Impulse Control Disorders in Parkinson’s Disease
“Ms. A” was diagnosed with Parkinson’s disease at 51 years of age, 5 years after seeing a neurologist for a variety of symptoms, ranging from assumed complications of bulging vertebral discs, cervical radiculopathy, headaches, and carpal tunnel syndrome. Ultimately, she was referred to a movement disorders neurologist who assessed her pain, decreased left upper extremity dexterity and arm swing, gait stiffness and weakness, and rigidity, as well as prominent depression and anxiety symptoms, and she received a diagnosis of mild idiopathic Parkinson’s disease (stage 1.5 on the Hoehn and Yahr scale). She received a prescription for a dopamine agonist (pergolide) as part of an open-label monotherapy study for young-onset Parkinson’s disease. However, she began to experience gastrointestinal side effects, and, in an effort to delay initiating levodopa therapy, her neurologist prescribed another dopamine agonist, pramipexole, at a dosage of 0.25 mg t.i.d. Pramipexole resulted in improvement in parkinsonism without gastrointestinal upset.
After approximately 4–6 months of pramipexole treatment, the patient noticed that sporadic trips to casinos with friends became more frequent, lasted longer than a few hours, and involved spending, on slot machines, more than her allotted $40 per trip. While at the casino, it became increasingly difficult for Ms. A’s spouse to persuade her to leave. She would feel euphoric while gambling, and she used her winnings for continued gambling. Given that she was by nature a conscientious and responsible individual with no history of significant gambling, this behavior was out of character. She did have chronic depression and anxiety symptoms that long predated onset of Parkinson’s disease and had been managed over the years with various psychotropic medications (including bupropion at the time of impulse control disorder onset) and psychotherapy. She asked her neurologist if pramipexole was linked to personality changes or compulsive behaviors and was informed that it was not a potential side effect.
Over the course of the next 2 years, Ms. A’s thrice-daily dose of pramipexole was gradually increased to 0.5 mg, then 0.75 mg, and finally 1 mg. During that period, Ms. A gambled away $1 million dollars and gained 40 pounds. Initially, she gambled with personal money, but after losing $150,000, she began taking money from the family’s business and taking out loans from credit card companies and from family members. Despite understanding that her actions were inappropriate and harmful, she was unable to stop. Her thoughts and desires were consumed with gambling. Instead of going to work, she would stay home to gamble on the Internet. If a casino or computer was unavailable, she would purchase $100 worth of “scratch-off” lottery tickets. She felt as if she were programmed to gamble; the drive was unquenchable. In addition to compulsive gambling, Ms. A, generally a healthy eater, found herself uncontrollably eating large amounts of junk food. As with her gambling behaviors, she felt driven to eat and lost enjoyment in food, even when eating her favorite dishes.
Feeling despondent and suicidal, Ms. A contacted her neurologist once again regarding her behaviors, and this time she was advised to immediately discontinue pramipexole. After just 10–14 days, she began feeling like her usual self; the drive to gamble and eat to excess dissipated, and she no longer had impulse control disorders. However, managing her Parkinson’s symptoms since that time has been a challenge. She is unable to tolerate any dopamine agonist medications; a brief trial of ropinirole resulted in similar behavioral changes and was quickly discontinued. Her Parkinson’s symptoms are currently managed with levodopa plus entacapone, with medication intake every 4 hours, resulting in gastrointestinal and cardiovascular side effects. She is not entirely satisfied with her Parkinson’s disease management, as she experiences worse motor function more of the time and has increased bradykinesia. Ms. A inquired about deep brain stimulation surgery but was told by multiple neurologists that she would not be a good candidate given the character of her motor symptoms. In addition to her gradually worsening and suboptimally treated motor symptoms, years later she still keenly feels the financial and emotional consequences from her time on pramipexole. Ms. A’s family business was forced to close, and she filed for bankruptcy. Family and personal relationships remain strained.
