Olanzapine Versus Placebo in Adult Outpatients With Anorexia Nervosa: A Randomized Clinical Trial
Abstract
Objective:
This study evaluated the benefits of olanzapine compared with placebo for adult outpatients with anorexia nervosa.
Methods:
This randomized double-blind placebo-controlled trial of adult outpatients with anorexia nervosa (N=152, 96% of whom were women; the sample’s mean body mass index [BMI] was 16.7) was conducted at five sites in North America. Participants were randomly assigned in a 1:1 ratio to receive olanzapine or placebo and were seen weekly for 16 weeks. The primary outcome measures were rate of change in body weight and rate of change in obsessionality, assessed with the Yale-Brown Obsessive Compulsive Scale (YBOCS).
Results:
Seventy-five participants were assigned to receive olanzapine and 77 to receive placebo. A statistically significant treatment-by-time interaction was observed, indicating that the increase in BMI over time was greater in the olanzapine group (0.259 [SD=0.051] compared with 0.095 [SD=0.053] per month). There was no significant difference between treatment groups in change in the YBOCS obsessions subscale score over time (−0.325 compared with −0.017 points per month) and there were no significant differences between groups in the frequency of abnormalities on blood tests assessing potential metabolic disturbances.
Conclusions:
This study documented a modest therapeutic effect of olanzapine compared with placebo on weight in adult outpatients with anorexia nervosa, but no significant benefit for psychological symptoms. Nevertheless, the finding on weight is notable, as achieving change in weight is notoriously challenging in this disorder.
Anorexia nervosa is a severe eating disorder characterized by the persistent maintenance of an abnormally low body weight in association with distinct psychological features, including distortion of body image and, often, a lack of recognition of the seriousness of the low body weight (1). In recent decades, family-based methods of treatment have been developed and demonstrated to be effective for younger patients (2). However, among adult patients, anorexia nervosa is often refractory to psychological treatment and is associated with one of the highest mortality rates among all psychiatric disorders (3).
Efforts to develop effective pharmacological interventions for anorexia nervosa have been disappointing (4). Although patients with the disorder frequently experience significant symptoms of anxiety and depression, placebo-controlled trials of antidepressants for acute treatment or for relapse prevention after successful acute treatment have shown little or no evidence of efficacy (5, 6). Six decades ago, open trials in anorexia nervosa of the first antipsychotic medication, chlorpromazine, initially generated great enthusiasm, which waned over time with the appreciation of significant side effects (7). Small controlled trials of the antipsychotics pimozide and sulpiride in the 1980s proved disappointing (8, 9). The introduction of second-generation antipsychotics rekindled interest in the potential utility of this class of medication, as has the documentation of dopaminergic disturbances in anorexia nervosa (10). In recent years, several small trials suggested potential benefits, especially for olanzapine, the use of which is associated with substantial weight gain in other disorders, such as schizophrenia. Bissada et al. (11) found that use of olanzapine in a 10-week structured day program was associated with a more rapid achievement of target body mass index (BMI) and a reduction in obsessional symptoms compared with placebo. Attia et al. (12), in an 8-week outpatient trial in 23 patients, reported that olanzapine was associated with more rapid weight gain than placebo. On the other hand, in a 10-week trial, Kafantaris et al. (13) found no benefit for olanzapine compared with placebo among 20 adolescents with anorexia nervosa, as did Brambilla et al. (14) in a trial in 30 adults. A recent meta-analysis suggested that the research to date does not support the use of second-generation antipsychotics for patients with anorexia nervosa (15); however, this conclusion is based only on small studies. Because of the lack of an effective pharmacological intervention and these mixed results, as well as biological plausibility, the present study was initiated to evaluate, in a large multisite placebo-controlled trial, the efficacy of olanzapine in promoting weight gain and improving psychological symptoms in outpatients with anorexia nervosa.
Methods
This 16-week randomized double-blind placebo-controlled trial of olanzapine compared with placebo for outpatients with anorexia nervosa was conducted at five sites from December 2010 to December 2016: New York State Psychiatric Institute/Columbia University Irving Medical Center, Weill Cornell Medical Center, University of Pittsburgh Medical Center, Johns Hopkins Medicine, and Toronto Center for Addiction and Mental Health. This was a parallel design with 1:1 randomization. The protocol was approved by the institutional review boards at each site, in accord with the Helsinki Declaration of 1975, and a data safety and monitoring board oversaw the conduct of the trial. Participants provided written informed consent after receiving a complete description of the study.
