Adverse Childhood Experiences: Implications for Offspring Telomere Length and Psychopathology
Abstract
Objective:
Adverse childhood experiences (ACEs) are associated with mental and physical health risks that, through biological and psychosocial pathways, likely span generations. Within an individual, telomere length (TL), an established marker of cellular stress and aging, is associated with both ACE exposure and psychopathology, providing the basis for an emerging literature suggesting that TL is a biomarker of the health risks linked to early-life adversity both within and across generations. The authors tested the effect of maternal ACEs on both the trajectory of infant TL and infant social-emotional problems at 18 months of age.
Methods:
Pregnant women were recruited, and maternal scores on the Adverse Childhood Experience questionnaire were obtained, along with demographic and prenatal stress measures. Postnatal visits with 155 mother-infant dyads occurred when infants were 4, 12, and 18 months of age. At each visit, infant buccal swabs were collected for TL measurement, and mothers completed measures of maternal depression. Mothers also completed the Child Behavior Checklist at the 18-month visit. Mixed-effects modeling was used to test how maternal ACEs influenced infant TL trajectory. Linear regression was used to test the association between maternal ACEs and infant internalizing and externalizing behaviors. Finally, the interaction between telomere attrition from 4 to 18 months and maternal ACEs was examined as a predictor of infant scores on the Child Behavior Checklist.
Results:
Higher maternal ACEs were associated with shorter infant TL across infancy and higher infant externalizing behavioral problems at 18 months. No associations were found with internalizing behavioral problems. Telomere attrition from 4 to 18 months interacted with maternal ACEs to predict externalizing behaviors. In infants whose mothers reported higher scores on the Adverse Childhood Experience questionnaire, greater telomere attrition predicted higher externalizing problems, even when accounting for maternal postnatal depression and prenatal stress.
Conclusions:
These data demonstrate an interactive pathway between maternal early-life adversity and infant TL that predicts emerging behavioral problems in the next generations.
In 1998, the first study demonstrating an association between self-reported exposures to abuse, neglect, and family dysfunction before age 18, scores on the Adverse Childhood Experience questionnaire, and a broad range of health outcomes, including cardiovascular disease, diabetes, and depression, was published (1). Since this seminal study, conducted by the Felitti et al. research group (1–4), the Adverse Childhood Experiences questionnaire has been used as a consistent, albeit blunt, predictor of both physical and mental health risks (2). Within an individual, a higher score on the questionnaire has been associated with substance abuse, suicide, poor perinatal outcomes, and maternal perinatal depression (2–5). Given the high prevalence of exposures to adverse childhood experiences (ACEs), their high cross-generational correlation, and their link to maternal mental health, consideration of intergenerational effects is warranted (6, 7).
Life-course theory suggests that the negative health risks linked to early adversity likely span generations through both direct and indirect pathways (8). Consistent with this theory, there is evidence of the intergenerational effects of ACE exposures (9–12). In previous studies, maternal exposure to childhood maltreatment and trauma was associated with elevated offspring psychopathology in adolescents as well as in children as young as 36 months, often with evidence that maternal factors, including depression, further influenced childhood risk (13–18). Beyond maltreatment, studies using broader definitions of early-life adversity provide further support for elevated intergenerational risk. In a recent analysis of the Alberta Pregnancy Outcomes and Nutrition study, maternal scores on the Adverse Childhood Experience questionnaire predicted both maternal depression and externalizing behavioral problems in offspring at 24 months (19). However, maternal depression only partially mediated the association between maternal Adverse Childhood Experience scores and externalizing behavioral problems, suggesting the influence of other pathways (19). Whether maternal scores elevate externalizing behavior specifically or psychopathology risk more generally, as well as how early these risk trajectories arise, remains unknown.
Exposure to early-life adversity is hypothesized to result in psychological and biological changes that challenge the adaptive capacity of an individual, putatively resulting in elevated health risk through epigenetic imprints and other mechanisms (20, 21). One epigenetic factor associated with various characterizations of early-life adversity, including ACEs, is telomere length (TL). Telomeres are highly conserved ribonucleoprotein complexes that cap eukaryotic chromosomes, protecting them from damage and serving as global epigenetic regulators (22). In the absence of telomere restoration, telomeres shorten with each replication, putatively leading to cellular senescence, apoptosis, or terminal differentiation (22, 23). TL also influences global transcriptomic regulation, suggesting a more complex role in both stress responsiveness and development (22, 24). TL is influenced by psychological stress, oxidative stress, genotoxic stress, and neuroendocrine hormones (25–29). Two meta-analytic studies have documented the association between early-life adversity and shorter TL within an individual (30, 31). Individuals with depression and anxiety are reported to have shorter TL, although some debate exists (32–36). In a cross-sectional study of adolescents, shorter TL was associated with higher internalizing and externalizing behavioral problems, and TL partially mediated the relationship between parent-child separation and later internalizing and externalizing behavior (37). Despite correlations between psychopathology and TL, the directionality of the relationship remains unknown. A recent study conducted by Wade et al. (38) found that, in mid- to late childhood, higher levels of internalizing problems predicted shorter TL during adolescence. Consistent with parental adversity influencing offspring risk of psychopathology, the effects of adversity on TL across generations have been reported. For example, Gotlib et al. (39) found that daughters of mothers with recurrent depression had shorter TL than daughters of mothers without depression, even in the absence of depression in the daughters themselves. Together, this body of research supports TL as an emerging biomarker capable of capturing both exposure to early-life adversity and heralding future psychopathology risk.
Given the evidence linking ACEs to mental health risk and TL within an individual and evidence of transgenerational transmission of the impact of maternal ACEs, we hypothesized that TL would influence the link between maternal ACEs and childhood socioemotional problems. We first tested the direct effect of maternal ACEs on infant TL across the first 18 months of life. We next examined the relationship between maternal ACEs and infant social-emotional problems at age 18 months. Lastly, we tested how telomere attrition interacted with maternal ACEs to predict externalizing problems while accounting for both maternal postnatal depression and prenatal maternal stress.
Methods
Study Design
In a prospective longitudinal study, we assessed 155 mother-infant dyads. Pregnant mothers were recruited at any point during pregnancy. Three postnatal mother-infant visits were conducted when the infant was 4, 12, and 18 months of age. The numbers of mother-infant dyads who completed individual follow-up time points are listed in Figure 1. Inclusion criteria for the analyses required a maternal prenatal visit and at least two postnatal visits.
