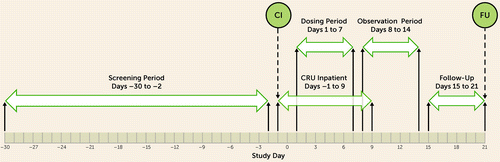
FIGURE 1. Trial study designa
a CI=check-in; CRU=clinical research unit; FU=follow-up.
Participants
A total of 62 adult patients (ages 18–65 years; 45% female) with a diagnosis of major depressive disorder participated in the trial. Patients were eligible if they met criteria for major depressive disorder as defined by DSM-5 criteria, if their diagnosis was confirmed by the Mini-International Neuropsychiatric Interview, version 7.0.2 (21), if they received a score ≥19 on the 17-item Hamilton Depression Rating Scale (HAM-D) (22, 23), and if they had a body mass index between 18 and 35. Patients were also required to have experienced a current major depressive episode lasting 8 weeks to 36 months and to have had an inadequate response to one to three courses of antidepressant treatment in the same major depressive episode as determined by an independent SAFER rater who administered the Antidepressant Treatment Response Questionnaire (24). Patients had to be on the same selective serotonin reuptake inhibitor, the same serotonin-norepinephrine reuptake inhibitor, or bupropion for at least 8 weeks prior to screening, maintaining the same adequate dosage for the last 4 weeks. Exclusion criteria included the following: more than three prescribed doses of opioids within 6 months; any opioid use within the last month prior to screening; evidence of clinically significant hepatic or renal impairment; history of clinically diagnosed hypotension requiring treatment; positive pregnancy test; use of antipsychotics, anticonvulsants, or mood stabilizers within 3 months prior to screening; and a history of bipolar disorder, psychotic disorder, posttraumatic stress disorder, borderline personality disorder, antisocial personality disorder, or similar disorder. A complete list of the trial inclusion and exclusion criteria is provided in the online supplement.
Randomization and Masking
Patients were assigned treatment by an unblinded pharmacist through an interactive web response system in a 1:1:1 randomization ratio to 25 mg of REL-1017, 50 mg of REL-1017, or placebo. The randomization code used in the interactive web response system was prepared by a statistician who was not working as a statistician on the study in any other capacity. The REL-1017 powder was dissolved in diet cranberry juice to mask the taste. Randomization was stratified for patients receiving concomitant antidepressants that were cytochrome 2B6 inhibitors (i.e., sertraline, paroxetine, and fluoxetine) but not for those who were not. Benzodiazepines and nonbenzodiazepine sleep aids taken as scheduled medications and at stable doses (not higher than the doses allowed in the label approved by the U.S. Food and Drug Administration [FDA]) for at least 30 days prior to check-in were allowed. Initiation of these medications during the trial was not allowed. Any medication taken consistently by the patient for 30 days prior to screening that was not a prohibited medication could be continued during the trial. Prohibited medications are detailed in the exclusion criteria (see the online supplement).
Procedures
All patients were admitted to an inpatient clinical research unit for approximately 10 days, during which time they received daily doses of REL-1017 or placebo for 7 consecutive days, along with a standard diet. On day 1, patients in the 25-mg and 50-mg dosing groups were administered a loading dose of 75 mg or 100 mg of REL-1017 or placebo. The loading dose was computed based on phase 1 pharmacokinetic data (12) in order to achieve a steady-state concentration by day 1. On days 2–7, patients received 25 mg of REL-1017, 50 mg of REL-1017, or placebo. Study drug was administered in the morning.
Patients remained under clinical supervision for at least 48 hours after the last dose on day 7. Patients were discharged on day 9 and returned to the clinical research unit 7 days after their last dose of study drug for follow-up evaluations. Study personnel telephoned each patient 14 days after the last dose of study drug and conducted a follow-up interview to assess the patient’s condition.