Patient Recruitment
Individuals were eligible to participate if they had a diagnosis of anorexia nervosa according to DSM-IV criteria (16) (with the exception of the requirement for amenorrhea), were between the ages of 18 and 65, and had a BMI ≥14.0 and ≤18.5. Diagnosis was assessed with the Structured Clinical Interview for DSM-IV Axis I Disorders (17) and confirmed by clinician interview. Individuals were excluded from participation if they had 1) a medical problem that required urgent attention, including a serum potassium level ≤2.5 mEq/L, a fasting blood glucose level ≥120 mg/dL or a nonfasting glucose level ≥140 mg/dL, an ECG with QTc >480 ms, or serum cholesterol or triglyceride levels ≥1.5 times the upper limit of normal; 2) a psychiatric problem that required immediate attention, including acute suicidality; 3) a current diagnosis of substance abuse or dependence, schizophrenia, schizophreniform disorder, or bipolar illness; 4) a neurological problem, including movement disorder (e.g., tardive dyskinesia), history of a seizure disorder, or dementia; or 4) an allergy to olanzapine or a documented failure to respond to or inability to tolerate olanzapine at 10 mg/day.
Eligible participants were required to have taken no antipsychotic medication or other medication known to affect weight during the previous 4 weeks. Participants could be on a stable dosage of other psychotropic medications and could be engaged in outpatient psychotherapy if they had not consistently gained weight (>3 lb) over the 4 weeks prior to study participation. Participants could not be in intensive outpatient treatments such as partial hospital or day programs.
Recruitment occurred primarily through clinical referrals and local media. If an initial telephone screening suggested potential eligibility, in-person screening with a study physician was scheduled.
Randomization
Randomization lists, stratified by site and subtype, were generated by a computer program using a random number generator seeded by time of day. Randomization assignments were kept by the pharmacy at each site. All clinical staff involved in the care of the patients, as well as study coordinators and statisticians, remained blind to medication assignment during the study. Olanzapine (2.5 mg) and matching placebo pills were provided by Eli Lilly.
Treatment
Medication was dispensed in a double-blind fashion after completion of baseline assessments. Participants met with a study psychiatrist weekly for 16 weeks. Medication was initiated at 2.5 mg/day (one pill/day) for 2 weeks, then increased to 5 mg/day (two pills/day) for 2 weeks. At week 4, the dosage was increased to the maximum of 10 mg/day (four pills/day). The titration was halted or the dosage decreased if the participant reported significant adverse effects.
Interactions with the psychiatrist during the weekly visits were guided by a manual (“MedPlus”) designed to enhance medication adherence. The 20- to 30-minute visits included a general review of symptoms and possible side effects and support for continued study participation. Participants also received two sessions of nutrition counseling during the first month after randomization.
Participants could receive up to 7 days of inpatient hospital care for acute problems without being withdrawn from the study. Treatment with study medication was terminated if the participant met study exclusion criteria (e.g., if BMI fell below 14.0), if the participant’s weight decreased at four consecutive visits or fell below 90% of study baseline weight, or if the participant voluntarily withdrew, for example, to obtain a higher level of care.
Outcome Measures
Height and weight were measured at baseline, and weight, the primary physical outcome measure, was measured at each weekly assessment. Overall illness severity and change were assessed by a study psychiatrist weekly using the 7-point Clinical Global Impressions (CGI) severity scale (1=not at all; 7=among the most extremely ill patients) and improvement scale (1=very much improved; 7=very much worse). These were anchored to assessments of weight, eating behavior, and mood (see the online supplement).
The primary psychological outcome measure was obsessionality, assessed with the Yale-Brown Obsessive Compulsive Scale interview (YBOCS) (18, 19). Symptoms of eating disorder severity, depression, and anxiety were examined using the following assessments, collected at baseline and 8 and 16 weeks after randomization: the Eating Disorder Examination (EDE) (20), the Center for Epidemiologic Studies Depression Scale (CES-D) (21), and the Zung Anxiety Inventory (22). To encourage data contribution from all participants for intent-to-treat analyses, including those who may have stopped taking study medication, participants were compensated for their time for completing assessments at weeks 8 ($50) and 16 ($100).
Safety and tolerability.
The severity of each of 22 somatic symptoms was assessed at each visit by a physician using a 4-point scale (none, mild, moderate, and severe). Participants were also screened for the presence of involuntary movements using the Abnormal Involuntary Movement Scale (23). Blood tests to assess potential metabolic problems were conducted monthly, as was ECG.
Adherence.
Medication diaries and monthly pill counts, as well as midpoint and end-of-study measurement of serum olanzapine levels, were used to assess adherence.