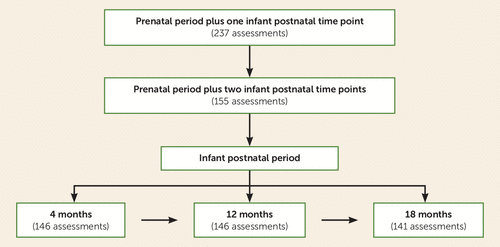
FIGURE 1. Mother-infant dyad visits at ages 4, 12, and 18 months in a study of maternal adverse childhood experiences and offspring telomere lengtha
a From a total of 237 mother-infant dyads who completed at least one postnatal visit, 155 dyads met study inclusion criteria, which required a prenatal time point and a minimum of two postnatal time points. Of the 155 mother-infant dyads who participated in the study, 103 dyads completed all three postnatal time points, and 52 completed two out of three postnatal visits.
Participants
A total of 237 mother-infant dyads were recruited from prenatal clinics, from woman, infant, and child clinics, and from other ongoing university studies. Women were enrolled at any point during pregnancy and were excluded if they were <18 years old or non-English speakers. Mothers provided information about multiple levels of their social ecology as well as that of their infant, including demographic data such as race and educational attainment, through an interview-assisted computer survey (Questionnaire Development System, Nova Research, Bethesda, Md.) administered face-to-face by a trained research assistant. Oral responses were recorded onto a computer by the trained interviewers. This study was approved by the institutional review board at Tulane University.
Measures Collected at the Prenatal Assessment
Maternal ACEs.
Prenatally, mothers reported their ACEs using the established self-report survey from the original ACE study, indicating “present” or “absent” for 10 types of early-life stress (e.g., parental mental illness, divorce) (1). A cumulative maternal Adverse Childhood Experience questionnaire score was the primary predictor variable (possible score range, 0–10).
Prenatal maternal stress (PNMS) index.
A PNMS index was generated from five indicators consisting of a unitary construct derived from a factor analysis, as previously reported (11). Indicators included the Pregnancy-Related Anxiety Scale (40), the Chronic Strain Questionnaire (41), the Prenatal Life Events Scale–Revised (42), the four-item version of the Perceived Stress Scale (43), and prenatal depression, assessed with the 10-item Edinburgh Depression Scale (44). For the indicator with a dichotomous clinical cutoff (Edinburgh Depression Scale), a score over the clinical threshold (>14) was coded as 1. For other indicators, participants with scores in the top quartile were coded as 1. A cumulative PNMS exposure score was generated from the sum of the five scales and ranged from 0 to 5.
Socioeconomic status index.
A sum score of socioeconomic status was indexed from education, employment, home ownership, income, savings, and government assistance status (possible score range, 0–6). Education level was scored as 0 for less than a high school diploma and as 1 for completion of high school or beyond. Employment status was scored as 0 for less than full-time employment and as 1 for full-time employment. Home ownership was scored as 0 for renting and as 1 for ownership. Income was scored as 0 for an annual income <$24,999 and as 1 for an annual income ≥$25,000. Savings was scored as 0 for <$500 and as 1 for ≥$500 or greater. Government assistance was scored as 0 for assistance and as 1 for no assistance.
Pregnancy-related data.
Medical covariates were abstracted from medical records. Gestational age at birth ranged from 34 to 42 weeks. A composite variable of pregnancy complications, which included gestational hypertension, gestational diabetes, fetal growth restriction, and eclampsia, was created. As a result of the small frequency of individual pregnancy complications, a dichotomous score was generated in which the pregnancy was scored as 1 if one or more complications were present and 0 if no complications were present (45).
Measures Collected at All Postnatal Time Points
Maternal postnatal depression.
Mothers completed the 10-item Edinburg Depression Scale contemporaneously with infant buccal DNA collection at 4 and 12 months of age (44). Maternal depression when the infants were 18 months old was assessed with the Beck Depression Inventory–II (BDI-II) (46). A dichotomous variable categorizing postnatal depression was generated using a threshold of 14 for the Edinburgh Depression Scale or 21 for the BDI-II. Mothers who scored above the threshold at any time point were categorized as positive for postnatal depression.
Infant buccal cell TL.
Infant buccal swabs for DNA were collected at 4, 12, and 18 months of age using Isohelix SK1 buccal swabs (Cell Projects, Kent, U.K.). Genomic DNA was isolated from buccal swabs using the QIAamp DNA Mini Kit protocol (Qiagen, Valencia, Calif., and Invitrogen, Carlsbad, Calif.). All DNA samples were evaluated for double-stranded integrity and concentration with the Qubit dsDNA BR assay kit (Invitrogen) and for purity with the NanoDrop-2000 (Thermo Fisher Scientific, Waltham, Mass.). DNA was stored at –20°C and underwent no more than three freeze-thaw cycles. The average relative TL was determined from the ratio of telomere repeat copy number to single gene (albumin) copy number by using an adapted monochrome multiplex quantitative real-time polymerase chain reaction (PCR) and a BioRad CFX96 (47). Longitudinal TL measurements were conducted as previously reported (48). Briefly, all infant buccal DNA samples from the same infant were run on the same plates in triplicate with a 7-point standard curve (0.0313–2 ng) using a single-pooled control buccal DNA sample for all plates. Each plate was run in duplicate with all samples in a different well position. Thus, six replicates, of both the single copy gene and the telomere repeat, were available for each individual at each time point. PCR efficiency criteria for telomere and albumin reactions were in the range of 90%–110%. Coefficients of variation were calculated within each triplicate (coefficients of variation criteria <10%) and between plates (coefficients of variation criteria <6%). Samples with unacceptably high coefficients of variation (>10% intra-assay or >6% interassay) were repeated. All samples were determined by the average of the triplicates from both plates. Additionally, all samples from each time point were required to pass quality control metrics for any sample measurement to be included.
Measures Collected Only at Age 18 Months
Infant psychopathology.
The Child Behavior Checklist was completed by mothers when their infants reached 18 months of age, concurrent with DNA collection (49). The internalizing and externalizing T scores on the Child Behavior Checklist were used in the analyses.