Outcomes
The primary objectives were safety and tolerability of 25 mg of REL-1017 and 50 mg of REL-1017 compared with placebo. Safety assessments included the evaluation of frequency and severity of adverse events, as well as changes in clinical laboratory parameters, electrocardiogram parameters, physical examinations, vital signs, weight, and body temperature. Other safety assessments included assessment of suicidal ideation and behavior with the Columbia-Suicide Severity Rating Scale (C-SSRS; a higher score indicates a higher intensity of suicidal ideation) (25); assessment of present-state dissociative symptoms with the Clinician-Administered Dissociative States Scale (CADSS; a higher score indicates a higher likelihood of the presence of a dissociative state) (26); assessment of opioid withdrawal with the Clinical Opiate Withdrawal Scale (27); and assessment of psychotic symptoms with the 4-item Positive Symptom Rating Scale.
The first efficacy endpoint was the change from baseline to day 7 in score on the Montgomery-Åsberg Depression Rating Scale (MADRS) (28). Other listed efficacy endpoints were change from baseline on the Symptoms of Depression Questionnaire (SDQ) score (29) and the Clinical Global Impressions severity scale (CGI-S) and improvement scale (CGI-I) scores (30).
Statistical Analysis
The safety analysis set population was used for all analyses of safety endpoints and included all randomly assigned patients who received any trial treatment (REL-1017 or placebo). The full analysis set population was used for all analyses of efficacy endpoints and consisted of all randomly assigned patients who received any trial treatment and had a baseline measurement and at least one postbaseline measurement (on the MADRS, SDQ, CGI-S, or CGI-I). The trial was not powered for efficacy detection; however, p values and effect size calculations were performed for efficacy measures, and no adjustments for multiple comparisons were made. In this trial, all randomly assigned patients were included in the safety analysis set and full analysis set populations.
The change from baseline in total score on each questionnaire (MADRS, SDQ, and CGI-S) on days 4, 7, and 14 was compared among treatment groups using a mixed model of repeated measurement, with a model equation containing treatment group, stratification (concomitant antidepressant drug), visit (day 2, day 4, day 7, and day 14), and interaction between treatment and visit as fixed terms; baseline MADRS score as a covariate; and visit as the repeated-measure factor. Least square means with standard errors, differences in least square means with standard errors, 90% confidence intervals for the difference in least square means, and corresponding p values are presented. In addition, Cohen’s d effect size and its 90% confidence interval were estimated to measure the magnitude of REL-1017 effect compared with placebo. A mixed model of repeated measurement based on the missing-at-random assumption was used to address missing efficacy data (for the MADRS, SDQ, CGI-S, and CGI-I). Patients who discontinued the trial prematurely were not replaced. All analyses were based on available data, and no imputation was made for missing data. Statistical analyses were performed using SAS, version 9.4.
RESULTS
Sixty-two patients were randomly assigned to the full analysis set population: 22 patients (35.5%) to the placebo group, 19 patients (30.6%) to the REL-1017 25-mg treatment group, and 21 patients (33.9%) to the REL-1017 50-mg treatment group (see Figure S1 in the online supplement). Of these, 57 patients (91.9%) completed the trial. Among the five patients who discontinued participation in the trial early, three were lost to follow-up (one patient in the placebo group and two patients in the REL-1017 50-mg group). Among the remaining two patients, one in the REL-1017 25-mg group withdrew from the trial, and one patient in the placebo group was withdrawn for not meeting inclusion criterion 1 (males and females between 18 and 65 years old, inclusive; see Table S1 in the online supplement).