Data Analysis
The study was designed to randomize 160 patients with equal allocation to olanzapine and placebo to provide >90% power to detect a difference of 0.57 lb/week between groups. This threshold was selected on the basis of the effect size calculated from previously published preliminary data (12).
Baseline characteristics were compared between the olanzapine and placebo groups using two-sample t tests for continuous variables. Linear mixed-effects models with random intercepts and random slopes were used to assess differences over time between the two groups on primary and secondary continuous measures following intent-to-treat principles, including all available assessments from all participants randomized. Each model included main effects of treatment and of time as well as their interaction to assess within-group change over time and between-group effects adjusting for study site and subtype.
Several exploratory analyses were also carried out. Per-protocol analyses were conducted including data only from participants who continued to take medication under double-blind conditions. The statistical tests for the per-protocol analyses were the same as the intent-to-treat analyses. Sequential propensity scores were estimated at each week and included as time-dependent weights in an inverse probability weighting method to compute per-protocol effect (24). CGI improvement and severity ratings were dichotomized as in other studies (25), with patients who were rated as much or very much improved categorized as responders and those rated as no change or worse categorized as nonresponders, and those with severity ratings of moderately or more severe categorized as severe and those with ratings of mildly or less severe categorized as nonsevere. CGI ratings were analyzed using a generalized linear mixed-effects model to estimate the odds of reduced CGI severity and of improvement ratings at week 16. Frequencies of somatic symptoms rated as moderate or severe and of laboratory test abnormalities were compared between groups at end of treatment (i.e., at the last visit when the participants were taking study medication) using chi-square tests. The Wilcoxon rank sum test was performed to compare medication dosage at week 16. Statistical analysis was conducted using SAS, version 9.4, and Stata, version 13.1, and all tests were two-sided with the significance threshold set at 0.05. Analyses of secondary outcome measures and exploratory analyses were not corrected for multiple comparisons.
Results
Patient Characteristics
Of 201 patients who provided informed consent, 152 were assigned to receive medication or placebo (75 in the olanzapine group and 77 in the placebo group) (Figure 1).
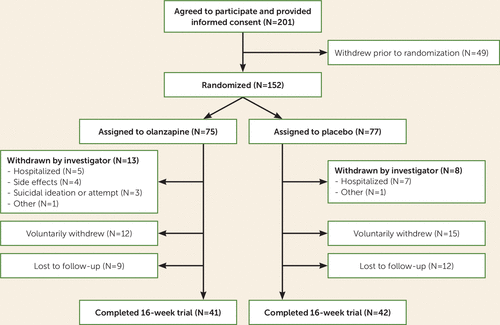
FIGURE 1. CONSORT diagram for a randomized clinical trial of olanzapine compared with placebo in adult outpatients with anorexia nervosa
Demographic and clinical characteristics at baseline in the placebo and olanzapine groups and across sites are summarized in Table 1. There were no statistically significant differences in characteristics between the placebo and olanzapine groups. There were statistically significant differences among sites in mean BMI, age, and YBOCS compulsions scale score. Co-occurring psychiatric diagnoses included mood disorder (current: 32.9%, past: 36.8%), prior alcohol abuse (7.2%) or dependence (11.8%), prior substance abuse (5.3%) or dependence (7.2%), anxiety disorder (current: 40.1%; past: 18.4%), and prior eating disorder other than anorexia nervosa (9.2%). The mean number of comorbid diagnoses was 2.1 (SD=1.4). Psychotropic medication use included antidepressants (N=45, 29.6%), sedative-hypnotics (N=23, 15.1%), or other psychotropic medications (N=19, 12.5%). Eighty-nine participants (58.6%) were taking no psychotropic medications.