Impulse control disorders (ICDs), compulsive gambling, and buying, sexual, and eating behaviors are an increasingly recognized serious complication in Parkinson’s disease. Related behaviors include punding (repetitive, purposeless behaviors), dopamine dysregulation syndrome (or compulsive medication overuse), and hobbyism (e.g., compulsive Internet use, artistic endeavors, writing). These disorders adversely affect quality of life, relationships, and caregiver burden and are associated with psychiatric comorbidity.
Pathological gambling was included as an ICD in DSM-IV-TR. In DSM-5, pathological gambling was moved from the ICD section to substance-related and addictive disorders; binge-eating disorder was added, and consideration was given to adding Internet addiction. ICDs have been conceptualized as “behavioral” addictions (1) because of their similarities to substance use disorders, with which they share risk factors, clinical features, cognitive changes, neurobiological substrates, and treatments. The term “impulse control disorders” covers the four major ICDs that occur in Parkinson’s disease, and “related behaviors” refers to other behaviors.
Epidemiology
In the DOMINION study (2), an interview was administered to assess the frequency of ICDs in 3,090 medicated Parkinson’s patients in the United States and Canada. One or more ICDs were identified in 13.6% of patients (gambling in 5.0%, compulsive sexual behavior in 3.5%, compulsive buying in 5.7%, and binge eating in 4.3%), and 3.9% of participants had two or more ICDs. While Asian studies have reported lower prevalence rates than the DOMINION study, studies in Finland, Denmark, Brazil, India, Germany, Spain, Russia, Australia, the United Kingdom, and Mexico have reported similar rates. Most studies suggest that ICDs are more common in treated patients than in the general population (3).
Regarding the prevalence of dopamine dysregulation syndrome and other impulsive-compulsive behaviors in Parkinson’s disease, in one study examining Parkinson’s patients who were being treated with higher levodopa equivalent daily doses, 14% met criteria for punding (4); in contrast, another study of unselected Parkinson’s patients reported a prevalence rate of 1.4% (5).
In one study, 39% of patients treated with dopamine agonists who did not have an ICD at baseline developed an ICD over a 4-year period, with a median onset time 23 months after initiation of a dopamine agonist (6). In a study of a dopamine agonist (rotigotine) in a 24-hour patch formulation, the cumulative ICD prevalence was 8.5%, with a median time to onset of 4–5 years (7).
Association Between ICDs and Parkinson’S Disease Treatments
Early case reports and cross-sectional studies suggested an association between use of newer dopamine agonists and the development of ICDs. In the DOMINION study, ICDs were more common in patients treated with a dopamine agonist (17.1%) than in patients not taking a dopamine agonist (6.9%). Furthermore, 18% of patients taking a dopamine agonist and levodopa had an ICD, compared with 14% of patients on a dopamine agonist without levodopa and 7% of patients taking only levodopa. The prevalence of ICDs was comparable in patients treated with pramipexole and those treated with ropinirole, the two commonly used dopamine agonists (17.7% and 15.5%, respectively). While some reports have shown a dose-response relationship between dopamine agonist use and ICDs (6, 7), this was not observed in the DOMINION study. There is preliminary evidence that long-acting dopamine agonists, in oral (8), patch (7), or pump (9) formulations, may decrease the risk of ICDs.
Use of levodopa (2) and amantadine (10) was associated with ICDs in the DOMINION study, but to a lesser extent than dopamine agonists. Dopamine dysregulation syndrome has been closely associated with the use of high-potency, shorter-acting dopaminergic therapies (e.g., levodopa and apomorphine). There is preliminary evidence that alternative delivery systems for levodopa, such as enteral suspension (Duopa), are less likely to be associated with ICDs (11). Additionally, an association between monoamine oxidase inhibitor-B use, specifically rasagiline, and ICDs has been reported (12).
