Mood Disorders and Outcomes of COVID-19 Hospitalizations
Abstract
Objective:
The authors sought to characterize the association between prior mood disorder diagnosis and hospital outcomes among individuals admitted with COVID-19 to six Eastern Massachusetts hospitals.
Methods:
A retrospective cohort was drawn from the electronic health records of two academic medical centers and four community hospitals between February 15 and May 24, 2020. Associations between history of mood disorder and in-hospital mortality and hospital discharge home were examined using regression models among any hospitalized patients with positive tests for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Results:
Among 2,988 admitted individuals, 717 (24.0%) had a prior mood disorder diagnosis. In Cox regression models adjusted for age, sex, and hospital site, presence of a mood disorder prior to admission was associated with greater in-hospital mortality risk beyond hospital day 12 (crude hazard ratio=2.156, 95% CI=1.540, 3.020; fully adjusted hazard ratio=1.540, 95% CI=1.054, 2.250). A mood disorder diagnosis was also associated with greater likelihood of discharge to a skilled nursing facility or other rehabilitation facility rather than home (crude odds ratio=2.035, 95% CI=1.661, 2.493; fully adjusted odds ratio=1.504, 95% CI=1.132, 1.999).
Conclusions:
Hospitalized individuals with a history of mood disorder may be at risk for greater COVID-19 morbidity and mortality and are at increased risk of need for postacute care. Further studies should investigate the mechanism by which these disorders may confer elevated risk.
While the pulmonary consequences of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) have contributed substantially to its morbidity and mortality, its extrapulmonary manifestations have also been documented. In addition to direct cardiac (1) and renal (2) effects, initial reports on small retrospective cohorts have suggested elevated risk of delirium (3, 4) and stroke (3, 5), as well as other neurologic presentations (4, 6).
Mood disorder diagnoses are known to be associated with poorer long-term outcomes for a range of disorders (7). Growing evidence that SARS-CoV-2 may affect brain function directly or indirectly likewise increases concern for interaction between preexisting disorders involving the CNS, including mood disorders, and adverse outcomes. Whether CNS features represent a direct consequence of infection, a secondary effect of immune activation and cytokine release syndrome (8), or some distinct mechanism secondary to COVID-19, they may complicate the management of acutely ill patients, particularly in resource-constrained environments (9).
Electronic health records (EHRs) have been applied in other contexts to enable rapid and efficient phenotyping (10, 11). While such phenotyping may be less precise than systematic prospective assessment, it is well suited for detecting and describing risk in settings where prospective assessment may not have focused on neuropsychiatric symptoms. Here, we examined all SARS-CoV-2-positive admissions across six Eastern Massachusetts hospitals. We sought to understand associations with prior mood disorder diagnoses, as well as the significance of estimated psychiatric or cognitive symptoms at admission. In particular, we aimed to determine whether past diagnosis of mood disorder or current symptoms were associated with differential hospital outcomes, as a means of understanding clinical features that might inform clinical decision making and identify higher-risk clinical subpopulations.
Methods
Cohort Derivation
The full cohort included all individuals with SARS-CoV-2 testing who were admitted to any of two academic medical centers and four community affiliate hospitals in Eastern Massachusetts between February 25 and May 24, 2020. For all of these individuals, any available narrative clinical notes from the admission date were extracted from the Research Patient Data Registry (Mass General Brigham Biobank, Cambridge, Mass.) (12) and used to generate a data mart (13). Data were augmented with age, sex, race, and ethnicity from the same source. Socioeconomic status was estimated by imputation of the Area Deprivation Index (ADI) on the basis of zip code (14); greater ADI values indicate greater deprivation. The enterprise laboratory feed was used to extract coronavirus test results. Prior mood disorder diagnosis was defined on the basis of ICD-10 codes, collapsed using the Healthcare Cost and Utilization Project Clinical Classifications Software (CSS), version 2019.1, including major depressive disorder (CSS code 5.8.2) and bipolar disorder (code 5.8.1). Anxiety disorder (code 5.2), substance use disorder (codes 5.11 and 5.12), and dementia/delirium (code 5.4) were similarly defined. For all of these diagnoses, only diagnostic codes preceding the hospitalization date were included. The body mass index (BMI) measurement closest to time of admission and current smoking status were also extracted from the Research Patient Data Registry. Overall burden of medical comorbidity was estimated using the Charlson comorbidity index, as previously described (15). The hospital systems’ enterprise data warehouse was used to determine intensive care unit admission, location prior to hospitalization (i.e., a skilled nursing facility or otherwise), and discharge disposition or mortality. No data were missing, with two exceptions: ADI could not be calculated for 17 individuals because their zip codes were unavailable, and BMI was not available for 18 individuals. To ensure robustness to missing data, three approaches were utilized in our analysis: median imputation, multiple imputation as implemented in mice 3.11.0 (16), and exclusion of the individuals with missing data. Results did not meaningfully differ across these methods.