| By Medication | By Site | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Olanzapine (N=75) | Placebo (N=77) | Columbia (N=55) | Cornell (N=16) | Hopkins (N=25) | Pittsburgh (N=23) | Toronto (N=33) | |||||||
| N | % | N | % | N | % | N | % | N | % | N | % | N | % | |
| Female | 70 | 94.6 | 75 | 97.4 | 52 | 94.6 | 15 | 93.8 | 24 | 100.0 | 22 | 95.7 | 32 | 97.0 |
| Restricting subtype | 36 | 48.0 | 39 | 50.7 | 23 | 41.8 | 11 | 68.8 | 17 | 68.0 | 11 | 47.8 | 13 | 39.4 |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Age (years) | 28.0 | 10.9 | 30.0 | 11.0 | 27.4 | 10.6 | 36.6 | 12.2 | 30.2 | 12.0 | 28.8 | 9.5 | 27.3 | 10.0 |
| Duration of illness (years) | 10.5 | 9.5 | 12.6 | 11.7 | 10.4 | 11.2 | 18.5 | 11.1 | 13.6 | 9.4 | 9.8 | 7.5 | 10.6 | 11.0 |
| Body mass index | 16.8 | 1.2 | 16.7 | 1.2 | 16.7 | 1.2 | 17.3 | 0.8 | 17.3 | 0.9 | 16.7 | 1.0 | 16.1 | 1.2 |
| Yale-Brown Obsessive Compulsive Scale | ||||||||||||||
| Total score | 16.5 | 10.5 | 16.4 | 10.0 | 17.2 | 10.2 | 17.5 | 8.8 | 13.3 | 10.1 | 13.8 | 11.7 | 19.0 | 9.3 |
| Obsessions subscale | 7.64 | 5.79 | 7.26 | 5.50 | 8.07 | 5.53 | 8.19 | 5.69 | 6.08 | 5.23 | 6.74 | 5.96 | 7.58 | 5.92 |
| Compulsions subscale | 8.85 | 5.60 | 9.18 | 5.39 | 9.13 | 5.53 | 9.31 | 4.25 | 7.20 | 5.21 | 7.09 | 6.37 | 11.42 | 4.70 |
| Eating Disorder Examination | ||||||||||||||
| Weight concerns subscale | 3.02 | 1.75 | 2.79 | 1.73 | 2.71 | 1.88 | 2.94 | 1.51 | 2.79 | 1.61 | 3.50 | 1.70 | 2.87 | 1.70 |
| Restraint subscale | 3.36 | 1.60 | 2.89 | 1.71 | 2.80 | 1.76 | 2.93 | 1.87 | 3.35 | 1.38 | 3.10 | 1.58 | 3.66 | 1.61 |
| Eating concerns subscale | 2.08 | 1.52 | 2.07 | 1.50 | 1.87 | 1.53 | 1.48 | 1.29 | 2.44 | 1.41 | 1.93 | 1.49 | 2.52 | 1.53 |
| Shape concerns subscale | 2.94 | 1.76 | 2.94 | 1.90 | 2.50 | 1.88 | 2.97 | 1.50 | 3.36 | 1.73 | 3.40 | 1.73 | 3.01 | 1.95 |
| CES-D | 25.9 | 12.7 | 28.3 | 13.7 | 26.2 | 13.3 | 23.2 | 10.1 | 25.4 | 13.8 | 27.7 | 14.7 | 31.6 | 12.7 |
| Zung Anxiety Inventory | 38.1 | 6.6 | 38.8 | 6.3 | 38.4 | 7.0 | 38.6 | 5.7 | 36.6 | 4.8 | 38.7 | 7.6 | 39.7 | 6.1 |
| CGI severity scale | 4.53 | 0.93 | 4.52 | 1.01 | 4.82 | 0.75 | 4.56 | 0.81 | 3.96 | 0.84 | 4.43 | 0.66 | 4.52 | 1.39 |
TABLE 1. Baseline demographic and clinical characteristics in a randomized clinical trial of olanzapine compared with placebo in adult outpatients with anorexia nervosaa
Primary Outcome Measures
As shown in Table 2, in the intent-to-treat, multilevel-model longitudinal analysis, which included all available data from all 152 randomized patients, olanzapine was associated with a significantly greater rate of weight gain (a BMI increase of 0.259 per month [SE=0.051]) than placebo (a BMI increase of 0.095 per month [SE=0.053]; F=4.98, df=1, 1435, p=0.026) (Figure 2). There was no significant interaction between treatment group and either anorexia nervosa subtype (restricting versus binge-purge, F=1.38, df=2, 1435, p=0.25) or treatment site (F=1.06, df=8, 1435, p=0.39) on rate of change of BMI. There was no significant difference between treatment groups in rate of change in YBOCS total score or YBOCS subscale scores.