Clinical and Demographic Correlates
Variables associated with ICDs include personal or familial history of alcohol use disorder or gambling, impulsive or novelty-seeking traits, younger age, male sex, early onset of Parkinson’s disease, being unmarried, and past or current cigarette smoking (2) (Figure 1). In the DOMINION study, another correlate of having an ICD was living in the United States compared with Canada, suggesting that environmental factors may play a role. Total ICD frequency was similar for men and women; there were notable sex differences in the frequency of specific ICDs, with compulsive sexual behavior more common in males and compulsive buying and binge eating more prevalent in women.
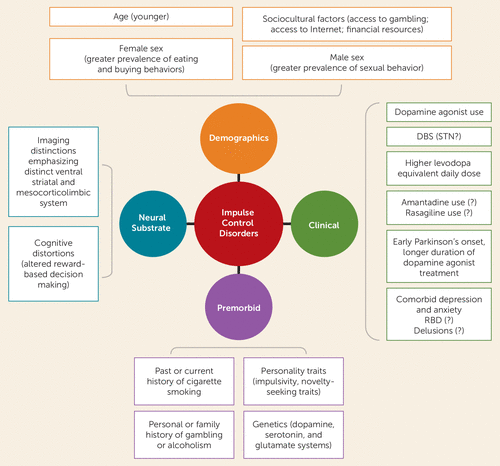
FIGURE 1. Correlates or risk factors for impulse control disorders and related disorders in Parkinson’s diseasea
a DBS=deep brain stimulation; RBD=REM sleep behavior disorder; STN=subthalamic nucleus.
Psychiatric Comorbidity
Patients with ICDs from the DOMINION study reported more depression, state and trait anxiety, obsessive-compulsive disorder, novelty seeking, and impulsivity compared with patients without ICDs (13). Other studies have also reported an association between ICDs and increased psychosis, anhedonia, alexithymia, and sleep disturbances. A strong association was shown between ICD symptoms (particularly pathological gambling) and REM sleep behavior disorder, a common Parkinson’s disease parasomnia (14). Apathy and ICDs are at opposite ends of a hypodopaminergic-hyperdopaminergic behavioral continuum (15), with evidence coming from a deep brain stimulation (DBS) study in which patients with ICDs experienced improvement in ICD symptoms but worsening in apathy when dopamine agonist treatment was discontinued (16).
Patients with dopamine dysregulation syndrome typically develop rapidly cycling mood disturbances (i.e., mood elevation or hypomania secondary to acute, short-acting dopaminergic therapy administration, followed by a dysphoric affective state when the medications wear off) (17). This syndrome is distinct from ICDs, as few Parkinson’s patients with ICDs are reported to use their Parkinson’s disease medications compulsively or to experience mood elevation.
Dopamine agonist withdrawal syndrome, a behavioral syndrome experienced by many patients with ICDs who discontinue treatment, shares psychiatric and physical features of withdrawal symptoms experienced in the context of substance use disorders (18). Symptoms include anxiety, panic attacks, agoraphobia, depression, dysphoria, diaphoresis, fatigue, pain, orthostatic hypotension, and drug cravings.
Cognition in Parkinson’s Patients With ICDs
Reports suggest that Parkinson’s patients with ICDs have deficiencies in cognitive tasks localizing to frontal regions and visual-spatial planning, including poorer working memory performance and a pattern of altered reward-punishment learning (19). Other studies reported no between-group differences in more specific inhibition and working memory tasks. The more conventional reward-based neuropsychological assessment is the Iowa Gambling Task, in which findings are also mixed.
Parkinson’s patients with ICDs who are taking their medications prefer more immediate rewards when exposed to a dopamine agonist than do control subjects, and they have been shown to demonstrate greater choice impulsivity in a delayed discounting task (13). In a functional MRI (fMRI) study that used a probabilistic gain and loss learning task, patients with ICDs responded to dopamine agonist exposure with an increased rate of learning for gain outcomes compared with control subjects (20). These findings have been extended in other studies, which show that patients with ICDs have a heightened preference for immediate over future rewards (21, 22).
