Psychiatric Comorbidity in Moyamoya Disease and Preliminary Guidelines For Treatment
“Ms. A” is a 54-year-old Chinese-American woman with no psychiatric history who presented to the emergency department in a “psychotic break.” She could not provide a coherent history and appeared withdrawn, selectively mute, and confused. Her husband reported that her father had died unexpectedly 2 weeks earlier from natural causes. When she returned to work after a few days off, she disappeared for several hours, without recollection of her activities.
Ms. A’s physical examination was normal, with stable vital signs with mild systolic hypertension (143/86 mmHg). Fully oriented to person, place, time, and situation, she denied acute psychiatric concerns apart from sadness over her father’s death. Her medical, social, and family histories were unremarkable. Findings from routine altered mental status screening, including CT, serological studies, and lumbar puncture, were negative. Urinalysis, however, showed pyuria. A diagnosis of cystitis was made, for which cephalexin was prescribed, and Ms. A was discharged home with her husband.
She returned to the emergency department 2 days later with worsening symptoms and new incontinence. Psychiatry was consulted and discharged her home to begin sertraline at 50 mg/day for observed depressive symptoms. At outpatient psychiatric follow-up 1 week later, her psychomotor retardation, mutism, and cognitive slowing were unchanged. She disclosed “feelings of inadequacy” and “sad” mood, describing her father’s death as “devastating.” She frequently repeated the clinician’s questions, but her orientation was intact. When asked to write her thoughts, she inscribed, “I am scared and excited,” over 100 times on a sheet of paper. She denied domestic violence when interviewed alone. Her sertraline dosage was increased to 100 mg/day, and lorazepam was started at 1 mg b.i.d. to treat presumptive catatonia.
Ms. A showed minimal response after 2 weeks, and her incontinence worsened, resulting in inpatient psychiatric admission. Given acute stressors and negative medical findings, the differential diagnosis included depressive catatonia, psychosis, dissociative disorder, and conversion disorder. She reported a chief complaint of “confusion,” scoring 15/30 on the Mini-Mental State Examination. Flat affect, selective mutism, echolalia, insomnia, bizarre behaviors (e.g., smearing toothpaste on the wall), and significant cognitive and psychomotor retardation remained despite increases in her dosages of sertraline and lorazepam and the addition of olanzapine (2.5–5 mg h.s.). Subjectively, she disclosed feeling “not normal” and anxious. Orientation to time and place fluctuated in no discernible pattern, with preserved self-identification.
Findings from additional workup, including an extensive autoimmune illness panel and EEG, were negative. MRI, however, demonstrated regions of diffusion restriction in the left superior frontal gyrus and in the anterior cerebral artery distribution, as well as small multifocal diffusion restriction in the left and right centrum semiovale in a watershed distribution. Corresponding T2-weighted fluid-attenuated inversion recovery hyperintense signal was observed in these areas (Figure 1A). Hemorrhagic transformation of the left basal ganglia was also noted. Magnetic resonance angiography and cerebral angiogram revealed bilateral stenosis of the internal carotid arteries with minimal filling of the anterior and middle cerebral vascular trees, as well as a network of collateral vessels at the terminal internal carotid arteries and posterior cerebral arteries, confirming bilateral moyamoya disease (Figure 1B). With blood pressure support, the patient’s mental status improved to a score of 29/30 on the Mini-Mental State Examination. She subsequently underwent cerebral artery bypass surgery.
Moyamoya disease is a cerebrovascular disorder of unknown etiology characterized by progressive unilateral or bilateral stenosis of the distal internal carotid arteries, frequently extending to the anterior and middle cerebral arteries. Cerebral angiogram reveals fragile compensatory collateral vessels forming along the base of the brain, resembling a diagnostic “puff of smoke”—moyamoya in Japanese (1, 2). The prevalence of moyamoya is highest in Korea, Japan, and China, although awareness of the disease has resulted in its increased characterization in other countries. In Japan, the incidence rate is estimated at 0.54 per 100,000 individuals, with a female-to-male ratio of 1.98 (3). Illness onset follows a bimodal distribution with a large peak centered at approximately age 5 and a smaller peak at age 40 (3). A family history of the disease is present in up to 15% of identified patients, and in this group the mean age at symptomatic onset is lower than in sporadic cases of moyamoya disease, indicating genetic anticipation (3, 4). Moyamoya disease may also occur secondary to atherosclerosis, autoimmune illness, neoplasms, Down’s syndrome, or irradiation, among other conditions, referred to as moyamoya syndrome (2).