The study protocol was approved by the Mass General Brigham Human Research Committee. No participant contact was required in this study, which relied on secondary use of data produced by routine clinical care, allowing for waiver of the informed consent requirement, as detailed by 45 CFR part 46.116
Symptom Characterization From Narrative Clinical Notes
Symptom domains were determined by identifying the presence of tokens curated by application of a previously described method for estimating transdiagnostic neuropsychiatric phenotypes via natural language processing (17). This method utilizes an expert-curated set of tokens associated with the National Institute of Mental Health (NIMH) Research Domain Criteria (RDoC) domains, informed by the NIMH RDoC Workgroup statements and expanded to include synonyms commonly found in health care notes. These estimated RDoC domain scores have been validated against clinician review and shown to predict longitudinal outcomes in psychiatric and nonpsychiatric populations (17–19), from emergency department, admission, and discharge notes (20, 21). For this study, we investigated negative valence (primarily anxiety and depressive symptoms), positive valence (substance use, impulsivity, and mania), and global cognition. Where multiple notes were available for the admission date (e.g., multiple emergency department notes), the maximum score in each domain was included in the analysis (i.e., only one score per domain per individual was analyzed).
Study Design and Analysis
For the primary analysis, we applied survival analysis (Kaplan-Meier survival curves, followed by Cox regression) to examine time to mortality. Specifically, Kaplan-Meier survival curves compared survival among individuals with and without mood disorder. Cox regression models were used to estimate association between mood disorder and mortality, with adjustment for age, sex, race, ethnicity, admission site (academic medical center compared with community hospital), ADI, and Charlson comorbidity index. Age and mood disorder, as indicated both by formal test and by visual inspection of Schoenfeld residuals, violated the assumption of proportional hazards for Cox regression. Numerous strategies exist for addressing this violation. We adopted a standard recommendation to partition time into two epochs in which this assumption is supported (22). Specifically, survival data were split a priori (at the point where 20% of events in the full cohort had occurred, based on the Kaplan-Meier survival curve for the full cohort) into two distinct epochs of follow-up (<12 days and ≥12 days) in which the proportionality assumption was supported. For comparison, analyses with alternate split points were also conducted secondarily. In follow-up analyses, estimates of neuropsychiatric symptoms at admission (i.e., negative valence, positive valence, and cognitive symptoms) were added to this model as a means of understanding whether current symptoms might account for some of the variance otherwise explained by the mood disorder diagnosis.
Multiple logistic regression was used to examine hospital discharge home without services compared with discharge to other sites indicative of persistent need for care (outside hospital, skilled nursing facility, rehabilitation hospital, and hospice). Three Cox regression models were generated, with adjustment for a progressively larger number of features: unadjusted, adjusted for sociodemographic features, and then adjusted for sociodemographic and clinical features. Once again, neuropsychiatric symptoms at admission were considered in the follow-up analysis to determine whether current (versus historical) symptoms might explain observed associations with mood disorder diagnosis. All analyses utilized R, version 3.6.3 (23). No correction for multiple hypothesis testing was applied.