Neurobiology
Dopamine function plays a critical role in the mediation of reward and reinforcement behaviors. The brain regions implicated in the development of ICDs include the caudal orbitofrontal cortex and possibly the ventromedial prefrontal cortex, which are involved in planning and judgment; the ventral striatum (particularly the dopamine pathway that projects from the ventral tegmental area to the nucleus accumbens), which is crucial for the reward system; the amygdala, which is involved in conditioned responses and emotional processing; and the medial dorsal and anterior nucleus of the thalamus (23). Alterations in cortico-striato-thalamo-cortical circuitry also contribute to the development of ICDs, with projections involving the more ventral components of the striatum (including the nucleus accumbens) implicated in urges and impulsivity, and those engaging the dorsal striatum more implicated in motor habits and compulsivity. Two important brain dopaminergic pathways that originate in the ventral tegmental area are the mesolimbic and mesocortical pathways, which are responsible for reward learning and executive decision making, respectively. While other neurotransmitters (e.g., serotonin, norepinephrine, and acetylcholine) are also affected early and significantly in the course of Parkinson’s disease, their role in ICD development has not been explored.
D1-like (D1 and D5) and D2-like (D2, D3, and D4) dopamine receptors are responsible for inhibitory and excitatory signaling, respectively. D1-like receptors localize to the direct pathway of movement, which is a neuronal circuit of the basal ganglia and several associated nuclei within the CNS; D2-like receptors localize to the indirect pathway of movement (24). Both D1-like and D2-like receptors are associated with prefrontal cortex–ventral striatum/amygdala pathways involved in decision making relevant to ICD behaviors. D2-like receptors enable flexible decision making and D1-like receptors promote persistence in choice biases. Disruption of D1-like modulation of prefrontal cortex–nucleus accumbens networks attenuates ICD-like behaviors in rodents (25). An imbalance between these pathways could lead to altered reward signaling and subsequent behavioral changes. Compared with levodopa, most first-line dopamine agonists have significantly higher D3:D2 and D3:D1 ratios of striatal receptor activation (26). D3 receptor–preferring dopamine agonists preferentially affect cerebral blood flow in the prefrontal and limbic cortex relative to a D2 receptor–preferring dopamine agonist (27). D3 receptors are concentrated in the ventral striatum, globus pallidus, ventral putamen, and medial dorsal nucleus of the thalamus, where they play a role in the mesolimbic reward pathways (28).
Two studies comparing de novo untreated Parkinson’s patients and healthy control subjects have found no differences between the groups in rates of ICD symptoms (29). However, several studies point to unique Parkinson’s disease–specific changes that may place some patients at increased risk for developing ICDs. First, Parkinson’s patients, when assessed while they are on their medications, commonly display a range of impairment in executive abilities, including action control, response inhibition, and delayed discounting, which localize to impaired frontal-striatal dopamine tracts (30). Second, de novo untreated Parkinson’s patients have also been shown to demonstrate intact punishment learning and impaired reward learning compared with healthy control subjects, but the introduction of dopamine agonist treatment reverses this pattern, leading to heightened reward learning and impaired punishment learning (31).
There is extensive neurobiological variability in Parkinson’s disease that may help explain differential risk for ICD development with exposure to dopaminergic therapy. Patients with early Parkinson’s disease who have greater dopamine transporter deficits, signifying more nigrostriatal dopamine deficiency, are at increased risk for ICD symptom development with initiation of Parkinson’s disease medications (32). Clinical neuroscience studies in Parkinson’s disease have focused on differential neurodegeneration of the striatum in early or mild Parkinson’s disease, with the dorsal striatum being more affected than the ventral striatum (33). It has been proposed that in mild Parkinson’s disease, medication-induced stimulation of the relatively intact ventral striatum receptors is associated with impaired performance on cognitive tasks that depend on ventral striatal activation (the dopamine “overdose” hypothesis), which manifests clinically as ICDs and related behaviors.