The safety analysis set population comprised 62 patients: 34 men (54.8%) and 28 women (45.2%). Twenty-two patients (35.5%) were Caucasian, 39 (62.9%) were Black or African American, and one (1.6%) was Asian. There were no notable differences in demographic and other baseline characteristics between the treatment groups (Table 1).
| Characteristic | Placebo (N=22) | REL-1017 25 mg (N=19) | REL-1017 50 mg (N=21) | All Patients (N=62) | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (years)b | 49.7 | 11.1 | 49.4 | 12.4 | 48.6 | 10.9 | 49.2 | 11.3 |
| N | % | N | % | N | % | N | % | |
| Sex | ||||||||
| Male | 11 | 50.0 | 11 | 57.9 | 12 | 57.1 | 34 | 54.8 |
| Female | 11 | 50.0 | 8 | 42.1 | 9 | 42.9 | 28 | 45.2 |
| Ethnicity | ||||||||
| Hispanic or Latino | 1 | 4.5 | 1 | 5.3 | 0 | 0.0 | 2 | 3.2 |
| Not Hispanic or Latino | 21 | 95.5 | 18 | 94.7 | 21 | 100.0 | 60 | 96.8 |
| Race | ||||||||
| Asian | 0 | 0 | 0 | 0 | 1 | 4.8 | 1 | 1.6 |
| Black or African American | 13 | 59.1 | 13 | 68.4 | 13 | 61.9 | 39 | 62.9 |
| Caucasian | 9 | 40.9 | 6 | 31.6 | 7 | 33.3 | 22 | 35.5 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Body mass indexc | 29.02 | 4.27 | 27.66 | 3.33 | 27.66 | 5.00 | 28.15 | 4.26 |
| Baseline HAM-D score | 25.6 | 3.5 | 25.1 | 3.5 | 25.0 | 3.8 | 25.3 | 3.6 |
TABLE 1. Baseline demographic and clinical characteristics of patients with major depressive disorder in the safety analysis seta
The mean baseline HAM-D score was 25.6 for the placebo group, 25.1 for the REL-1017 25-mg dosing group, and 25.0 for the REL-1017 50-mg dosing group. A list of the most common concomitant medications is provided in Table S10 in the online supplement. All patients enrolled in the study (N=62; 100%) met the inclusion criterion of having failed to benefit from one to three treatments for their current episode of major depressive disorder. The majority of patients (N=54; 87.1%) had failed to benefit from one prior treatment during their current episode. Seven patients (11.3%) had failed to benefit from two treatments, with one (4.5%), two (10.5%), and four (19.0%) patients in the placebo, REL-1017 25-mg, and REL-1017 50-mg groups, respectively. One patient (4.8%) in the REL-1017 50-mg group had failed to benefit from three prior treatments.
There were no serious adverse events, and no patient experienced treatment-emergent adverse events that resulted in treatment discontinuation or discontinuation from the trial. A total of 36 (58.1%) patients experienced at least one treatment-emergent adverse event. Treatment-emergent adverse events were reported by 12 (54.5%) patients in the placebo group, nine (47.4%) patients in the REL-1017 25-mg group, and 15 (71.4%) patients in the REL-1017 50-mg group (Table 2). The most common treatment-emergent adverse events that occurred in at least 5% of all patients were headache, constipation, nausea, and somnolence (Table 2).