Results
In total, 2,988 SARS-CoV-2-positive individuals were hospitalized by May 24, 2020 (Table 1). The cohort was 53.4% male, 51.1% white, and 28.1% Hispanic, with a mean age of 62.8 years (SD=16.8). The majority (55.3%) were hospitalized at academic medical centers; 15.4% died during hospitalization. Of 2,481 individuals discharged, 1,352 (54.5%) were discharged to rehabilitation or skilled nursing facilities (Table 2).
| Characteristic | Not Deceased in Hospital (N=2,529) | Deceased in Hospital (N=459) | Total (N=2988) | p | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Mood disorder | 580 | 22.9 | 137 | 29.8 | 717 | 24.0 | 0.001 |
| Male | 1,334 | 52.7 | 263 | 57.3 | 1,597 | 53.4 | 0.072 |
| White | 1,235 | 48.8 | 293 | 63.8 | 1,528 | 51.1 | <0.001 |
| Hispanic | 771 | 30.5 | 69 | 15.0 | 840 | 28.1 | <0.001 |
| Academic hospital site | 1,446 | 57.2 | 207 | 45.1 | 1,653 | 55.3 | <0.001 |
| Current smoker | 142 | 5.6 | 22 | 4.8 | 164 | 5.5 | 0.477 |
| Anxiety disorder | 496 | 19.6 | 110 | 24.0 | 606 | 20.3 | 0.033 |
| Substance use disorder | 336 | 13.3 | 65 | 14.2 | 401 | 13.4 | 0.613 |
| Dementia/delirium | 313 | 12.4 | 152 | 33.1 | 465 | 15.6 | <0.001 |
| Admitted from skilled nursing facility | 129 | 5.1 | 71 | 15.5 | 200 | 6.7 | <0.001 |
| Mean | SD | Mean | SD | Mean | SD | ||
| Age (years) | 60.13 | 18.29 | 77.73 | 12.57 | 62.83 | 18.65 | <0.001 |
| Area Deprivation Indexa | 87.75 | 20.01 | 85.59 | 21.52 | 87.42 | 20.26 | 0.036 |
| Body mass indexb | 29.56 | 7.55 | 28.22 | 8.01 | 29.36 | 7.63 | <0.001 |
| Comorbidity index | 2.25 | 3.11 | 4.24 | 4.10 | 2.56 | 3.36 | <0.001 |
TABLE 1. Comparison of patients hospitalized for COVID-19 who did or did not die in the hospital
| Characteristic | Discharged Home (N=1,125) | Not Discharged Home (N=1,229) | Total (N=2,354) | p | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Mood disorder | 178 | 15.8 | 340 | 27.7 | 518 | 22.0 | <0.001 |
| Male | 622 | 55.3 | 623 | 50.7 | 1,245 | 52.9 | 0.026 |
| White | 458 | 40.7 | 656 | 53.4 | 1,114 | 47.3 | <0.001 |
| Hispanic | 459 | 40.8 | 300 | 24.4 | 759 | 32.2 | <0.001 |
| Academic hospital site | 692 | 61.5 | 627 | 51.0 | 1,319 | 56.0 | <0.001 |
| Current smoker | 60 | 5.3 | 73 | 5.9 | 133 | 5.6 | 0.524 |
| Anxiety disorder | 183 | 16.3 | 261 | 21.2 | 444 | 18.9 | 0.002 |
| Substance use disorder | 118 | 10.5 | 175 | 14.2 | 293 | 12.4 | 0.006 |
| Dementia/delirium | 28 | 2.5 | 222 | 18.1 | 250 | 10.6 | <0.001 |
| Intensive care unit stay | 121 | 10.8 | 351 | 28.6 | 472 | 20.1 | <0.001 |
| Mean | SD | Mean | SD | Mean | SD | ||
| Age (years) | 51.09 | 15.96 | 66.70 | 16.92 | 59.24 | 18.22 | <0.001 |
| Area Deprivation Indexa | 90.51 | 17.92 | 85.59 | 21.26 | 87.95 | 19.88 | <0.001 |
| Body mass indexb | 30.50 | 6.46 | 28.89 | 8.17 | 29.66 | 7.44 | <0.001 |
| Charlson comorbidity index | 1.34 | 2.37 | 2.84 | 3.37 | 2.12 | 3.03 | <0.001 |
TABLE 2. Comparison of patients hospitalized for COVID-19 who were discharged home and those discharged to a skilled nursing or rehabilitation facility
Time to death among hospitalized individuals with and without a documented mood disorder diagnosis prior to hospital admission is illustrated in Figure 1. The shape of this Kaplan-Meier survival curve illustrates minimal difference in early course but substantially greater risk among individuals with a mood disorder beginning by hospital day 10 (Kaplan-Meier log-rank test: p<0.001). Three Cox regression models are presented in Table 3: unadjusted, adjusted for sociodemographic features, and adjusted for sociodemographic features plus clinical features. To account for time-varying effects of age and mood diagnosis (i.e., magnitude of risk change during different periods of hospitalization), data were split into pre- and post-day 12. Across all three models, prior mood disorder was significantly associated with risk of death later in hospitalization. The crude hazard ratio was 2.156 (95% CI=1.540, 3.020), the hazard ratio adjusted for sociodemographic features was 1.997 (95% CI=1.415, 2.820), and the fully adjusted hazard ratio was 1.540 (95% CI=1.054, 2.250). (Alternate definitions of early compared with late hospitalization periods [i.e., considering ±24 hours] also yielded elevated hazard ratios for mortality in fully adjusted models; see Table S1 in the online supplement.) Likewise, when current mood and cognitive symptoms at admission were added to Cox models, the risk-increasing effect of mood disorder diagnosis after 12 days persisted (hazard ratio=1.594, 95% CI=1.076, 2.363).