Brain positron emission tomography (PET) studies with 11C raclopride, which binds to dopamine D2–like receptors in the striatum, have found that Parkinson’s patients with pathological gambling have reduced striatal D2–like binding potential as well as a relatively greater decrease in binding potential during performance of a gambling task in the ventral but not the dorsal striatum (34). A recent study using the D3 receptor–preferring agonist [11C]-(+)-PHN) PET imaging ligand found Parkinson’s patients with ICDs to have decreased binding in the ventral striatum, suggesting either lower ventral striatum D3 receptor availability or increased dopamine release in the ventral striatum in patients with ICDs (35).
Task-related or resting-state fMRI studies of patients taking Parkinson’s disease medications further support the localization to the ventral striatum and anterior cingulate as key ICD brain regions. Using a probabilistic gain and loss learning task, Parkinson’s patients with ICDs responded to dopamine agonist exposure with increased striatal reward prediction error (20). When engaging in risk, patients with ICDs exhibit decreased ventral striatum activity compared with Parkinson’s disease control patients. After exposure to gambling-related visual cues, Parkinson’s patients with pathological gambling had increased activation in the ventral striatum and cingulate cortex (36). Altered blood flow in patients with ICDs is supported by studies employing arterial spin labeling perfusion and blood-oxygenation-level-dependent fMRI, where neural responses to risk taking during performance on the Balloon Analogue Risk Task localized to the right ventral striatum (37).
Associations have been reported between ICD behaviors in Parkinson’s disease and dopamine receptor (DRD3), serotonin receptor (HTR2A), and glutamate receptor (GRIN2B) genes (38). In a study of newly diagnosed Parkinson’s patients starting dopaminergic therapy, the heritability of incident ICD behaviors was 57% (39). Adding genotypes from the 13 candidate single-nucleotide polymorphisms to clinical risk factors significantly increased ICD predictability, with opioid (OPRK1), serotonin (HTR2A), and dopamine (DDC) genotypes the strongest predictive genetic factors.
In Parkinson’s patients with ICDs who had undergone subthalamic nucleus (STN) DBS surgery, oscillatory activity was observed in the theta-alpha band (4–10 Hz) in the ventral-intermediate portion of the STN, with cortico-thalamic coherence in the prefrontal cortex (40). This led the study authors to hypothesize that excessive dopaminergic therapy may lead to psychiatric complications through its effects on the ventral portion of the STN and motor complications (e.g., dyskinesias) through its effects on the dorsal portion of the STN.
Assessment and Diagnosis
There is evidence that ICD behaviors in Parkinson’s patients continue to be underrecognized and undermanaged in clinical practice. There could be several reasons for continuing underrecognition. Routine screening is not common, and patients may not report symptoms because they are embarrassed, have limited awareness of their behaviors, or do not suspect an association with Parkinson’s disease medications. Additionally, agreement between patient and informant reporting of symptoms is not high (41).
Several instruments have been used to assess for ICD symptoms. One is the Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease (QUIP), and a validated rating scale version of this instrument is also available (the QUIP-RS) (42). The Movement Disorder Society–Unified Parkinson’s Disease Rating Scale includes a single item for dopamine dysregulation syndrome, which covers excessive gambling, sexual behaviors, hobbyism, punding, and dopamine dysregulation syndrome. The Ardouin Scale of Behavior in Parkinson’s Disease, administered as an interview, assesses behaviors that are thought to represent the spectrum of hyper- and hypodopaminergic behaviors that occur in Parkinson’s disease (15).
Management
Given the association between ICDs in Parkinson’s disease and potential financial, legal, and medical complications, worse quality of life and function, strained interpersonal relationships, increased caregiver burden (43), and high rates of psychiatric comorbidity, it is imperative that the symptoms be identified and treated promptly.
Case reports and anecdotal experience suggest that ICDs often resolve with reduction of the dosage of the existing dopamine agonist, particularly with complete discontinuation of dopamine agonist treatment (often with a compensatory increase in levodopa dosage) (44). However, it may be difficult for some patients to discontinue dopamine agonist therapy, in part because of a physiological and psychological withdrawal syndrome (18).