| Placebo (N=23) | REL-1017 25 mg (N=19) | REL-1017 50 mg (N=21) | All Patients (N=62) | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | N | % | N | % | N | % | N | % |
| Patients with a serious adverse event | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Patients with a severe treatment-emergent adverse event | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Patients with at least one adverse event | 12 | 54.5 | 9 | 47.4 | 15 | 71.4 | 36 | 58.1 |
| Treatment-emergent adverse events occurring in three or more patients | ||||||||
| Constipation | 3 | 13.6 | 1 | 5.3 | 3 | 14.3 | 7 | 11.3 |
| Nausea | 2 | 9.1 | 1 | 5.3 | 2 | 9.5 | 5 | 8.1 |
| Diarrhea | 3 | 13.6 | 0 | 0.0 | 0 | 0.0 | 3 | 4.8 |
| Headache | 3 | 13.6 | 2 | 10.5 | 3 | 14.3 | 8 | 12.9 |
| Somnolence | 2 | 9.1 | 1 | 5.3 | 1 | 4.8 | 4 | 6.5 |
| Dizziness | 1 | 4.5 | 1 | 5.3 | 1 | 4.8 | 3 | 4.8 |
| Back pain | 0 | 0.0 | 1 | 5.3 | 2 | 9.5 | 3 | 4.8 |
TABLE 2. Treatment-emergent adverse events by preferred term in patients with major depressive disorder in the safety analysis seta
In the assessment of suicidal behavior, the C-SSRS was measured at screening and on the day before the start of treatment and on days 1, 2, 8, and 9 (discharge), day 14 (end of observation period), and day 21 (follow-up). No patient had suicidal behavior during the trial, and three patients, one per treatment group, answered “yes” to the question “wish to be dead” at least once. In the assessment of opioid withdrawal, the Clinical Opiate Withdrawal Scale was administered at days 8, 9, and 14. Mean scores were comparable across treatment groups and were ≤1 at days 8, 9, and 14, indicating that opioid withdrawal was not present. In the assessment of present-state dissociative symptoms, the CADSS was administered before dosing and 2 hours after dosing on day 1, 2 hours after dosing on day 7, and prior to discharge on day 9. No dose-related trends were observed in CADSS scores. In the assessment of psychotic symptoms, the 4-item Positive Symptom Rating Scale was administered before dosing and 2 hours after dosing on day 1, 2 hours after dosing on day 7, and prior to discharge on day 9. Patients had no psychotic symptoms during the trial. There was no adverse event of special interest in the REL-1017 treatment groups compared with the placebo group. There were no instances of clinically meaningful QTc prolongation. Additional tables and a discussion of safety results are presented in the online supplement.
This study was not powered for efficacy detection, and p values are presented for descriptive purposes. For the full analysis set population, the mean MADRS score at baseline was 33.8 (SD=4.0) in the placebo group, 32.9 (SD=6.0) in the REL-1017 25-mg group, and 35.2 (SD=3.9) in the REL-1017 50-mg group (see Table S2 in the online supplement). For MADRS scores on day 7, the least square mean difference compared with the placebo group was −8.7 (90% CI=−14.3, −3.1, p=0.0122; effect size=0.8) for the REL-1017 25-mg group and −7.2 (90% CI=−12.7, −1.8, p=0.0308; effect size=0.7) for the REL-1017 50-mg group (Table 3, Figure 2A). On day 14, the least square mean difference compared with the placebo group was −9.4 (90% CI=−15.4, −3.5, p=0.0103; effect size=0.9) for the REL-1017 25-mg group and −10.4 (90% CI=−16.1, −4.6, p=0.0039; effect size=1.0) for the REL-1017 50-mg group. There was improvement on MADRS scores on day 4 in both REL-1017 groups, and the improvement continued through day 7 and day 14, 7 days after treatment discontinuation, with a p value ≤0.0308 and effect sizes ranging from 0.7 to 1.0 (Table 3, Figure 2A). The mean change from baseline (day 1) in MADRS scores showed more improvement at day 7 (end of dosing period) for both the 25-mg and 50-mg REL-1017 dosing groups (−16.8 [SD=11.3] for the REL-1017 25-mg treatment group and −16.6 [SD=9.8] for the REL-1017 50-mg treatment group) compared with the placebo group (−8.8 [SD=12.5]).