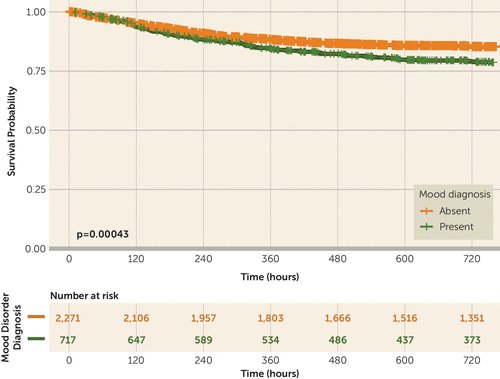
FIGURE 1. Kaplan-Meier survival curves representing time to mortality by prior mood disorder diagnosis among patients admitted to the hospital with COVID-19
| Crude Model | Sociodemographic Model | Fully Adjusted Model | ||||
|---|---|---|---|---|---|---|
| Variable | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI | Hazard Ratio | 95% CI |
| Mood disordera | ||||||
| <12 hospital days | 1.169 | 0.910, 1.502 | 1.076 | 0.833, 1.391 | 0.876 | 0.653, 1.175 |
| ≥12 hospital days | 2.156 | 1.540, 3.020 | 1.997 | 1.415, 2.820 | 1.540 | 1.054, 2.250 |
| Agea | ||||||
| <12 hospital days | 1.067 | 1.057, 1.077 | 1.064 | 1.053, 1.075 | ||
| ≥12 hospital days | 1.040 | 1.027, 1.054 | 1.039 | 1.025, 1.053 | ||
| Sex (male) | 1.379 | 1.137, 1.674 | 1.351 | 1.104, 1.653 | ||
| Race (white) | 1.143 | 0.922, 1.416 | 1.129 | 0.905, 1.408 | ||
| Ethnicity (Hispanic) | 0.861 | 0.650, 1.142 | 0.955 | 0.716, 1.273 | ||
| Area Deprivation Index | 1.006 | 1.001, 1.011 | 1.005 | 1.000, 1.010 | ||
| Hospital site (academic) | 0.690 | 0.567, 0.839 | 0.606 | 0.493, 0.745 | ||
| Admitted from skilled nursing facility | 1.517 | 1.144, 2.010 | ||||
| Body mass index | 1.020 | 1.007, 1.034 | ||||
| Current smoker | 1.327 | 0.815, 2.162 | ||||
| Charlson comorbidity index | 1.038 | 1.010, 1.065 | ||||
| Anxiety disorderb | 1.049 | 0.804, 1.369 | ||||
| Substance use disorderb | 0.897 | 0.657, 1.226 | ||||
| Dementia/deliriumb | 1.215 | 0.967, 1.528 | ||||
TABLE 3. Cox regression models of time to death in the hospital, censored at the end of follow-up evaluation, among patients admitted with COVID-19
We then examined probability of hospital discharge to sites other than home, compared with discharge home, among those who survived to discharge, as a measure of post-acute morbidity. This cohort is summarized in Table 2. Results from logistic regression models, unadjusted and then incorporating sociodemographic features and clinical features, are presented in Table 4. As with mortality analyses, history of a mood disorder diagnosis was associated with a statistically significant increase in risk for discharge to rehabilitation compared with a discharge home. The odds ratio for discharge to a location other than home without services was 2.035 (95% CI=1.661, 2.493) in unadjusted models, 1.921 (95% CI=1.526, 2.417) in models adjusted for sociodemographic features, and 1.504 (95% CI=1.132, 1.999) in fully adjusted models. Risk associated with a mood disorder diagnosis persisted when admission estimates of negative valence, positive valence, and cognition were added to the full model (odds ratio=1.419, 95% CI=1.058, 1.905).