The relationship between DBS surgery and ICDs appears complex and controversial. Most case series and studies have reported that STN DBS is associated with improvement in ICD symptoms, likely attributable to significant postoperative reductions in dopaminergic therapy. However, more recent case series have suggested that deep brain stimulation itself may cause or exacerbate ICDs in a minority of patients. In support of this clinical finding, cognitive neuroscience research has demonstrated that DBS patients, when their stimulators are turned on, are more impulsive in their decision making when in high-conflict conditions, irrespective of their Parkinson’s medication status (45).
There are few clinical trials data to support the use of neuropsychiatric drugs or behavioral interventions for ICDs in Parkinson’s disease. Case reports have shown conflicting results on response to a variety of classes of medications, including selective serotonin reuptake inhibitors, bupropion, antipsychotics, mood stabilizers, and zonisamide. A small placebo-controlled study reported benefit for amantadine as a treatment for pathological gambling in Parkinson’s disease (46), but amantadine was associated with the presence of ICDs in the DOMINION study (10).
Opioid antagonists modulate dopamine in the ventral tegmental area. Opioid receptor antagonists affecting mu and kappa receptors, such as naltrexone and nalmefene, have shown benefit in some studies in treating compulsive gambling, sexual behavior, alcoholism, and compulsive buying in non-Parkinson’s patients. A randomized controlled trial of naltrexone for ICDs in Parkinson’s disease did not show benefit on the primary endpoint (clinician’s rating of change in symptoms), but it did show a statistically significant benefit for naltrexone on the QUIP-RS (47). Regarding nonpharmacologic treatments, a randomized controlled study of cognitive-behavioral therapy showed benefit (48).
Conclusions and Future Directions
Data suggest that Parkinson’s disease medications, and perhaps DBS in a subset of patients, are associated with the development of ICDs and related behaviors. Susceptibility to these disorders has been associated with specific demographic and clinical characteristics, cognitive deficits, brain abnormalities, and possibly genetic risk factors. These behavioral addictions have significant clinical and neurobiological overlap with substance use disorders in the general population, with a shared neural substrate of altered dopaminergic activity in the striatum and deficits in prefrontal cortex–related cognitive abilities. ICDs and related behaviors can have serious consequences. Moreover, ICDs in Parkinson’s disease may become permanent, because affected patients may be unable to discontinue dopamine agonist therapy because of motor worsening or dopamine agonist withdrawal syndrome.
Given the potentially devastating consequences and lack of effective treatments, prevention and treatment strategies are critical. A multidisciplinary approach is recommended, including aggressive patient and caregiver education, vigilant monitoring with prompt discontinuation of dopamine agonists in affected patients if possible, and the judicious use of dopamine agonists in general, with consideration of alternative treatments. The use of emerging empirically validated treatments for ICDs should be considered, particularly in patients for whom discontinuation of dopamine agonist therapy is not an option.