| Measurea | Olanzapine (N=75) | Placebo (N=77) | Comparison | Effect Sizeb | |||
|---|---|---|---|---|---|---|---|
| F | df | p | Effect size | 95% CI | |||
| BMI | 0.259 | 0.095 | 4.98 | 1, 1435 | 0.026 | 0.629 | 0.106, 1.151 |
| Yale-Brown Obsessive Compulsive Scale | |||||||
| Total | –0.800 | –0.762 | 0.01 | 1, 92 | 0.94 | –0.027 | –0.419, 0.365 |
| Obsessions subscale | –0.325 | –0.017 | 0.27 | 1, 93 | 0.60 | –0.063 | –0.443, 0.319 |
| Compulsions subscale | –0.499 | –0.561 | 0.05 | 1, 92 | 0.82 | –0.011 | –0.395, 0.373 |
| Weight concerns subscale | 0.018 | –0.062 | 1.22 | 1, 91 | 0.27 | 0.300 | –0.045, 0.644 |
| Eating Disorder Examination | |||||||
| Restraint subscale | –0.094 | –0.122 | 0.14 | 1, 91 | 0.71 | 0.342 | 0.0005, 0.683 |
| Eating concerns subscale | –0.084 | –0.081 | 0.00 | 1, 90 | 0.96 | 0.051 | –0.284, 0.385 |
| Shape concerns subscale | 0.083 | –0.105 | 7.10 | 1, 91 | 0.01 | 0.387 | 0.055, 0.720 |
| CES-D | –0.585 | –0.934 | 0.29 | 1, 269 | 0.59 | –0.106 | –0.493, 0.280 |
| Zung Anxiety Inventory | –0.225 | –0.216 | 0.00 | 1, 237 | 0.98 | –0.133 | –0.600, 0.334 |
| t | df | p | Odds ratio | 95% CI | |||
| CGI | |||||||
| Severity scalec | 0.29 | 0.41 | 0.41 | 1356 | 0.68 | 0.716 | 0.146, 3.521 |
| Improvement scaled | 0.28 | 0.091 | 1.68 | 1213 | 0.094 | 3.118 | 0.825, 11.782 |
TABLE 2. Estimate of change per month in body mass index (BMI) and psychological measures and of odds of change in CGI ratings and effect sizes in a randomized clinical trial of olanzapine compared with placebo in adult outpatients with anorexia nervosaa
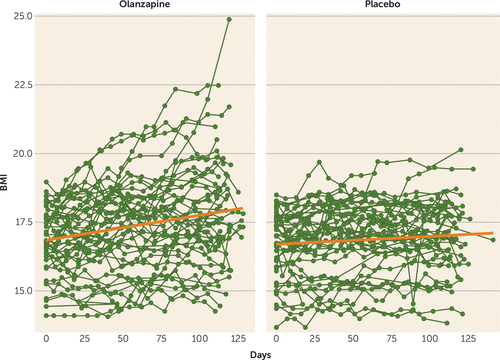
FIGURE 2. Change in body mass index (BMI) in a randomized clinical trial of olanzapine compared with placebo in adult outpatients with anorexia nervosaa
a Each fine line represents all available BMI values for one patient. The heavier lines depict the rate of change in BMI in the olanzapine and placebo groups, as estimated by multilevel-model longitudinal analysis.
Secondary Outcome Measures
Individuals in the olanzapine group had a threefold greater likelihood of being rated much or very much improved at week 16, although this difference did not reach statistical significance (odds ratio=3.118, 95% CI=0.825, 11.782, p=0.094) (see Table 2); there was no significant difference between the treatment groups in likelihood of a reduction in CGI severity score. There was no significant difference between the groups in rate of change in the EDE subscale scores for weight concerns, restraint, or eating concerns, in CES-D score, or in Zung Anxiety Inventory score. There was a significantly greater rate of increase on the EDE shape concerns subscale in the olanzapine group (0.083 per month [SE=0.049]) compared with the placebo group (20.105 per month [SE=0.053]; F=7.10, df=1, 91, p=0.01); there was no significant association between the increase in EDE shape concerns and rate of increase in BMI (F=2.10, df=1, 91, p=0.15). The results of the per-protocol analysis showed a similar pattern for both primary and secondary outcome measures (see Table S1 in the online supplement).
Adherence to Protocol
Ninety-eight participants (64.5%) continued to take the study medication at the midpoint assessment (week 8) and 83 (54.6%) at the termination visit (week 16). There was no significant difference over time between the olanzapine and placebo groups in the likelihood of discontinuing study medication (log-rank p=0.65; see Figure S1 in the online supplement).
The average medication dosage, in terms of pills per day, at week 16 was significantly lower in the olanzapine group, at 3.11 pills/day (SD=1.07) (7.77 mg of olanzapine per day), compared with 3.76 pills/day (SD=0.67) in the placebo group (Wilcoxon rank sum statistic S=1863; p<0.001). Among patients in active treatment in the study, the mean plasma level of olanzapine in the olanzapine group was 21.0 ng/mL (SD=12.8) at week 8 and 22.0 ng/mL (SD=18.2) at week 16.