| Crude Model | Sociodemographic Model | Fully Adjusted Model | ||||
|---|---|---|---|---|---|---|
| Variable | Odds Ratio | 95% CI | Odds Ratio | 95% CI | Odds Ratio | 95% CI |
| Mood disordera | 2.035 | 1.661, 2.493 | 1.921 | 1.526, 2.417 | 1.504 | 1.132, 1.999 |
| Age | 2.640 | 2.363, 2.950 | 2.427 | 2.145, 2.747 | ||
| Sex (male) | 1.005 | 0.836, 1.209 | 0.986 | 0.815, 1.192 | ||
| Race (white) | 0.967 | 0.791, 1.182 | 0.926 | 0.755, 1.137 | ||
| Ethnicity (Hispanic) | 0.848 | 0.685, 1.050 | 0.890 | 0.718, 1.105 | ||
| Area Deprivation Index | 0.998 | 0.904, 1.103 | 1.016 | 0.918, 1.125 | ||
| Hospital site (academic) | 0.833 | 0.688, 1.009 | 0.825 | 0.679, 1.002 | ||
| Body mass index | 1.044 | 0.949, 1.148 | ||||
| Current smoker | 1.376 | 0.893, 2.119 | ||||
| Charlson comorbidity index | 1.141 | 1.018, 1.279 | ||||
| Anxiety disordera | 0.823 | 0.614, 1.102 | ||||
| Substance use disordera | 1.186 | 0.853, 1.649 | ||||
| Dementia/deliriuma | 3.146 | 2.016, 4.910 | ||||
TABLE 4. Logistic regression model of hospital discharge to rehabilitation compared with discharge home among patients admitted with COVID-19
Discussion
In this study of 2,988 patients with COVID-19 hospitalized by May 24, 2020, across six hospitals, we found that a mood disorder diagnosis prior to hospitalization was associated with elevated risk for mortality and greater likelihood of requiring posthospitalization rehabilitation. These risks were not entirely attributable to sociodemographic differences, nor to differences in burden of medical or neuropsychiatric comorbidity, BMI, or smoking history.
Incorporating current neuropsychiatric symptoms, based on a validated natural language processing approach, suggested that symptoms reflected in emergency department documentation (19) did not explain the observed mood disorder effects. We recently showed that with onset of COVID-19 in the Boston area, documentation of psychiatric symptoms in general was dramatically reduced in the emergency department setting (24); hence, we cannot exclude the possibility that some of the elevation in risk was attributable to acute symptoms as well as longer-term symptoms.
Also notable is the time dependence of elevated mortality risk, evident in the survival curves: while there was little or no difference in early hospitalization, there was marked divergence by week 2. Why the substantial effect of mood disorder history became apparent later in the hospital course merits further investigation. It may, for example, reflect the commonly observed consequences of cytokine storm (25) and broader immune-mediated effects among individuals who initially appeared to improve during hospitalization.
We likewise identified elevated risk for requiring postdischarge rehabilitation among individuals with mood disorders, rather than discharge directly home. As with mortality, this risk persisted despite adjustment for sociodemographic features and aspects of comorbidity. These effects also were not attributable to mood or cognition symptoms at admission estimated by natural language processing.
Taken together, our results underscore the pressing need to better understand potential CNS effects of COVID-19 and how they may interact with preexisting psychiatric illness. For example, in the most comprehensive study to date, among 58 intensive care unit patients with COVID-19, nearly 70% experienced agitation or confusion (3), and MRI identified bilateral frontotemporal hypoperfusion among 11 symptomatic patients. Beyond the potential impact of hyperperfusion or hypoxia, the systemic immune response, particularly cytokine release syndrome (8), may contribute to CNS effects of COVID-19. A range of cytokines are implicated in other systemic COVID-19 effects, including interleukin-6, which plays a key role in microglia-neuron interactions (26), particularly after acute injury. A role for cellular signaling between neutrophils and macrophages in COVID-19 has also been suggested (27), which could further implicate microglia, brain-specific macrophages, in mediating COVID morbidity and sequelae. Given the complex and bidirectional relationship between major depression and inflammation (28), numerous mechanisms may contribute to elevated risk for adverse outcomes among individuals with mood disorders.