Statistical Analysis
Descriptive, bivariable, and multivariate analyses were conducted with SAS, version 9.4 (SAS Institute, Cary, N.C.). Descriptive statistics characterized participants overall. Excluded mother-infant dyads who completed the prenatal visit but only one of the two postnatal visits did not differ significantly on any demographic outcome from mother-infant dyads included in the study. Infant TL at any time point did not differ from a normal distribution as judged by the Q-Q plots. Externalizing and internalizing behavioral problems were normally distributed as judged by the Q-Q plots. Spearman’s correlation coefficients were used to examine the relationship between covariates and outcomes. Single imputation was performed for missing TL measures using the mean TL by infant sex and age at the missing TL time point; this was conducted only for infants who were missing either a 4-month or 18-month TL measurement (N=15, 3.6%) and for whom there was a completed Child Behavior Checklist form. All models were run with and without imputed TL data and did not differ substantively (50).
Clustering of infant TL over time.
Multilevel mixed-effects linear regression models produced intraclass correlation coefficients to estimate the degree of within-individual correlation of TL across time points. Mixed-effects linear regression models were performed using PROC MIXED in SAS to account for clustering of TL measurements within an individual, and the CL option was used to produce 95% confidence intervals.
Maternal ACEs and infant TL trajectory.
Mixed-effects linear regression models with an unstructured covariance matrix were used to examine the effect of maternal ACEs on infant TL over time. Model fit was assessed with the Akaike and Bayesian information criteria. Mixed-effects linear regression models using randomly distributed individual-specific intercepts first tested the unadjusted associations of infant age, age-squared to account for nonlinear trajectories, and maternal ACEs with infant TL. The model below was used:

To examine the trajectory over time by maternal ACEs and race, interaction terms between maternal ACEs or race (i.e., X) and age were included.
To select covariates included in the adjusted model, covariates with either significant bivariable associations or established empirical associations with the dependent outcome (i.e., externalizing problems) or the predictor variables (i.e., maternal ACEs and TL) were included. The following variables were tested but did not account for a significant portion of variance (i.e., did not contribute to statistical models by explaining a significant portion of the dependent outcome): pregnancy complications, prenatal smoking, trimester at recruitment, gestational age at birth, maternal pre-pregnancy body mass index (BMI), and maternal age. PNMS score and maternal depression demonstrated significant associations and were therefore included as covariates. Sex, as well as race and socioeconomic status, were included a priori given meta-analytic findings related to TL and evidence that ACEs may be elevated disproportionately in individuals of low socioeconomic status and in minority populations (6, 51). To maintain parsimony between analyses, the same covariate structure was used across all models. Models were run with all covariates as well as with the final covariate structure to ensure consistency of effects.
Maternal ACE exposure and infant psychopathology.
Generalized linear models using maximum likelihood estimates were used to examine the effect of maternal ACEs on infant externalizing and internalizing behavior, independently. The models were run with the same covariate structure as the maternal ACEs and infant TL trajectory model: PNMS score, infant sex, socioeconomic status, race, and maternal depression. The strength and direction of the independent predictor (i.e., maternal ACEs) for both infant TL and externalizing problems did not change with differing covariate structure, and therefore we report on the final model only.
Moderation of maternal ACE exposure and infant psychopathology by telomere attrition.
To explore whether the association between maternal ACEs and infant externalizing behavioral problems was moderated by telomere attrition, we generated a change score that was the difference between the 4-month and 18-month TL assessment (∆TL=4-month TL − 18-month TL). Generalized linear regression models failed to detect a relationship between telomere attrition and externalizing problems, and therefore moderation, and not mediation, analyses were conducted. Moderation analyses were used to test how the interaction of maternal ACEs and telomere attrition predicted externalizing problems. Models were adjusted for the same covariates as previous models and included PNMS score, infant sex, socioeconomic status, race, and maternal depression. To deconstruct the magnitude of telomere attrition as a moderator, a dummy variable partitioning telomere attrition into quartiles was created, with the first quartile representing the least amount of telomere attrition and the fourth quartile representing the greatest amount of telomere attrition. An interaction of the four-level quartile telomere attrition dummy variable and maternal ACEs tested the directionality of the interaction in relation to externalizing behavior.
Results
A total of 413 infant TL measurements were obtained from the 155 infants included in the analyses. Overall, 103 infants had TL measurements from all three time points, and 52 infants had measurements from two time points. Of the 52 infants missing one TL measurement, 11 TL measurements were missing at 4 months of age, 22 were missing at 12 months, and 19 were missing at 18 months. Missing TL measurements were either the result of missing one visit (N=31) or infant noncompliance with the buccal swab, resulting in a DNA concentration that was too low for analysis (N=21). Of the 15 imputed TL measurements, 11 were from the 4-month time point and four were from the 18-month time point. Including imputed TL measurements yielded a total of 428 TL measurements for analysis. Infants missing a TL measurement did not differ significantly on any demographic or other outcomes from infants with all TL measurements.
The demographic and clinical characteristics of the study sample are summarized in Table 1. Correlations between study variables are presented in Table S1 in the online supplement. The majority of mothers were recruited during the third trimester of pregnancy (N=96, 61.9%), with 34.2% recruited in the second trimester (N=53) and 3.9% in the first trimester (N=6). The mothers’ mean Adverse Childhood Experience questionnaire score was 2.29 (SD=2.08). Higher scores were associated with higher PNMS scores (ρ=0.39, p<0.0001) and lower socioeconomic status (ρ=–0.34, p<0.0001). Maternal Adverse Childhood Experience questionnaire scores were not associated with maternal race (ρ=–0.09, p=0.25). Higher Adverse Childhood Experience questionnaire scores (odds ratio=1.26, 95% CI=1.04, 1.52, p=0.019) and higher PNMS scores (odds ratio=2.10, 95% CI=1.54, 2.86, p<0.0001) were both associated with increased risk for maternal postnatal depression. Twenty-seven mothers (17.4%) were positive for depression at any postnatal visit (Table 1), 12 mothers (8.3%) were positive for depression when their infant was 4 months old, four (2.7%) were positive when their infant was 12 months old, and 16 (11.4%) were positive when their infant was 18 months old. Mothers who were positive for postnatal depression reported lower socioeconomic status (ρ=–0.30, p=0.0001). Infant TL was significantly correlated across time points. Infants who were racially classified as white or other exhibited shorter TL measurements at 12 months of age compared with black infants (ρ=–0.24, p=0.006), and white infants exhibited a greater rate of telomere attrition compared with black infants (β=–0.249, 95% CI=–0.450, –0.049, p=0.015). No sex differences in TL or telomere attrition were observed.