1 : “Behavioral” addictions: do they exist? Science 2001; 294:980–982Crossref, Medline, Google Scholar
2 : Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol 2010; 67:589–595Crossref, Medline, Google Scholar
3 : New onset heightened interest or drive for gambling, shopping, eating, or sexual activity in patients with Parkinson’s disease: the role of dopamine agonist treatment and age at motor symptoms onset. J Psychopharmacol 2007; 21:501–506Crossref, Medline, Google Scholar
4 : Punding in Parkinson’s disease: its relation to the dopamine dysregulation syndrome. Mov Disord 2004; 19:397–405Crossref, Medline, Google Scholar
5 : Punding prevalence in Parkinson’s disease. Mov Disord 2007; 22:1179–1181Crossref, Medline, Google Scholar
6 : Prospective cohort study of impulse control disorders in Parkinson’s disease. Mov Disord 2013; 28:327–333Crossref, Medline, Google Scholar
7 : Impulse control disorder related behaviours during long-term rotigotine treatment: a post hoc analysis. Eur J Neurol 2016; 23:1556–1565Crossref, Medline, Google Scholar
8 : A European multicentre survey of impulse control behaviours in Parkinson’s disease patients treated with short- and long-acting dopamine agonists. Eur J Neurol 2016; 23:1255–1261Crossref, Medline, Google Scholar
9 : A retrospective evaluation of the frequency of impulsive compulsive behaviors in Parkinson’s disease patients treated with continuous waking day apomorphine pumps. Mov Disord Clin Pract 2016; 4:323–328Crossref, Medline, Google Scholar
10 : Amantadine use associated with impulse control disorders in Parkinson disease in cross-sectional study. Ann Neurol 2010; 68:963–968Crossref, Medline, Google Scholar
11 : Levodopa infusion improves impulsivity and dopamine dysregulation syndrome in Parkinson’s disease. Mov Disord 2013; 28:2007–2010Crossref, Medline, Google Scholar
12 : Impulse control disorder in patients with Parkinson’s disease under dopamine agonist therapy: a multicentre study. J Neurol Neurosurg Psychiatry 2014; 85:840–844Crossref, Medline, Google Scholar
13 : Impulse control disorders in Parkinson disease: a multicenter case-control study. Ann Neurol 2011; 69:986–996Crossref, Medline, Google Scholar
14 : Increased risk of impulse control symptoms in Parkinson’s disease with REM sleep behaviour disorder. J Neurol Neurosurg Psychiatry 2015; 86:174–179Crossref, Medline, Google Scholar
15 : [Assessment of hyper- and hypodopaminergic behaviors in Parkinson’s disease]. Rev Neurol (Paris) 2009; 165:845–856 (French)Crossref, Medline, Google Scholar
16 : Non-motor dopamine withdrawal syndrome after surgery for Parkinson’s disease: predictors and underlying mesolimbic denervation. Brain 2010; 133:1111–1127Crossref, Medline, Google Scholar
17 : Hedonistic homeostatic dysregulation in patients with Parkinson’s disease on dopamine replacement therapies. J Neurol Neurosurg Psychiatry 2000; 68:423–428Crossref, Medline, Google Scholar
18 : Dopamine agonist withdrawal syndrome in Parkinson disease. Arch Neurol 2010; 67:58–63Crossref, Medline, Google Scholar
19 : Risk and learning in impulsive and nonimpulsive patients with Parkinson’s disease. Mov Disord 2010; 25:2203–2210Crossref, Medline, Google Scholar
20 : Mechanisms underlying dopamine-mediated reward bias in compulsive behaviors. Neuron 2010; 65:135–142Crossref, Medline, Google Scholar
21 : Intact reward learning but elevated delay discounting in Parkinson’s disease patients with impulsive-compulsive spectrum behaviors. Neuropsychopharmacology 2010; 35:2155–2164Crossref, Medline, Google Scholar
22 : The risky business of dopamine agonists in Parkinson disease and impulse control disorders. Behav Neurosci 2011; 125:492–500Crossref, Medline, Google Scholar
23 : The neurobiology and genetics of impulse control disorders: relationships to drug addictions. Biochem Pharmacol 2008; 75:63–75Crossref, Medline, Google Scholar
24 : Functional implications of dopamine D1 vs D2 receptors: a “prepare and select” model of the striatal direct vs indirect pathways. Neuroscience 2014; 282:156–175Crossref, Medline, Google Scholar