| Measure, Time Point, and Group | N | Least Square Meanb | SE | Difference of Least Square Mean Drug Versus Placeboc | 90% CI | Effect Sized | pe |
|---|---|---|---|---|---|---|---|
| MADRS | |||||||
| Day 7 | |||||||
| Placebo | 21 | –8.7 | 2.3 | ||||
| REL-1017 25 mg | 19 | –17.4 | 2.5 | –8.7 | –14.3, –3.1 | 0.8 | 0.0122 |
| REL-1017 50 mg | 21 | –15.9 | 2.4 | –7.2 | –12.7, –1.8 | 0.7 | 0.0308 |
| Day 14 | |||||||
| Placebo | 20 | –7.4 | 2.4 | ||||
| REL-1017 25 mg | 16 | –16.8 | 2.7 | –9.4 | –15.4, –3.5 | 0.9 | 0.0103 |
| REL-1017 50 mg | 18 | –17.8 | 2.6 | –10.4 | –16.1, –4.6 | 1.0 | 0.0039 |
| SDQ | |||||||
| Day 7 | |||||||
| Placebo | 21 | –37.9 | 6.4 | ||||
| REL-1017 25 mg | 19 | –52.4 | 7.1 | –14.5 | –30.0, 1.0 | 0.5 | 0.1237 |
| REL-1017 50 mg | 21 | –52.9 | 6.6 | –15.0 | –30.1, 0.1 | 0.5 | 0.1017 |
| Day 14 | |||||||
| Placebo | 20 | –31.8 | 5.6 | ||||
| REL-1017 25 mg | 16 | –55.0 | 6.4 | –23.2 | –36.9, –9.4 | 0.9 | 0.0066 |
| REL-1017 50 mg | 18 | –58.6 | 6.0 | –26.8 | –40.1, –13.5 | 1.1 | 0.0014 |
| CGI-S | |||||||
| Day 7 | |||||||
| Placebo | 21 | –0.8 | 0.3 | ||||
| REL-1017 25 mg | 19 | –1.7 | 0.3 | –1.0 | –1.6, –0.3 | 0.7 | 0.0245 |
| REL-1017 50 mg | 21 | –1.7 | 0.3 | –0.9 | –1.6, –0.2 | 0.7 | 0.0253 |
| Day 14 | |||||||
| Placebo | 20 | –0.7 | 0.3 | ||||
| REL-1017 25 mg | 16 | –1.6 | 0.3 | –0.9 | –1.7, –0.2 | 0.7 | 0.0454 |
| REL-1017 50 mg | 18 | –2.0 | 0.3 | –1.3 | –2.0, –0.6 | 0.9 | 0.0043 |
| CGI-I | |||||||
| Day 7 | |||||||
| Placebo | 21 | 3.2 | 0.2 | ||||
| REL-1017 25 mg | 19 | 2.4 | 0.2 | –0.8 | –1.3, –0.2 | 0.8 | 0.0177 |
| REL-1017 50 mg | 21 | 2.3 | 0.2 | –0.9 | –1.4, –0.3 | 0.9 | 0.0072 |
| Day 14 | |||||||
| Placebo | 20 | 3.3 | 0.3 | ||||
| REL-1017 25 mg | 16 | 2.6 | 0.3 | –0.7 | –1.3, 0.0 | 0.6 | 0.0895 |
| REL-1017 50 mg | 18 | 2.3 | 0.3 | –1.0 | –1.6, –0.4 | 0.8 | 0.0109 |
TABLE 3. Efficacy assessment at day 7 (end of dosing period, postdose) and day 14 (end of observation period) in patients with major depressive disorder in the full analysis set populationa
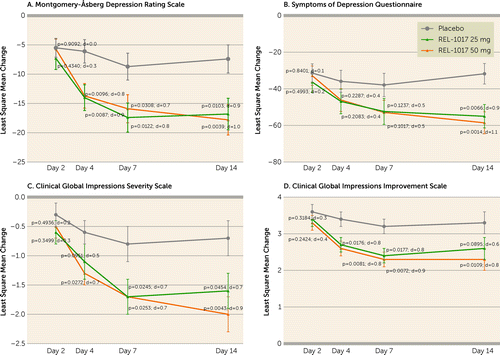
FIGURE 2. Efficacy endpoints in patients receiving placebo (N=22), REL-1017 25 mg/day (N=19), or REL-1017 50 mg/day (N=21)a
a Panels A–D show the change from baseline in the least square mean from day 2 through day 14, compared with placebo, in the full analysis set population for the Montgomery-Åsberg Depression Rating Scale, the Symptoms of Depression Questionnaire, the Clinical Global Impressions severity scale, and the Clinical Global Impressions improvement scale, respectively. Error bars indicate standard error of the mean. The p values and Cohen’s d values indicate drug treatment compared with placebo.