| Characteristic | |||
|---|---|---|---|
| Mean | SD | Range | |
| Maternal conception age (years) | 28.49 | 5.47 | 17–42 |
| Gestational age at birth (weeks) | 39.17 | 1.54 | 34–43 |
| Socioeconomic status index | 3.16 | 1.90 | 0–6 |
| Cumulative adverse childhood experiences | 2.29 | 2.08 | 0–9 |
| Cumulative prenatal maternal stress index | 1.27 | 1.42 | 0–5 |
| N | % | ||
| Race | |||
| Black | 52.3 | 81 | |
| White | 37.7 | 57 | |
| Other | 11.0 | 17 | |
| Infant sex | |||
| Male | 53.5 | 83 | |
| Female | 46.5 | 72 | |
| Maternal postnatal depression | 18.7 | 29 | |
| Pregnancy complications | 18.7 | 29 |
TABLE 1. Demographic and clinical characteristics and relevant covariates of mother-infant dyads in a study of maternal adverse childhood experiences and offspring telomere length (N=155)
The mean t score for externalizing problems was 48.57 (SD=11.14, range=28–86) and, for internalizing problems, 44.77 (SD=10.07, range=29–73). With a clinical threshold score of 60, 15.4% (N=21) of infants exhibited clinically relevant externalizing problem scores, and 8.8% (N=12) exhibited clinically relevant internalizing problem scores. Higher Adverse Childhood Experience questionnaire scores were associated with higher externalizing problems (ρ=0.23, p=0.006) but not internalizing problems (ρ=0.16, p=0.070). PNMS score was not associated with externalizing (ρ=0.10, p=0.24) or internalizing (ρ=0.14, p=0.095) problems. Maternal postnatal depression was significantly associated with higher externalizing (ρ=0.19, p=0.031) and internalizing (ρ=0.26, p=0.002) problems. Externalizing and internalizing problems were not associated with telomere attrition or TL at any single time point.
Clustering of TL within each infant (empty model).
The intraclass correlation coefficient of infant TL, using only non-imputed TL measurements, from the empty mixed-effects model was 12.0% (imputed TL intraclass correlation coefficient=9.3%). When accounting for infant age and a nonlinear time factor in the empty model, the intraclass correlation coefficient increased to 18.4% (imputed TL intraclass correlation coefficient=17.3%).
Effect of ACE exposure on infant TL (model 1).
Linear and nonlinear trajectories of TL models were run independently. Including only linear age in the model, TL shortened over time (β=–0.371, 95% CI=–0.468, –0.274, p<0.0001). Including only nonlinear age (i.e., age-squared) in the model, TL also exhibited a significant nonlinear trajectory (β=–0.171, 95% CI=–0.223, –0.119, p<0.0001). The model fit indices for the unadjusted model including linear age, nonlinear age, and maternal Adverse Childhood Experience questionnaire scores were 704.7 and 710.8 (Akaike and Bayesian information criteria, respectively). In the unadjusted model, maternal Adverse Childhood Experience questionnaire scores predicted shorter infant TL across time points (Table 2) (model 1: β=–0.031, 95% CI=–0.059, –0.003, p=0.033) but did not influence the trajectory of TL over time, as assessed by testing the interaction of maternal Adverse Childhood Experience questionnaire scores and infant age (β=0.014, 95% CI=–0.030, 0.059, p=0.53).
| Model 1: Main Effectsb | Model 2: Adjusted Modelb | |||
|---|---|---|---|---|
| Variable | β | p | Β | p |
| Age | –1.757 | <0.0001 | –1.143 | <0.0001 |
| Age-squared | 0.744 | <0.0001 | 0.738 | <0.0001 |
| ACE score | –0.031 | 0.033 | –0.039 | 0.021 |
| PNMS score | 0.027 | 0.30 | ||
| Sex | –0.052 | 0.39 | ||
| Race | –0.041 | 0.43 | ||
| Socioeconomic status index | –0.014 | 0.45 | ||
| Maternal depression | 0.004 | 0.96 | ||
TABLE 2. Statistical models assessing the impact of maternal Adverse Childhood Experience questionnaire (ACE) score on infant telomere lengtha
Adjusted effect of maternal ACE exposure on infant TL (adjusted model).
The adjusted model fit indices were 722.6 and 728.7 (Akaike and Bayesian information criteria, respectively). Neither PNMS score (Table 2) (model 2: β=0.027, 95% CI=–0.029, 0.077, p=0.30) nor maternal postnatal depression (β=0.004, 95% CI=–0.051, 0.183, p=0.96) was associated with infant TL. After adjusting for covariates, higher maternal Adverse Childhood Experience questionnaire scores remained predictive of shorter infant TL (β=–0.039, 95% CI=–0.072, –0.006, p=0.021).
Effect of maternal ACE exposure on infant psychopathology.
Higher maternal Adverse Childhood Experience questionnaire scores predicted higher infant externalizing behavioral problems (Table 3) (β=1.528, 95% CI=0.562, 2.495, p=0.002) but not internalizing problems (β=0.650, 95% CI=–0.211, 1.511, p=0.14). The model fit indices for externalizing problems were 1,040.51 and 1,063.81 (Akaike and Bayesian information criteria, respectively), and the indices for internalizing problems were 1,009.0 and 1,032.3. PNMS score was not associated with externalizing behavior (β=–0.824, 95% CI=–2.400, 0.753, p=0.31), but maternal postnatal depression exhibited a significant association with externalizing problems (β=6.211, 95% CI=0.978, 11.445, p=0.020). Because the maternal Adverse Childhood Experience questionnaire score was only associated with externalizing problems, internalizing behaviors were not tested in the moderation analyses.
| Externalizing Behavior | ||||||
|---|---|---|---|---|---|---|
| Main Effects | Main Effects With Telomere Attrition | Moderation by Telomere Attrition | ||||
| Variable | β | p | β | p | Β | p |
| Intercept | 45.313 | <0.0001 | 45.602 | <0.0001 | 47.422 | <0.0001 |
| ACE score | 1.528 | 0.002 | 1.485 | 0.003 | 0.936 | 0.091 |
| Telomere attrition | –0.912 | 0.51 | –4.696 | 0.036 | ||
| ACE by telomere attrition | 1.410 | 0.034 | ||||
| PNMS score | –0.824 | 0.31 | –0.811 | 0.31 | –0.670 | 0.40 |
| Sex | –0.794 | 0.66 | –0.728 | 0.69 | –1.176 | 0.51 |
| Race | –0.276 | 0.85 | –0.256 | 0.86 | –0.403 | 0.78 |
| Socioeconomic status index | 0.261 | 0.64 | 0.279 | 0.61 | 0.472 | 0.39 |
| Maternal depression | 6.21 | 0.020 | 6.335 | 0.018 | 5.840 | 0.027 |
TABLE 3. Moderation of maternal Adverse Childhood Experience questionnaire (ACE) score on externalizing problems by telomere attrition (N=136)a
Examination of the interrelationships between maternal ACEs and telomere attrition to predict externalizing behavioral problems.