25 : Prefrontal dopamine D1 and D2 receptors regulate dissociable aspects of decision making via distinct ventral striatal and amygdalar circuits. J Neurosci 2017; 37:6200–6213Crossref, Medline, Google Scholar
26 : Dopamine receptor agonists in current clinical use: comparative dopamine receptor binding profiles defined in the human striatum. J Neural Transm (Vienna) 2003; 110:1119–1127Crossref, Medline, Google Scholar
27 : A possible substrate for dopamine-related changes in mood and behavior: prefrontal and limbic effects of a D3-preferring dopamine agonist. Proc Natl Acad Sci USA 2002; 99:17113–17118Crossref, Medline, Google Scholar
28 : Molecular cloning and characterization of a novel dopamine receptor (D3) as a target for neuroleptics. Nature 1990; 347:146–151Crossref, Medline, Google Scholar
29 : Screening for impulse control disorder symptoms in patients with de novo Parkinson disease: a case-control study. Neurology 2013; 80:176–180Crossref, Medline, Google Scholar
30 : Decision-making impairments in patients with Parkinson’s disease. Behav Neurol 2004; 15:77–85Crossref, Medline, Google Scholar
31 : Reward-learning and the novelty-seeking personality: a between- and within-subjects study of the effects of dopamine agonists on young Parkinson’s patients. Brain 2009; 132:2385–2395Crossref, Medline, Google Scholar
32 : Incident impulse control disorder symptoms and dopamine transporter imaging in Parkinson disease. J Neurol Neurosurg Psychiatry 2016; 87:864–870Crossref, Medline, Google Scholar
33 : Reversal learning in Parkinson’s disease depends on medication status and outcome valence. Neuropsychologia 2006; 44:1663–1673Crossref, Medline, Google Scholar
34 : Increased striatal dopamine release in Parkinsonian patients with pathological gambling: a [11C] raclopride PET study. Brain 2009; 132:1376–1385Crossref, Medline, Google Scholar
35 : [11C]-(+)-PHNO PET imaging of dopamine D(2/3) receptors in Parkinson’s disease with impulse control disorders. Mov Disord 2015; 30:160–166Crossref, Medline, Google Scholar
36 : Parkinson’s disease and pathological gambling: results from a functional MRI study. Mov Disord 2010; 25:2449–2453Crossref, Medline, Google Scholar
37 : Decreased ventral striatal activity with impulse control disorders in Parkinson’s disease. Mov Disord 2010; 25:1660–1669Crossref, Medline, Google Scholar
38 : DRD and GRIN2B polymorphisms and their association with the development of impulse control behaviour among Malaysian Parkinson’s disease patients. BMC Neurol 2015; 15:59Crossref, Medline, Google Scholar
39 : Clinical-genetic model predicts incident impulse control disorders in Parkinson’s disease. J Neurol Neurosurg Psychiatry 2016; 87:1106–1111Crossref, Medline, Google Scholar
40 : Involvement of the subthalamic nucleus in impulse control disorders associated with Parkinson’s disease. Brain 2011; 134:36–49Crossref, Medline, Google Scholar
41 : Patient versus informant reporting of ICD symptoms in Parkinson’s disease using the QUIP: validity and variability. Parkinsonism Relat Disord 2011; 17:153–155Crossref, Medline, Google Scholar
42 : Questionnaire for Impulsive-Compulsive Disorders in Parkinson’s Disease–Rating Scale. Mov Disord 2012; 27:242–247Crossref, Medline, Google Scholar
43 : Carer burden in apathy and impulse control disorders in Parkinson’s disease. Int J Geriatr Psychiatry 2012; 27:160–166Crossref, Medline, Google Scholar
44 : Long-term follow-up of impulse control disorders in Parkinson’s disease. Mov Disord 2008; 23:75–80Crossref, Medline, Google Scholar
45 : Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science 2007; 318:1309–1312Crossref, Medline, Google Scholar
46 : Pathological gambling in Parkinson disease is reduced by amantadine. Ann Neurol 2010; 68:400–404Crossref, Medline, Google Scholar
47 : Naltrexone for impulse control disorders in Parkinson disease: a placebo-controlled study. Neurology 2014; 83:826–833Crossref, Medline, Google Scholar
48 : Trial of CBT for impulse control behaviors affecting Parkinson patients and their caregivers. Neurology 2013; 80:792–799Crossref, Medline, Google Scholar