Results of the other efficacy endpoints were similar to that for the MADRS. For the SDQ, the least square mean difference on day 14 showed improvement in the REL-1017 25-mg group (−23.2, p=0.0066; effect size=0.9) and REL-1017 50-mg group (−26.8, p=0.0014; effect size=1.1) (Table 3, Figure 2B). For the CGI-S, the changes from baseline on day 7 and day 14 (end of observation period) showed improvement among patients in both the REL-1017 25-mg and REL-1017 50-mg treatment groups compared with the placebo group (Table 3, Figure 2C). On day 7 after dosing (end of dosing period), the CGI-I scale revealed a mean score of 3.2 (SE=0.2) for the placebo group, compared with a mean score of 2.4 (SE=0.2) for the REL-1017 25-mg group and 2.3 (SE=0.2) for the REL-1017 50-mg group (Table 3, Figure 2D).
Remission rates (where remission was defined as a MADRS score ≤10) on day 14, the last day of efficacy assessment, were 5%, 31% (p=0.035), and 39% (p=0.01) for the placebo group, the REL-1017 25-mg group, and the REL-1017 50-mg, respectively. The number needed to treat to achieve remission on day 14 was 4 for the REL-1017 25-mg group and 3 for the REL-1017 50-mg group.
SDQ subscales were used to measure subsets of assessed symptoms: lassitude, mood, cognitive/social functioning (SDQ-1); anxiety, agitation, anger, and irritability (SDQ-2); desire to be dead (SDQ-3); disruptions in sleep quality (SDQ-4); and changes in appetite and weight (SDQ-5). On day 14 (end of observation period), the difference of the least square mean (90% CI) between the placebo group and the REL-1017 25-mg and REL-1017 50-mg groups showed improvement for both tested doses on the SDQ-1 (p=0.0025, effect size=1.0, and p=0.0009, effect size=1.1, respectively), the SDQ-2 (p=0.0398, effect size=0.7, and p=0.0012, effect size=1.1, respectively), and the SDQ-4 (p=0.0055, effect size=1.0, and p=0.0029, effect size=1.0, respectively).
Additional tables and a discussion of efficacy results are presented in the Appendix and Efficacy Results section in the online supplement.
DISCUSSION
This trial in patients with major depressive disorder and no significant psychiatric comorbidity confirmed the favorable safety, tolerability, and pharmacokinetic profiles of REL-1017 observed in phase 1 trials (12). Additionally, REL-1017 induced robust, rapid, and sustained therapeutic effects when given as an adjunctive treatment to patients with major depressive disorder and inadequate response to at least one, and up to three, adequate antidepressant treatments. Importantly, the selected dosages achieved antidepressant effects without typical opioid or psychotomimetic symptoms and without withdrawal effects on abrupt discontinuation. The sustained therapeutic efficacy after treatment discontinuation on day 7, extending at least to day 14, supports preclinical findings, suggesting that REL-1017 may improve depressive phenotypes by modulating neural plasticity (19) and may signal potential disease-modifying effects, rather than symptomatic effects caused by direct mu opioid receptor occupancy or monoaminergic effects. Effects determined by mu opioid receptor occupancy by an opioid agonist typically cease and rebound after drug discontinuation. While a 7-day treatment course may be too short to result in opioid withdrawal symptoms after abrupt discontinuation, depressive symptoms masked by opioid agonist actions would be expected to cease and rebound 24–48 hours after treatment discontinuation.
The lack of typical opioid effects and the lack of a separation in dose response between the tested 25-mg and 50-mg dosages are also suggestive of a mechanism of action unrelated to direct mu opioid receptor effects, which tend to increase with the opioid dose (8). Lack of dose separation and sustained improvement 7 days after discontinuation may signal a mechanism related to the hypothesized NMDAR channel-blocking effects of REL-1017 (9), similar to ketamine (19), which has sustained therapeutic effects and has also shown a lack of meaningful separation in dose response for two intravenous doses (0.5 mg/kg and 1 mg/kg) in major depressive disorder (31).