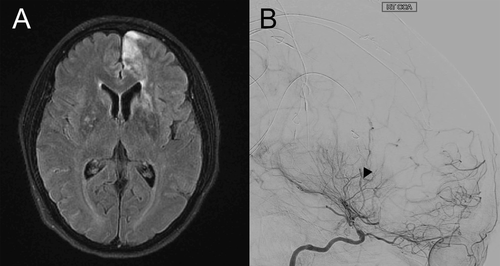
FIGURE 1. MRI and cerebral angiogram of the patient after admission to the inpatient psychiatric unita
a Panel A is an image from brain MRI with contrast, T2-weighted fluid-attenuated inversion recovery, axial section. Hyperintense signal is noted along the left superior frontal gyrus and the left and right centrum semiovale in areas of diffusion restriction. Panel B is a cerebral angiogram of the right common carotid artery. Severe narrowing of the internal carotid artery above the ophthalmic artery origin is observed as well as a network of developing collateral vessels (arrow).
Ischemia secondary to hypoperfusion is the primary clinical feature, resulting in repeated transient ischemic attacks or stroke, particularly in the cerebral cortex (e.g., frontal, parietal, and temporal lobes) (2, 5). Hemorrhagic stroke may also occur from rupture of fragile collateral vessels or aneurysms with intraventricular, intraparenchymal, and subarachnoid distributions (6). Embolism is uncommon. Disruption of the anterior circulation results in hemiparesis, speech disturbance, and hemisensory impairments (1). Headache is a common presenting and persisting symptom even with treatment (7). Involvement of the posterior circulation is more infrequent and is associated with greater illness severity (8). Cognitive dysfunction is common, with impairments observed in processing speed, verbal memory, verbal fluency, and executive function (9, 10). Ischemic symptoms are more common in children, and hemorrhagic symptoms predominate in older adults (11, 12).
Studies have suggested that moyamoya disease may manifest with a different phenotype in non–East Asian countries (13). In California and Washington, the incidence is estimated at 0.086 per 100,000 persons (14). An analysis of 2,280 admissions for moyamoya disease from the Nationwide Inpatient Sample, the largest inpatient care database in the United States, calculated a hospital admission rate of 0.57 per 100,000 persons per year (15). Predominantly females were affected (72%), with a mean and median age of 31.6 years at presentation. Overall, 49% were white, 24% black, 11% Hispanic, 11% Asian, and 5% other. Children and adults were more likely to present initially with ischemic rather than hemorrhagic illness. The authors of that report acknowledge, however, that these phenotypic differences may be due to an increased number of cases with moyamoya syndrome. In the Mayo Clinic moyamoya disease/moyamoya syndrome cohort, individuals are also primarily female (72.3%) and Caucasian (85.1%), with higher incidences of autoimmune diseases in comparison with the general population (22.3% and 3.2%, respectively), including type 1 diabetes and Graves’ disease (16). Failure to recognize moyamoya syndrome may falsely lower incidence rates in the United States.
Moyamoya disease is a chronic illness with a highly variable natural course ranging from a slow, asymptomatic illness to rapid decompensation. A Japanese prospective study estimated a natural progression rate of approximately 20% over 6 years (17). In symptomatic patients, surgical revascularization is the treatment of choice to prevent cerebral infarction and restore perfusion and reserve capacity (18). Revascularization occurs either via direct artery-to-artery anastomosis or indirect arteriosynangiosis. Conservative treatment utilizes long-term aspirin, antiplatelet, or statin therapy, which has been shown to be insufficient to stop disease progression compared with surgery, after which the rate of symptomatic progression may be as low as 2.3% (18, 19). The role of surgical intervention in hemorrhagic disease is controversial, with rates of rebleeding in up to 18% of patients regardless of treatment modality (20, 21). Rebleeding is associated with extremely poor prognosis (6). In children and adults, functional status at the time of surgical intervention is a better predictor of favorable outcome and quality of life than age at presentation (12, 22). Early intervention is critical to reducing the risk of permanent neurological and cognitive deficits and improving long-term prognosis and life expectancy.