We note multiple important limitations to this study. While use of EHRs allows for an unbiased detection of symptoms—for example, via natural language processing applied to clinical notes—this method lacks the precision of more systematic investigation, which will be needed to characterize neuropsychiatric phenotypes in more detail. Indeed, our results suggest the importance of such measures as larger-scale COVID-19 cohort investigations are designed. The approach we applied here, while validated across multiple settings, will only succeed when neuropsychiatric symptoms are actually documented in clinical notes. Second, absent an appropriate comparator group, we cannot determine the extent to which adverse outcomes may reflect nonspecific consequences of severe illness in general (i.e., while we controlled for both psychiatric and general medical comorbidity, there may still be residual confounding associated with mood disorder history). Third, because this cohort was treated in the midst of a surge in demand in the Boston area, it is possible that these effects will not generalize to hospitals with more routine flow of admissions, which may be expected in areas less affected by COVID-19. However, these limitations are shared in all research to date retrospectively characterizing experience with this novel pathogen.
These multisite results may nonetheless guide clinical and translational approaches to COVID-19 in multiple respects. First, they suggest the need to consider strategies to address brain involvement in COVID-19, even when other consequences may be more apparent. For example, detection and management of delirium may be particularly challenging when severely ill patients are being cared for in settings less accustomed to tracking mental status (e.g., newly created intensive care units) (9). These are critical symptoms to track because it is possible that neurologic symptoms (specifically cognitive symptoms, including inattention) may persist to hospital discharge and beyond in a subset of patients (3), contributing to the need for scarce institutional discharges rather than discharge home. Second, they suggest the importance of including symptoms of neurologic and psychiatric illness in COVID-19 surveillance efforts. To date, most reports of so-called asymptomatic presentations focus on pulmonary symptoms or general symptoms of infection alone (29, 30). To this end, efforts to organize consortia to investigate such symptoms as part of routine care may be critical (31, 32).
In aggregate, this large, multihospital retrospective cohort study suggests that psychiatric comorbidity, and mood disorders in particular, must be carefully considered in hospitalized COVID-19 patients. The mechanism by which a preexisting mood disorder may influence hospital course and outcome merits further investigation in large clinical cohorts, as well as at a neurobiological level.
1 : Potential effects of coronaviruses on the cardiovascular system: a review . JAMA Cardiol 2020 ; 5 : 831 – 840 Crossref, Medline, Google Scholar
2 : Should COVID-19 concern nephrologists? why and to what extent? the emerging impasse of angiotensin blockade . Nephron 2020 ; 144 : 213 – 221 Crossref, Medline, Google Scholar
3 : Neurologic features in severe SARS-CoV-2 Infection . N Engl J Med 2020 ; 382 : 2268 – 2270 Crossref, Medline, Google Scholar
4 Mao L, Wang M, Chen S, et al.: Neurological manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study. medRxiv 2020. doi: 10.1101/2020.02.22.20026500. https://www.medrxiv.org/content/10.1101/2020.02.22.20026500v1 Google Scholar
5 : Incidence of thrombotic complications in critically ill ICU patients with COVID-19 . Thromb Res 2020 ; 191 : 145 – 147 Crossref, Medline, Google Scholar
6 : A first case of meningitis/encephalitis associated with SARS-Coronavirus-2 . Int J Infect Dis 2020 ; 94 : 55 – 58 Crossref, Medline, Google Scholar
7 : Depression . Lancet 2018 ; 392 : 2299 – 2312 Crossref, Medline, Google Scholar
8 : Cytokine release syndrome in severe COVID-19 . Science 2020 . 6490 : 473 – 474 Crossref, Google Scholar