While maternal Adverse Childhood Experience questionnaire scores predicted externalizing behavioral problems and overall TL, telomere attrition did not predict externalizing behavior (β=–0.912, 95% CI=–3.625, 1.802, p=0.51). We therefore tested the effect of the interaction of maternal Adverse Childhood Experience questionnaire scores and telomere attrition on externalizing problems (model fit indices: 1,039.6 and 1,068.8 for Akaike and Bayesian information criteria, respectively). This interaction was statistically significant (Table 3 and Figure 2A) (β=1.410, 95% CI=0.110, 2.710, p=0.034). In infants with greater telomere erosion, the relationship between maternal Adverse Childhood Experience questionnaire scores and externalizing problems was strengthened. Maternal postnatal depression persisted as a significant predictor of externalizing problems in this model (β=5.840, 95% CI=0.667, 11.013, p=0.027).
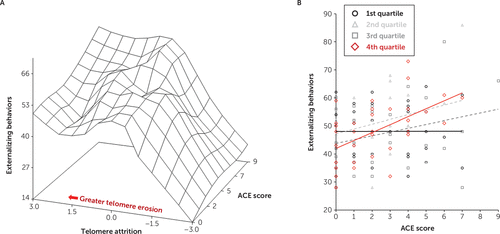
FIGURE 2. Moderation of maternal Adverse Childhood Experience questionnaire score and infant externalizing problems by telomere attritiona
a Panel A shows a surface plot of the moderation of maternal Adverse Childhood Experience questionnaire (ACE) score and externalizing behavior by telomere attrition. A spline interpolation was used to smooth the surface plot. Telomere attrition was defined as the difference in the 4-month telomere length (TL) from the 18-month TL (i.e., telomere attrition=4-month TL − 18-month TL). Panel B shows where telomere attrition was partitioned into quartiles in which the least amount of telomere attrition is represented by the first quartile (black), and the fourth quartile (red) represents the greatest amount of telomere attrition. For each quartile of telomere attrition, maternal ACE scores are plotted with externalizing behavior, and linear trend lines for each quartile are fitted to facilitate data visualization.
To examine how the magnitude of telomere attrition influenced the relationship between maternal Adverse Childhood Experience questionnaire scores and infant externalizing problems, a quartile split classified telomere attrition in which the first quartile represented the least amount of telomere attrition and the fourth quartile represented the greatest telomere attrition. The model fit indices were 1,044.1 and 1,084.8 (Akaike and Bayesian information criteria, respectively). When compared with the least amount of telomere attrition (i.e., first quartile), the greatest telomere attrition (i.e., fourth quartile) interacted with maternal Adverse Childhood Experience questionnaire scores to predict externalizing behavioral problems, such that higher maternal scores predicted higher externalizing problems (Figure 2B) (see also Table S2 in the online supplement) (β=2.902, 95% CI=0.500, 5.305, p=0.018). The fourth quartile of telomere attrition was the only quartile significantly different from the first quartile (see also Table S2 in the online supplement).
Discussion
This is the first study, to our knowledge, to examine the association between maternal Adverse Childhood Experience questionnaire scores and infant socioemotional problems in the context of a biomarker of cellular stress and aging. Despite being a blunt and retrospective indicator of early-life adversity, maternal Adverse Childhood Experience questionnaire scores predicted both infant TL and externalizing behavioral problems. These results are surprisingly consistent with the existing literature describing the effects of maternal life-course adversity on subsequent generations (11, 52, 53). Higher maternal Adverse Childhood Experience questionnaire scores were associated with shorter infant TL, a relationship that was not explained by demographic factors, PNMS score, or postnatal maternal depression. These results converge with our previous findings that maternal preconception adversity and PNMS score, despite their correlation, exhibit distinct effects on the next generation (11, 12) and that alterations in early telomere dynamics contribute to later child socioemotional outcomes (37, 38). Beyond these findings, the significant relationship between maternal Adverse Childhood Experience questionnaire scores and both PNMS score and postnatal depression warrants additional investigation given their independent implications for maternal and child health.
Early TL and TL trajectories appear to both predict later TL and future health risks (54, 55). However, to date, only three previous studies (55-57), to our knowledge, have examined the trajectory of TL in children, and all three of these studies included only two time points, limiting the ability to detect nonlinear patterns. In the present study of infants ranging between 4 and 18 months of age, TL exhibited an overall linear decrease and a quadratic trajectory. This nonlinear pattern is consistent with the early-life trajectory of leukocyte TL reported for nonhuman primates (58-60). These cross-species results suggest that TL exhibits complex trajectories across development, similar to other indicators of growth and aging. Inclusion of multiple TL measurements in longitudinal studies is therefore recommended. The effect of maternal preconception adversity (i.e., ACEs) on children’s early TL trajectories, coupled with the relevance of early TL for future health risks, highlights the need to extend these studies into later childhood and examine both risk and protective factors (36).
Consistent with the recent study by Letourneau et al. (19), we found a direct positive association between maternal Adverse Childhood Experience questionnaire scores and externalizing behavioral problems among offspring but no relationship with internalizing behavioral problems. Despite our expectations, PNMS score did not predict externalizing or internalizing behavior or TL. Postnatal depression, however, uniquely predicted both internal and externalizing behavior, even when accounting for maternal Adverse Childhood Experience questionnaire scores. However, maternal postnatal depression did not interact with telomere attrition to predict externalizing problems, suggesting that although maternal postnatal depression is related to infant externalizing behavior and maternal ACEs, the lasting influence on child behavioral problems is not solely transmitted through maternal depression. Despite the strong correlation between maternal Adverse Childhood Experience questionnaire score, PNMS score, and postnatal depression, each appears to demonstrate unique relationships to TL and externalizing and internalizing behavioral problems among offspring. These results complement the few existing studies that implicate complex pathways between maternal life-course adversity and offspring outcomes, providing innovative directions for future research (61).