Psychiatric Manifestations of Moyamoya Disease
Exclusively psychiatric presentations of moyamoya disease are exceedingly rare in the literature. In adults, coincident complaint of depression or anxiety in assessment of new focal neurological symptoms is more common (23, 24). In children, months to years of mood lability, hyperactivity, impaired concentration, and poor academic performance have also been described (25, 26). Of those cases identified where psychiatric symptoms preceded neurological deficits, none of the patients reported a prior history of mental illness. The clinical presentations are highly variable, although psychosis appears to be a more frequent manifestation in children and young adults. One case report (27) describes an 11-year-old Indian boy who developed insomnia, disorganization, agitation, and internal preoccupation after a febrile illness, treated as presumptive schizophrenia for 1 week until he developed extrapyramidal symptoms. EEG revealed slow waves in the right frontal and temporal region, and angiography demonstrated bilateral moyamoya disease. The patient’s symptoms improved with surgical treatment and antipsychotic medication, albeit with continued impulsivity and restlessness. In a similar case (28), a 23-year-old Vietnamese man with auditory hallucinations and paranoid delusions received antipsychotic treatment for 2 years before an MRI for a research study revealed bilateral moyamoya disease. His symptoms resolved with antipsychotic treatment and conservative management.
The degree of psychosis can be profound in some instances. One case report (29) describes a 12-year-old Caucasian boy who developed acute-onset insomnia, hyperverbal speech, tangential thought processes, severe mood lability, auditory hallucinations of laughter and babies crying, visual hallucinations of dolls, Capgras delusion regarding his mother, paranoid delusions of being observed and hypnotized, and the belief that he could receive messages through the television. Symptoms started 9 days after he participated in a 1500-meter run. The patient’s EEG was normal, but MRI and MR angiography showed unilateral moyamoya disease. His psychotic symptoms resolved with antipsychotic treatment without surgical revascularization.
Acute psychiatric symptoms in the setting of known moyamoya disease may also signal new ischemia. One report (30) describes an 8-year-old boy with moyamoya disease who presented to outpatient psychiatry with irritability, paranoia, internal preoccupation, poor self-care, insomnia, and hallucinations of cartoon characters accusing or scolding him throughout the day. MRI subsequently revealed new ischemic changes in the left middle cerebral artery distribution. His symptoms resolved with antipsychotic treatment.
In adults, refractory depression and psychosis prompting expanded neurological investigation have revealed moyamoya disease, sometimes years after symptom onset (24, 31). A report from Korea (32) describes the case of a 45-year-old woman with depression and transient episodes of confabulation and inappropriate behavior provoked by arguments with her husband. The authors initially thought her symptoms were secondary to depression until she developed right-sided weakness and MRI showed bilateral moyamoya disease. She received treatment with antiplatelet agents, statins, and a mood stabilizer, which partially resolved her mood lability.
Psychiatric Comorbidity
Most large studies of moyamoya disease have excluded psychological measures in examination of long-term outcomes. From the case literature and the limited number of small studies, depression and anxiety appear to be the most common psychiatric sequelae of illness (23, 33–36). A sample of Korean pediatric cases reported comorbid depression, anxiety, impulsivity, impaired concentration, and social adaptivity issues (37). In a study of American adults with moyamoya disease who were evaluated before surgical revascularization, 36% were found to have mild depression and 28% to have moderate to severe depression, as measured by the Beck Depression Inventory (BDI) (9). A large percentage of participants reported a history of stroke (83%), although degree of neurocognitive dysfunction did not differ between the stroke and nonstroke groups and showed no correlation with the depression. Another study of American adults with moyamoya disease (38) revealed a 20% rate of at least mild depression in a cohort with a mixed history of stroke. Among the portion of the cohort without evidence of ischemic stroke on MRI, 29% reported suffering clinically significant depression and 28% clinically significant anxiety (10). Patients and families indicated apathy as the predominant behavioral issue (10).
Anxiety also appears to be a common comorbidity. A Chinese study of adults with moyamoya disease (39) compared quality of life with intraventricular hemorrhagic moyamoya disease managed conservatively to matched healthy control subjects and patients with spontaneous intraventricular hemorrhage. One year after initial stroke, those with hemorrhagic moyamoya disease reported higher rates of sensitivity, poor stress tolerance, poor interpersonal relationships, and pessimism compared with both control groups, and 73% reported depression. The patients with moyamoya disease also scored higher for obsessive-compulsive, depressive, and phobic anxiety symptoms. Baseline measures of mood and personality were not available for comparison. A study (40) of Korean males (59.5% ischemic presentation, 2% hemorrhagic) with a mixture of moyamoya disease management types (70.2% surgical and 29.8% antiplatelet or no treatment) showed significantly increased anxiety, depression, and somatization compared with healthy control subjects. An association was additionally observed between degree of encephalomalacic changes in the frontal lobe and paranoia. Only one study has directly examined the impact of revascularization on depression (41). As measured by the BDI in a group of U.S. adults with moyamoya disease, 19% indicated improved depression, 11% worsened depression, and 70% no change in depression at 6-month follow-up after revascularization.