In a recent clinical study, naltrexone dramatically blocked the antidepressant effects of ketamine but not the dissociative effects, suggesting that ketamine’s acute antidepressant effects require a functional opioid system (32). Recent experimental work showed that ketamine does not act as an opioid, but its antidepressant effects require both NMDAR and opioid receptor signaling, suggesting that interactions between these two neurotransmitter systems are necessary to achieve antidepressant effects (33). As a result of similar postulated mechanisms of action of ketamine and esmethadone in experimental models of depressive-like behavior (19), it is also conceivable that a functional endorphin system may be necessary for the therapeutic effects of other antidepressant NMDAR channel blockers, including REL-1017.
The results of this trial suggest that dissociative effects may not be necessary for the antidepressant effects of NMDAR channel blockers. The lack of dissociative side effects in patients treated with REL-1017 suggests potential sparing of physiologically operating NMDARs. In contrast, higher-potency NMDAR channel blockers, such as ketamine and esketamine, cause temporary dissociative symptoms in the majority of patients at doses effective for major depressive disorder, indicating that the NMDAR block exerted by these drugs may extend, at least temporarily, to physiologically operating NMDARs (5).
Additionally, as pointed out by different authors of ketamine studies (6, 32, 34), the potential role of the opioid system and other receptor systems in the antidepressant effects of putative NMDAR antagonists, including ketamine and REL-1017, will require further studies in order to be fully elucidated. REL-1017 will be tested in studies appropriately designed to adequately characterize its abuse potential before undergoing consideration by the FDA for approval for patients with major depressive disorder.
The main limitations of this trial are its relatively small sample size and its relatively short duration. However, the robust effect sizes and the sustained improvement on all efficacy measures through day 14 suggest that these limitations did not undermine the assay sensitivity and the clinical implications of the trial results.
In summary, in this phase 2a trial in patients with major depressive disorder and inadequate responses to antidepressant treatments, REL-1017 confirmed favorable safety, tolerability, and pharmacokinetic profiles and showed a robust signal for rapid and sustained antidepressant effects that warrant confirmation in larger and longer trials. Ongoing phase 3 trials to confirm the efficacy and safety of REL-1017 are registered at ClinicalTrials.gov.
1 : Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study Lancet 2015; 386(9995):743–800Crossref, Medline, Google Scholar
2 : Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry 2018; 75:336–346Crossref, Medline, Google Scholar
3 : Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am 1996; 19:179–200Crossref, Medline, Google Scholar
4 : NMDA antagonists under investigation for the treatment of major depressive disorder. Expert Opin Investig Drugs 2014; 23:1181–1192Crossref, Medline, Google Scholar
5 : Antidepressant efficacy and tolerability of ketamine and esketamine: a critical review. CNS Drugs 2018; 32:411–420Crossref, Medline, Google Scholar
6 : Pharmacological and behavioral divergence of ketamine enantiomers: implications for abuse liability. Mol Psychiatry (Online ahead of print, April 15, 2021) Crossref, Medline, Google Scholar
7 : Synthetic substances with morphine-like effect: clinical experience; potency, side-effects, addiction liability. Bull World Health Organ 1957; 17:569–863Medline, Google Scholar
8 : Mu opioids and their receptors: evolution of a concept. Pharmacol Rev 2013; 65:1257–1317Crossref, Medline, Google Scholar
9 : The d- and l-isomers of methadone bind to the non-competitive site on the N-methyl-d-aspartate (NMDA) receptor in rat forebrain and spinal cord. Neurosci Lett 1997; 223:5–8Crossref, Medline, Google Scholar