9 : Collaborative delirium prevention in the age of COVID-19 . J Am Geriatr Soc 2020 ; 68 : 947 – 949 Crossref, Medline, Google Scholar
10 : Using electronic health records to drive discovery in disease genomics . Nat Rev Genet 2011 ; 12 : 417 – 428 Crossref, Medline, Google Scholar
11 : Using electronic medical records to enable large-scale studies in psychiatry: treatment resistant depression as a model . Psychol Med 2012 ; 42 : 41 – 50 Crossref, Medline, Google Scholar
12 : Calculating the benefits of a Research Patient Data Repository . AMIA Annu Symp Proc 2006 ; 2006 : 1044 Google Scholar
13 : Serving the enterprise and beyond with informatics for integrating biology and the bedside (i2b2) . J Am Med Inform Assoc 2010 ; 17 : 124 – 130 Crossref, Medline, Google Scholar
14 : Making neighborhood-disadvantage metrics accessible: the Neighborhood Atlas . N Engl J Med 2018 ; 378 : 2456 – 2458 Crossref, Medline, Google Scholar
15 : Validation of a combined comorbidity index . J Clin Epidemiol 1994 ; 47 : 1245 – 1251 Crossref, Medline, Google Scholar
16 : Mice: multivariate imputation by chained equations in R . J Stat Softw 2011 ; 45 : 1 – 67 Google Scholar
17 : High throughput phenotyping for dimensional psychopathology in electronic health records . Biol Psychiatry 2018 ; 83 : 997 – 1004 Crossref, Medline, Google Scholar
18 : Research Domain Criteria scores estimated through natural language processing are associated with risk for suicide and accidental death . Depress Anxiety 2019 ; 36 : 392 – 399 Crossref, Medline, Google Scholar
19 : Association between child psychiatric emergency room outcomes and dimensions of psychopathology . Gen Hosp Psychiatry 2019 ; 59 : 1 – 6 Crossref, Medline, Google Scholar
20 : Differences among Research Domain Criteria score trajectories by Diagnostic and Statistical Manual categorical diagnosis during inpatient hospitalization . PLoS One 2020 ; 15 :
21 : Stratifying risk for dementia onset using large‐scale electronic health record data: a retrospective cohort study. Alzheimers Dement 2020 ; 16 : 531 – 540 Crossref, Medline, Google Scholar
22 : Variables with time-varying effects and the Cox model: some statistical concepts illustrated with a prognostic factor study in breast cancer . BMC Med Res Methodol 2010 ; 10 : 20 Crossref, Medline, Google Scholar
23 R Core Team: R: a language and environment for statistical computing. Vienna, Austria. R Foundation for Statistical Computing, 2019. www.R-project.org Google Scholar
24 : Electronic health record documentation of psychiatric assessments in Massachusetts emergency department and outpatient settings during the COVID-19 pandemic . JAMA Netw Open 2020 ; 3(6):e2011346Google Scholar
25 : COVID-19: consider cytokine storm syndromes and immunosuppression . Lancet 2020 ; 395 : 1033 – 1034 Crossref, Medline, Google Scholar
26 : Repopulating microglia promote brain repair in an IL-6-dependent manner . Cell 2020 ; 180 : 833 – 846.e16 Crossref, Medline, Google Scholar
27 : Targeting potential drivers of COVID-19: neutrophil extracellular traps . J Exp Med 2020 ; 217 : 217 Crossref, Google Scholar
28 : Inflammation: depression fans the flames and feasts on the heat . Am J Psychiatry 2015 ; 172 : 1075 – 1091 Link, Google Scholar
29 : Spread of SARS-CoV-2 in the Icelandic population . N Engl J Med 2020 ; 382 : 2302 – 2315 Crossref, Medline, Google Scholar
30 Lavezzo E, Franchin E, Ciavarella C, et al.: Suppression of COVID-19 outbreak in the municipality of Vo, Italy. medRxiv 2020; 2020.04.17.20053157Google Scholar
31 Wadman M, Couzin-Frankel J, Kaiser J: How does coronavirus kill? clinicians trace a ferocious rampage through the body, from brain to toes. Science 2020. https://www.sciencemag.org/news/2020/04/how-does-coronavirus-kill-clinicians-trace-ferocious-rampage-through-body-brain-toes Google Scholar
32 I2b2 TranSMART Foundation: Consortium for clinical characterization of Covid19 by her (4CE). https://i2b2transmart.org/covid-19-community-project Google Scholar