There was no significant difference between the olanzapine and placebo groups in number of patients hospitalized during the study (eight [10.7%] and three [3.9%], respectively; χ2=2.59, p=0.11); nor was there a difference between the olanzapine and placebo groups in number of days hospitalized among those who were admitted to hospital (5.1 days [SD=1.6] compared with 4.3 days [SD=2.1]; t=0.67, p=0.52). Three of the 75 patients in the olanzapine group were withdrawn for suicidal ideation or a suicide attempt, compared with none in the placebo group (p=0.12, Fisher’s exact test).
Somatic Effects
At study termination, there were no significant differences in the frequency of abnormal results between the olanzapine and placebo groups on blood tests to assess metabolic abnormalities (serum concentrations of cholesterol, triglycerides, high-density lipoproteins (HDL), low-density lipoproteins (LDL), the ratio of cholesterol to HDL, hemoglobin A1C, ALT, and AST (see Table S2 in the online supplement).
At study termination, a significantly smaller fraction of the patients in the olanzapine group were rated as having moderate or severe symptoms of trouble concentrating (14.5% compared with 32.7%; χ2=5.45, p=0.02), difficulty sitting still (6.5% compared with 18.2%; χ2=3.81, p=0.05), trouble falling asleep (9.7% compared with 30.9%; χ2=8.32, p=0.004), and trouble staying asleep (14.5% compared with 40.0%; χ2=9.72, p=0.002). There were also lower frequencies of complaints of headache (4.8% compared with 14.5%; χ2=3.22, p=0.07), tremors or shakiness (0% compared with 5.5%; χ2=3.47, p=0.06), and nervousness (14.5% compared with 27.3%; χ2=2.91, p=0.09) in the olanzapine group, although these differences did not reach statistical significance (see Table S3 in the online supplement).
Discussion
This multisite randomized controlled trial of olanzapine compared with placebo is, to our knowledge, the largest medication trial conducted to date in anorexia nervosa. The study was carried out at five sites that have experience in the treatment of anorexia nervosa, and the study enrolled patients with a range of other psychiatric disorders and receiving treatment with a variety of psychotropic medications, lending support to the generalizability of the findings to treatment-seeking adults with anorexia nervosa.
The results of both the intent-to-treat and per-protocol analyses indicate that, in an outpatient setting, olanzapine offers modest benefit in weight gain, with patients in the olanzapine group gaining approximately 0.165 BMI points more per month than those in the placebo group, equivalent to approximately 1 lb more per month for a woman of average height (5 feet 5 inches). In other words, patients on olanzapine gained 0.259 BMI points per month on average, equivalent to ∼1.5 lb per month. There was also a tendency for patients in the olanzapine group to be more likely to be rated by their clinician as much or very much improved over time, which did not reach statistical significance. These results confirm and extend the results of several smaller trials (11, 12). Unlike Bissada et al. (11), however, we were unable to detect a therapeutic effect of olanzapine compared with placebo on obsessionality, a characteristic psychological feature of anorexia nervosa.
The olanzapine was well tolerated, and the rates of medication discontinuation did not differ significantly between the olanzapine and placebo groups (see Figure S1 in the online supplement). That these rates of participation were adequate to detect a significant drug effect contrasts with previous concerns regarding the successful execution of randomized controlled medication studies in anorexia nervosa (26). Three patients in the olanzapine group, compared with none in the placebo group, were withdrawn because of suicidal ideation (N=2) or suicide attempt (N=1). This difference was not statistically significant, and individuals with anorexia nervosa are known to be at increased risk for suicide (27). In other clinical populations, data do not suggest an association between olanzapine and suicide (28). Blood measures of lipid, hepatic, and glucose metabolism showed no differences at the end of study in rates of abnormality in the olanzapine and placebo groups. The frequency of several somatic symptoms was significantly reduced at the end of study in the olanzapine group compared with the placebo group, suggesting that olanzapine may have relieved some of the physical symptoms often experienced by individuals with anorexia nervosa.
We found a weight gain effect associated with olanzapine, but it was more modest than the substantial, usually undesirable, weight gain seen when olanzapine is used to treat other disorders (29). Whether the weight gain associated with olanzapine in this study is secondary to the same mechanisms responsible for the more substantial weight gain described in other populations is unknown. Our results suggest that weight gain and other effects that have been described in association with olanzapine (e.g., sedation, metabolic effects) are relatively mild in anorexia nervosa, generally well tolerated, and potentially therapeutic.