Among infants with greater telomere attrition from 4 to 18 months of age, higher maternal Adverse Childhood Experience questionnaire scores were associated with higher externalizing behavioral problems at 18 months. Beyond the influence of maternal postnatal depression, our data indicate that the risk of social-emotional problems as a function of maternal ACEs is compounded by biological processes likely related to TL dynamics, such as inflammation and oxidative stress (62). Given that a substantial proportion of the variance in externalizing problems remains unexplained, additional biological and psychosocial factors, particularly within the postnatal environment, are expected to further influence transgenerational risks.
There are unique strengths to this study. First, while the Adverse Childhood Experience questionnaire has shortcomings, its established predictive value for health outcomes and its brevity make it an attractive screening instrument for both clinical practice and research (63). Second, our results extend previous links between maternal early-life adversity and offspring social-emotional problems to an earlier developmental period, highlighting the need for infant mental health screening and intervention programs, especially in high-risk families (15, 19). Third, this is the largest infant longitudinal TL cohort to date, and it provides evidence, consistent with nonhuman primate studies, of nonlinear patterns of TL trajectory in early development. Finally, this is the first study, to our knowledge, to link maternal preconception adversity to both infant externalizing problems and TL.
Despite the study’s strengths, it has several limitations. While reports associating maternal BMI and TL measured in gestational tissues exist, we did not find an association of maternal prepregnancy BMI with infant TL, and maternal BMI was not associated with internalizing or externalizing problems. Models including maternal BMI did not alter the significance of these findings (64). We were also only able to partially account for fetal and maternal pregnancy complications. Fetal growth restriction is related to cellular turnover in the placenta and potentially influences cellular turnover and TL in the infant; however, rates of fetal growth restriction were too low for meaningful analyses. Prenatal surveys were conducted at different time points during pregnancy, and we did not collect data on psychotropic medication use or lifetime psychiatric diagnoses; however, adjusting for the trimester of the pregnancy interview did not alter the results. Although we controlled for maternal depression, other factors related to the early caregiving environment, including hostile parenting and the attachment relationship, could influence the relationship between maternal early-life adversity and offspring outcomes. Future studies exploring the effects of early adversity across generations should mirror current efforts to understand the ability of the postnatal environment to moderate the impact of childhood adversity within an individual.
We acknowledge that maternal Adverse Childhood Experience questionnaire scores, PNMS score, depression, and infant externalizing problems were all maternal-reported measures. The differential association between these scores and the collection of data at different time points partially diminishes the concern about bias due to there being a single informant; however, there remains a need for studies that utilize more objective assessments. While reliance on retrospective reports of early-childhood adversity is often cited as a challenge to the body of research using the Adverse Childhood Experience questionnaire, Reuben et al. (65) found moderate agreement between prospective and retrospective scores and noted that both predicted adult outcomes, albeit with somewhat different relationships. Beyond the issue of reporting bias, the Adverse Childhood Experience questionnaire does not capture the severity, chronicity, or age at which the exposures occurred, since such screening measures, including this questionnaire, must balance the ease of collection with these limitations. Despite these limitations, the predictive capacity of the Adverse Childhood Experience questionnaire score across health outcomes remains remarkably strong.
In addition, the absence of a direct relationship between maternal Adverse Childhood Experience questionnaire scores and infant internalizing behavioral problems was unexpected. Previous studies have suggested that in the toddler age range, the sensitivity of parental reports of child internalizing problems is limited, and, for the most part, internalizing behavioral problems are reported at lower rates than externalizing problems. The use of semistructured interviews (e.g., the Preschool Age Psychiatric Assessment) or other instruments that more specifically target internalizing problems in this age range may clarify whether this relationship is specific to externalizing problems or whether measurement methodology affects the ability to detect relationships with internalizing problems (66, 67). As a result of the initial study design, accurate measurements of postnatal physical growth (e.g., height, weight, and head circumference) were not obtained at all visits. Although we are not aware of any existing data indicating that infant growth rate from 4 to 18 months is associated with the trajectory of TL, or other markers of cellular aging, this should be considered for future studies, particularly for studies that include low birth weight or preterm infants, because “catch up” growth is associated with elevated cellular turnover and has been linked with shorter islet cell telomeres in rodents (68-70). We did not detect sex differences in infant TL, despite evidence in other studies. However, to our knowledge, no studies have reported sex differences in this age range. One potential explanation for this is that sex differences, at least in buccal cell TL measurement, appear later in development. Another consideration, given our findings of racial differences in TL, is that our study was underpowered to examine both sex and race differences, and race may obfuscate sex differences. Disentangling the interactive and independent effects of sex and race in relation to transmission of adversity across generations is an important future research direction.
When considering our measurement of TL, several specific limitations are notable. Changes in TL in buccal epithelial cells may not reflect changes in other cell types, and therefore caution is warranted when interpreting these findings. This remains a challenge to most human studies in which the specific tissue of interest, in this case the brain, is not accessible for study. The use of a change score in the moderation analyses is also a limitation, particularly in light of the nonlinear trajectory of TL. In addition, we did not use a true “baseline” TL measurement because our first TL measure occurred at 4 months of age. Recognizing the increasing importance of baseline TL in understanding the trajectory of TL across the life course, studies capturing a true baseline TL at birth are needed. Because buccal DNA was not available at birth in this cohort, we tested whether TL measured from DNA obtained from newborn blood spots influenced our findings. Despite the correlation between infant buccal TL with bloodspot TL, including bloodspot TL in the models did not influence the results, and we ultimately decided against inclusion in the final analyses because of concerns related to tissue specificity.