10 : Serotonin and norepinephrine uptake inhibiting activity of centrally acting analgesics: structural determinants and role in antinociception. J Pharmacol Exp Ther 1995; 274:1263–1270Medline, Google Scholar
11 : Feasability and safety of transfer from racemic methadone to (R)-methadone in primary care: clinical results from an open study. World J Biol Psychiatry 2009; 10:217–224Crossref, Medline, Google Scholar
12 : Characterization of the safety and pharmacokinetic profile of dextromethadone, a novel N-methyl-d-aspartate receptor antagonist in healthy, opioid-naïve subjects: results of two phase 1 studies. J Clin Psychopharmacol 2019; 39:226–237Crossref, Medline, Google Scholar
13 : The addiction liability of some drugs of the methadon series. J Pharmacol Exp Ther 1948; 93:305–313Medline, Google Scholar
14 US Drug Enforcement Administration: Drug and chemical information section: methadone. Springfield, Va, US Drug Enforcement Administration, Diversion, Control Division. https://www.deadiversion.usdoj.gov/drug_chem_info/methadone/methadone.pdfGoogle Scholar
15 : Morphine, oxycodone, methadone and its enantiomers in different models of nociception in the rat. Anesth Analg 2006; 102:1768–1774Crossref, Medline, Google Scholar
16 : An analysis of precipitated withdrawal in rats acutely dependent on morphine. Jpn J Pharmacol 1985; 37:307–316Crossref, Medline, Google Scholar
17 : Opioid-induced inhibition of the human 5-HT and noradrenaline transporters in vitro: link to clinical reports of serotonin syndrome. Br J Pharmacol 2018; 175:532–543Crossref, Medline, Google Scholar
18 : The N-methyl-d-aspartate receptor antagonist d-methadone acutely improves depressive-like behavior in the forced swim test performance of rats. Exp Clin Psychopharmacol 2020; 28:196–201Crossref, Medline, Google Scholar
19 : N-Methyl-d-aspartate receptor antagonist d-methadone produces rapid, mTORC1-dependent antidepressant effects. Neuropsychopharmacology 2019; 44:2230–2238Crossref, Medline, Google Scholar
20 : REL-1017 (esmethadone) increases circulating BDNF levels in healthy subjects of a phase 1 clinical study. Front Pharmacol 2021; 12:671859Crossref, Medline, Google Scholar
21 : The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59(Suppl 20):22–33Medline, Google Scholar
22 : A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
23 : The Hamilton Rating Scales for Depression: a critical review of clinimetric properties of different versions. Psychother Psychosom 2020; 89:133–150Crossref, Medline, Google Scholar
24 : Massachusetts General Hospital SAFER criteria for clinical trials and research. Harv Rev Psychiatry 2013; 21:269–274Crossref, Medline, Google Scholar
25 : The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 2011; 168:1266–1277Link, Google Scholar
26 : Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS). J Trauma Stress 1998; 11:125–136Crossref, Medline, Google Scholar
27 : The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs 2003; 35:253–259Crossref, Medline, Google Scholar
28 : A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Crossref, Medline, Google Scholar
29 : Reliability and validity of the Symptoms of Depression Questionnaire (SDQ). CNS Spectr 2014; 19:535–546Crossref, Medline, Google Scholar
30 : The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont) 2007; 4:28–37Medline, Google Scholar
31 : Double-blind, placebo-controlled, dose-ranging trial of intravenous ketamine as adjunctive therapy in treatment-resistant depression (TRD). Mol Psychiatry 2020; 25:1592–1603Crossref, Medline, Google Scholar
32 : Attenuation of antidepressant effects of ketamine by opioid receptor antagonism. Am J Psychiatry 2018; 175:1205–1215Link, Google Scholar
33 : Opioid system is necessary but not sufficient for antidepressive actions of ketamine in rodents. Proc Natl Acad Sci USA 2020; 117:2656–2662Crossref, Medline, Google Scholar
34 : Central antinociception induced by ketamine is mediated by endogenous opioids and μ- and δ-opioid receptors. Brain Res 2014; 8:69–75Crossref, Google Scholar