Unfortunately, we found no evidence that olanzapine had a significant impact on the characteristic psychopathological features of anorexia nervosa, such as obsessionality and overconcern with gaining weight. In fact, the shape concerns subscale score of the EDE increased more among patients in the olanzapine group than among those in the placebo group. However, since the olanzapine and placebo groups did not differ significantly in change over time in the other secondary measures, including the three other subscales of the EDE, and because there was not a significant relationship between change in the shape concerns subscale and rate of change in BMI, we cannot be certain that the difference between groups on the shape concerns subscale was not due to chance.
The failure in this study to replicate the effect of olanzapine on obsessionality noted by Bissada et al. (11) may also be related to the challenges in measuring the psychological features of anorexia nervosa, as compared with objective measurement of weight. The underlying neurobiological mechanisms of anorexia nervosa are poorly understood. This, combined with the multiple neurobiological targets of olanzapine, raises challenges in selecting the most useful metrics for measuring psychological variables that may be associated with the change seen with olanzapine. Exploration of the psychological and genetic factors associated with response to olanzapine may be useful, both for personalizing care and for identifying targets for studying mechanisms of illness and developing new treatment approaches.
The strengths of this study, including its relatively large sample size, the use of multiple sites, and the inclusion of patients with multiple other psychiatric disorders and on other psychotropic medications, suggest that the results are generalizable. Several limitations should also be noted, including a significant dropout rate, short duration, and the study’s being conducted at tertiary centers that are familiar with this patient population. Nonetheless, our results suggest that olanzapine may provide a modest therapeutic benefit for adult outpatients with anorexia nervosa, a group much in need of effective treatment strategies. Olanzapine alone clearly does not constitute a sufficient treatment intervention and should be provided in the context of appropriate psychological and behavioral therapy. Furthermore, although longer than most previous trials of medication in anorexia nervosa, this study assessed the impact of olanzapine over only 16 weeks. Future studies should examine how best to combine olanzapine with other treatment interventions and assess longer-term outcomes. More broadly, this study underscores the challenges of treating anorexia nervosa and the need for research to improve our understanding of its relative refractoriness to both psychological and pharmacological treatments.
Patient Perspective
“Ms. A” is a 22-year-old college graduate who moved to New York City after college and began work as a teacher in a dance studio. Although she was normal in weight throughout childhood and early adolescence, she became concerned with body shape and weight during high school and developed more significant food restriction, along with occasional episodes of subjective binge eating, during college. Since starting work, she had become uncomfortable eating in front of coworkers and roommates, heard from a cousin that she appeared “too thin,” and was disturbed by a near constant preoccupation with food. She entered the study hoping that a medication could help her keep her job and improve other aspects of life in her newly adopted city.
Ms. A presented with a BMI of 17.9. She arrived early for most sessions, wanting to have enough time to complete the research questionnaires, “so that others with the same thought loops in their head” might benefit from her research participation. At her baseline evaluation, she had a score of 23 on the Center for Epidemiological Studies Depression Scale (CES-D), above the cutoff used to indicate risk of clinically significant depression. Her score on the Body Shape Questionnaire was 84, indicating modest preoccupation and dissatisfaction with body shape. She told her psychiatrist that she was not sure that her preoccupations about food were fully captured on the study assessments. During the second week of receiving study medication at a dosage of one pill per day, Ms. A spoke enthusiastically of the relief she felt, as some of the preoccupying thoughts of food and meal planning seemed to have quieted down. She let her doctor know that she was sleeping better, and, while she liked this change, she was worried that some daily midday lethargy may be due to the medication, and she did not like this change at all. Her daytime sleepiness became more pronounced after she increased her daily dosage to two pills, and she insisted on decreasing her dosage back to the single pill by week 4, remaining on this low dosage throughout the remainder of the 16-week trial. Ms. A’s weight increased slowly during the trial, changing by approximately 7.5 lb during the study period, and her BMI reached 19.6 at study termination. Ms. A continued to report “improved thinking” throughout her participation in the study. Her research assessments demonstrated improved mood, with CES-D scores of 5 at the midpoint assessment and 2 at study termination. Other assessments did not reflect improvement as dramatically. For example, her Body Shape Questionnaire score improved only slightly to 65 at study’s end. On the Clinical Global Impressions improvement scale, her study physician rated the patient as “much improved” at 16 weeks.