In summary, our data, when combined with findings from other studies, confirm maternal life-course experiences as a potent predictor of offspring mental and physical well-being. Too often, studies examine maternal exposures at only one time point (e.g., prenatal or postnatal), and, equally problematic, rely on either biological or behavioral outcomes. Mitigating the lasting effects of maternal adversity on child mental health clearly requires more comprehensive models that are appropriately reflective of the ever-changing interactions between biology, development, and the environment. Our results suggest that screening for maternal ACEs in obstetric, pediatric, and child mental health settings may provide an important indicator of risk for both the mother and the child, especially during infancy. Data from both human and preclinical research provide strong support for the need to integrate a multigenerational life-course perspective into our understanding of the biopsychosocial etiology of mental illness. Encouraging the widespread utilization of practical screening tools that have clinical utility and capture stressors across the life course and the broader environment in which children develop may enhance our ability to understand the origins of early mental illness and the effectiveness, rather than the efficacy, of current intervention and prevention efforts. This same model also offers an unprecedented opportunity for research to capture resiliency factors, such as the early caregiving environment, that may buffer the negative effects of maternal early adversity at the molecular, physiologic, behavioral, and neurocognitive levels.
1 : Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the Adverse Childhood Experiences (ACE) Study. Am J Prev Med 1998; 14:245–258Crossref, Medline, Google Scholar
2 : Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord 2004; 82:217–225Crossref, Medline, Google Scholar
3 : Adverse childhood experiences, alcoholic parents, and later risk of alcoholism and depression. Psychiatr Serv 2002; 53:1001–1009Link, Google Scholar
4 : Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the Adverse Childhood Experiences Study. JAMA 2001; 286:3089–3096Crossref, Medline, Google Scholar
5 : Adverse childhood experiences and risk for first-episode major depression during the menopause transition. J Clin Psychiatry 2017; 78:e298–e307Crossref, Medline, Google Scholar
6 : Prevalence of adverse childhood experiences from the 2011–2014 behavioral risk factor surveillance system in 23 states. JAMA Pediatr 2018; 172:1038–1044Crossref, Medline, Google Scholar
7 : Childhood adversity, parental vulnerability and disorder: examining inter-generational transmission of risk. J Child Psychol Psychiatry 2002; 43:1075–1086Crossref, Medline, Google Scholar
8 : Lifecourse health development: past, present and future. Matern Child Health J 2014; 18:344–365Crossref, Medline, Google Scholar
9 : Intergenerational associations of parent adverse childhood experiences and child health outcomes. Pediatrics 2018; 141:
10 : Maternal adverse childhood experiences and infant development. Pediatrics 2018; 141:
11 : Thinking across generations: unique contributions of maternal early life and prenatal stress to infant physiology. J Am Acad Child Adolesc Psychiatry 2017; 56:922–929Crossref, Medline, Google Scholar
12 : The transgenerational transmission of maternal adverse childhood experiences (ACEs): insights from placental aging and infant autonomic nervous system reactivity. Psychoneuroendocrinology 2019; 106:20–27Crossref, Medline, Google Scholar
13 : Impact of maternal early life maltreatment and maternal history of depression on child psychopathology: mediating role of maternal sensitivity? Child Psychiatry Hum Dev 2019; 50:278–290Crossref, Medline, Google Scholar
14 : Maternal childhood maltreatment history and child mental health: mechanisms in intergenerational effects. J Clin Child Adolesc Psychol 2018; 47:S47–S62Crossref, Medline, Google Scholar
15 : Maternal childhood abuse predicts externalizing behaviour in toddlers: a prospective cohort study. Scand J Public Health 2014; 42:263–269Crossref, Medline, Google Scholar
16 : Association between maternal childhood trauma and offspring childhood psychopathology: mediation analysis from the ALSPAC cohort. Br J Psychiatry 2017; 211:144–150Crossref, Medline, Google Scholar
17 : Maternal childhood maltreatment and offspring emotional and behavioral problems: maternal and paternal mechanisms of risk transmission. Child Maltreat 2014; 19:67–78Crossref, Medline, Google Scholar
18 : Maternal trauma exposure and childhood anxiety outcomes: examining psychosocial mechanisms of risk. J Abnorm Child Psychol 2019; 47:645–657Crossref, Medline, Google Scholar
19 : Intergenerational transmission of adverse childhood experiences via maternal depression and anxiety and moderation by child sex. J Dev Orig Health Dis 2019; 10:88–99Crossref, Medline, Google Scholar
20 : Strategies for measuring stress in studies of psychiatric and physical disorders, in Measuring Stress: A Guide for Health and Social Scientists. New York, Oxford University Press, 1995, pp 3–26Google Scholar
21 : Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones (Athens) 2009; 8:7–22Crossref, Medline, Google Scholar
22 : Telomeres and telomerase: their mechanisms of action and the effects of altering their functions. FEBS Lett 2005; 579:859–862Crossref, Medline, Google Scholar
23 : Cellular senescence and tissue aging in vivo. J Gerontol A Biol Sci Med Sci 2002; 57:B251–B256Crossref, Medline, Google Scholar
24 : Transcriptional outcome of telomere signalling. Nat Rev Genet 2014; 15:491–503Crossref, Medline, Google Scholar
25 : Diurnal and stress-reactive dehydroepiandrosterone levels and telomere length in youth. Endocr Connect 2016; 5:107–114Crossref, Medline, Google Scholar
26 : Growing up or growing old? cellular aging linked with testosterone reactivity to stress in youth. Am J Med Sci 2014; 348:92–100Crossref, Medline, Google Scholar
27 : Telomere length and early severe social deprivation: linking early adversity and cellular aging. Mol Psychiatry 2012; 17:719–727Crossref, Medline, Google Scholar
28 : Telomeres are favoured targets of a persistent DNA damage response in ageing and stress-induced senescence. Nat Commun 2012; 3:708Crossref, Medline, Google Scholar
29 : Oxidative stress shortens telomeres. Trends Biochem Sci 2002; 27:339–344Crossref, Medline, Google Scholar
30 : Association between childhood trauma and accelerated telomere erosion in adulthood: a meta-analytic study. J Psychiatr Res 2017; 93:64–71Crossref, Medline, Google Scholar
31 : Early life adversity and telomere length: a meta-analysis. Mol Psychiatry 2017Medline, Google Scholar
32 : Association between anxiety but not depressive disorders and leukocyte telomere length after 2 years of follow-up in a population-based sample. Psychol Med 2013; 43:689–697Crossref, Medline, Google Scholar
33 : Depression, anxiety and telomere length in young adults: evidence from the National Health and Nutrition Examination Survey. Mol Psychiatry 2015; 20:520–528Crossref, Medline, Google Scholar
34 : Do symptoms of depression predict telomere length? evidence from the west of Scotland Twenty–07 Study. Psychosom Med 2013; 75:288–296Crossref, Medline, Google Scholar
35 : Depression and telomere length: a meta-analysis. J Affect Disord 2016; 191:237–247Crossref, Medline, Google Scholar
36 : Major depressive disorder and accelerated cellular aging: results from a large psychiatric cohort study. Mol Psychiatry 2014; 19:895–901Crossref, Medline, Google Scholar
37 : Associations between early life parent-child separation and shortened telomere length and psychopathological outcomes during adolescence. Psychoneuroendocrinology 2019; 103:195–202Crossref, Medline, Google Scholar
38 : Telomere length and psychopathology: specificity and direction of effects within the Bucharest Early Intervention Project. J Am Acad Child Adolesc Psychiatry (Epub ahead of print, March 4, 2019)Google Scholar
39 : Telomere length and cortisol reactivity in children of depressed mothers. Mol Psychiatry 2015; 20:615–620Crossref, Medline, Google Scholar
40 : Psychological adaptation and birth outcomes: the role of personal resources, stress, and sociocultural context in pregnancy. Health Psychol 1999; 18:333–345PubMedCrossref, Medline, Google Scholar
41 : Shedding light on the mechanisms underlying health disparities through community participatory methods: the stress pathway. Perspect Psychol Sci 2013; 8:613–633PubMedCrossref, Medline, Google Scholar
42 : Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Health Psychol 2008; 27:604–615PubMedCrossref, Medline, Google Scholar
43 : A global measure of perceived stress. J Health Soc Behav 1983; 24:385–396PubMedCrossref, Medline, Google Scholar
44 : Detection of postnatal depression: development of the 10-item Edinburgh Postnatal Depression Scale. Br J Psychiatry 1987; 150:782–786Crossref, Medline, Google Scholar
45 : Differences in placental telomere length suggest a link between racial disparities in birth outcomes and cellular aging. Am J Obstet Gynecol 2017; 216:294.e291–294.e298PubMedCrossref, Google Scholar
46 : Beck Depression Inventory–II. San Antonio, Tex, Psychological Corp, 1996, pp 78204–72498Google Scholar
47 : Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res 2009; 37:e21–e21Crossref, Medline, Google Scholar
48 : Accelerated telomere shortening: tracking the lasting impact of early institutional care at the cellular level. Psychiatry Res 2016; 246:95–100Crossref, Medline, Google Scholar
49 : Manual for the Child Behavior Checklist: and Revised Child Behavior Profile. Burlington, Vt, Department of Psychiatry, University of Vermont, 1983Google Scholar
50 : Missing data analysis: making it work in the real world. Annu Rev Psychol 2009; 60:549–576Crossref, Medline, Google Scholar
51 : Race/ethnicity and telomere length in the Multi-Ethnic Study of Atherosclerosis. Aging Cell 2009; 8:251–257Crossref, Medline, Google Scholar
52 : Impact of physical abuse on internalizing behavior across generations. J Child Fam Stud 2017; 26:2753–2761Crossref, Medline, Google Scholar
53 : Early childhood adversity and pregnancy outcomes. Matern Child Health J 2016; 20:790–798Crossref, Medline, Google Scholar
54 : Reflections on telomere dynamics and ageing-related diseases in humans. Philos Trans R Soc Lond B Biol Sci 2018; 373:
55 : Early and late childhood telomere length predict subclinical atherosclerosis at age 14 yrs: the CardioCAPS study. Int J Cardiol 2019; 278:250–253Crossref, Medline, Google Scholar
56 : Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol Psychiatry 2013; 18:576–581Crossref, Medline, Google Scholar
57 : Telomere length change plateaus at 4 years of age in Latino children: associations with baseline length and maternal change. Mol Genet Genomics 2016; 291:1379–1389Crossref, Medline, Google Scholar
58 : Longitudinal data on telomere length in leukocytes from newborn baboons support a marked drop in stem cell turnover around 1 year of age. Aging Cell 2007; 6:121–123Crossref, Medline, Google Scholar
59 : Shaping long-term primate development: telomere length trajectory as an indicator of early maternal maltreatment and predictor of future physiologic regulation. Dev Psychopathol 2017; 29:1539–1551Crossref, Medline, Google Scholar
60 : Population mixture model for nonlinear telomere dynamics. Phys Rev E Stat Nonlin Soft Matter Phys 2008; 78:
61 : Sex differences in effects of maternal risk and protective factors in childhood and pregnancy on newborn telomere length. Psychoneuroendocrinology 2018; 95:74–85Crossref, Medline, Google Scholar
62 : Mothers’ childhood hardship forecasts adverse pregnancy outcomes: role of inflammatory, lifestyle, and psychosocial pathways. Brain Behav Immun 2017; 65:11–19Crossref, Medline, Google Scholar
63 American Academy of Pediatrics: Addressing adverse childhood experiences and other types of trauma in the primary care setting. Itasca, Ill, American Academy of Pediatrics, 2014Google Scholar
64 : Maternal pre-pregnancy body mass index and newborn telomere length. BMC Med 2016; 14:148Crossref, Medline, Google Scholar
65 : Lest we forget: comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. J Child Psychol Psychiatry 2016; 57:1103–1112Crossref, Medline, Google Scholar
66 : Prevalence of social-emotional and behavioral problems in a community sample of 1- and 2-year-old children. J Am Acad Child Adolesc Psychiatry 2001; 40:811–819Crossref, Medline, Google Scholar
67 : Parent-child agreement on child psychiatric symptoms assessed via structured interview. J Child Psychol Psychiatry 1986; 27:181–190Crossref, Medline, Google Scholar
68 : Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ 2005; 331:929Crossref, Medline, Google Scholar
69 : Insulin sensitivity and secretion are related to catch-up growth in small-for-gestational-age infants at age 1 year: results from a prospective cohort. J Clin Endocrinol Metab 2003; 88:3645–3650Crossref, Medline, Google Scholar
70 : Poor maternal nutrition followed by accelerated postnatal growth leads to telomere shortening and increased markers of cell senescence in rat islets. FASEB J 2009; 23:1521–1528Crossref, Medline, Google Scholar