1 : Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Washington, DC, American Psychiatric Association, 2013Crossref, Google Scholar
2 : Randomized clinical trial comparing family-based treatment with adolescent-focused individual therapy for adolescents with anorexia nervosa. Arch Gen Psychiatry 2010; 67:1025–1032Crossref, Medline, Google Scholar
3 : Mortality rates in patients with anorexia nervosa and other eating disorders: a meta-analysis of 36 studies. Arch Gen Psychiatry 2011; 68:724–731Crossref, Medline, Google Scholar
4 : The role of psychotropic medications in the management of anorexia nervosa: rationale, evidence, and future prospects. CNS Drugs 2016; 30:419–442Crossref, Medline, Google Scholar
5 : Fluoxetine after weight restoration in anorexia nervosa: a randomized controlled trial. JAMA 2006; 295:2605–2612Crossref, Medline, Google Scholar
6 : Does fluoxetine augment the inpatient treatment of anorexia nervosa? Am J Psychiatry 1998; 155:548–551Link, Google Scholar
7 : A new treatment of anorexia nervosa. BMJ 1960; 1:1770–1773Crossref, Medline, Google Scholar
8 : Neuroleptics in the short-term treatment of anorexia nervosa: a double-blind placebo-controlled study with sulpiride. Br J Psychiatry 1984; 144:288–292Crossref, Medline, Google Scholar
9 : Pimozide combined with behavior therapy in the short-term treatment of anorexia nervosa: a double-blind placebo-controlled cross-over study. Acta Psychiatr Scand 1982; 66:445–450Crossref, Medline, Google Scholar
10 : Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11c]raclopride. Biol Psychiatry 2005; 58:908–912Crossref, Medline, Google Scholar
11 : Olanzapine in the treatment of low body weight and obsessive thinking in women with anorexia nervosa: a randomized, double-blind, placebo-controlled trial. Am J Psychiatry 2008; 165:1281–1288Link, Google Scholar
12 : Olanzapine versus placebo for out-patients with anorexia nervosa. Psychol Med 2011; 41:2177–2182Crossref, Medline, Google Scholar
13 : A placebo-controlled pilot study of adjunctive olanzapine for adolescents with anorexia nervosa. J Child Adolesc Psychopharmacol 2011; 21:207–212Crossref, Medline, Google Scholar
14 : Olanzapine therapy in anorexia nervosa: psychobiological effects. Int Clin Psychopharmacol 2007; 22:197–204Crossref, Medline, Google Scholar
15 : Second-generation antipsychotic drugs in anorexia nervosa: a meta-analysis of randomized controlled trials. Psychother Psychosom 2015; 84:110–116Crossref, Medline, Google Scholar
16 : Diagnostic and Statistical Manual of Mental Disorders, 4th ed, Text Revision. Washington, DC, American Psychiatric Association, 2000Google Scholar
17 : Structured Clinical Interview for DSM-IV Axis I Disorders. New York, New York State Psychiatric Institute, Biometrics Research, 1998Google Scholar
18 : The Yale-Brown Obsessive Compulsive Scale, I: development, use, and reliability. Arch Gen Psychiatry 1989; 46:1006–1011Crossref, Medline, Google Scholar
19 : The Yale-Brown Obsessive Compulsive Scale, II: validity. Arch Gen Psychiatry 1989; 46:1012–1016Crossref, Medline, Google Scholar
20 : The Eating Disorder Examination, in Binge Eating: Nature, Assessment, and Treatment. Edited by Fairburn CG, Wilson GT. New York, Guilford, 1993, pp 317–360Google Scholar
21 : The CES-D Scale. Appl Psychol Meas 1977; 1:385–401Crossref, Google Scholar
22 : A rating instrument for anxiety disorders. Psychosomatics 1971; 12:371–379Crossref, Medline, Google Scholar
23 : Assessment of tardive dyskinesia using the Abnormal Involuntary Movement Scale. J Nerv Ment Dis 1985; 173:353–357Crossref, Medline, Google Scholar
24 : Per-protocol analyses of pragmatic trials. N Engl J Med 2017; 377:1391–1398Crossref, Medline, Google Scholar
25 : Optimizing treatment of complicated grief: a randomized clinical trial. JAMA Psychiatry 2016; 73:685–694Crossref, Medline, Google Scholar
26 : Predictors of treatment acceptance and completion in anorexia nervosa: implications for future study designs. Arch Gen Psychiatry 2005; 62:776–781Crossref, Medline, Google Scholar
27 : The incidence of eating disorders in a Danish register study: associations with suicide risk and mortality. J Psychiatr Res 2015; 65:16–22Crossref, Medline, Google Scholar
28 : Do atypical antipsychotics have antisuicidal effects? A hypothesis-generating overview. Int J Mol Sci 2016; 17:1700Crossref, Google Scholar
29 : Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005; 353:1209–1223Crossref, Medline, Google Scholar