Mood lability, irritability, psychomotor agitation, insomnia, and impulsivity resembling bipolar disorder developing days to years after illness onset has also been described, although less commonly (35, 42, 43). Rarer are hallucinations and paranoia (23, 42, 44). Psychic akinesia has also been described (45, 46). Typically associated with lesions in the prefrontal cortex or basal ganglia, the condition is characterized by severely flattened affect, apathy, and amotivation. Similar symptoms may be seen in schizophrenia and Parkinson’s disease. Unlike depression, psychic akinesia occurs without subjective sad or negative thinking and may be accompanied by obsessive-compulsive behaviors and motor tics (47).
Psychopharmacologic Treatment For Psychiatric Symptoms in Moyamoya Disease
Although patients with moyamoya disease are frequently treated with a variety of psychotropic medications, no controlled trials have examined the efficacy of specific treatments in the management of associated psychiatric symptoms (9). Review of the case literature indicates that several features of a psychotropic medication should be considered prior to initiation in patients with moyamoya disease, including a medication’s ability to alter vascular hemodynamics and affect cerebral perfusion, induce confusion or paradoxical disinhibition, lower the seizure threshold, worsen mood lability, and block dopamine D2 receptors. As psychiatric phenotypes are highly heterogeneous in moyamoya disease, no absolute contraindications exist for any individual medication. Medication selection must also consider the individual’s specific disease characteristics—degree and location of occlusions, prior ischemic or hemorrhagic injuries, and seizure history. Specific recommendations evidenced in the literature are summarized below by medication class.
Mood Stabilizers
Although less common than depression and anxiety, secondary mania is associated with moyamoya disease, characterized by mood lability (typically irritability, although euphoria is also described), psychomotor agitation, impulsivity, insomnia, and/or psychosis (26, 32, 35, 42, 43). Use of carbamazepine (43), divalproex sodium (26, 33), and oxcarbazepine (25) has been reported, with diminished irritability and impulsivity in moyamoya disease (32). Given evidence that older agents produce cognitive and behavioral side effects (48), preference would be for medications that have more established mood stabilization benefits, such as lamotrigine and divalproex sodium.
Antidepressants
Moyamoya disease–associated depression, anxiety, and psychic akinesia respond variably to antidepressants (9, 28, 35, 36). Given the risk for depression secondary to severe medical illness, comorbid anxiety, or obsessive-compulsive symptoms, early antidepressant treatment is recommended, particularly given the relatively safe side effect profile of these medications. Furthermore, anxiety carries a risk for panic attacks, including crying and hyperventilation, which may result in repeated hypoperfusion stress on collateral or anastomotic vessels, raising risk for new ischemia. Use of amitriptyline (9, 23) and sertraline (9, 28, 36) is commonly reported. Selective serotonin reuptake inhibitors are favored because of data on improved motor coordination following ischemic injury, a relative lack of anticholinergic side effects compared with tricyclic antidepressants, and a low impact on blood pressure compared with some serotonin-norepinephrine reuptake inhibitors (49). Atypical agents such as trazodone and mirtazapine appear to be effective in targeting mood and insomnia.
Antipsychotics
Patients with moyamoya disease display high neuroleptic sensitivity, with frequent reports of extrapyramidal symptoms and neuroleptic malignant syndrome, especially with first-generation agents (26–29). Impaired basal ganglia function due to abnormal vessel collateralization or ischemia may explain this sensitivity, which is similar in Lewy body dementia. Antipsychotics, however, are effective in reducing hallucinations and paranoia (23, 27–30, 44). Second-generation agents, such as quetiapine, are preferred given a lower risk for extrapyramidal complications. Trial reduction after prolonged resolution of symptoms appears reasonable, particularly if symptoms occurred prior to surgical intervention. Antipsychotic treatment may also assist with motor tics, if present (23).
Benzodiazepines
Treatment with benzodiazepines is commonly described in the treatment of moyamoya disease–related anxiety and agitation, but their efficacy as a monotherapy has been reported to be poor, owing to rapid tolerance and possible paradoxical disinhibition (9, 23, 29, 33, 43). For short-term management of anxiety or emergent agitation, however, benzodiazepines appear to be a safe option.
Stimulants
Stimulants or stimulant-like medications (e.g., modafinil) offer theoretical benefit in depression, apathy, impulsivity, and neurocognition in moyamoya disease. An Internet search reveals frequent anecdotal reports of patients with moyamoya disease receiving stimulants to assist with cognitive difficulties. Given the potential for hypertension, vasoconstriction, and irritability, conservative management would restrict stimulant trials to medically and psychiatrically stable patients after surgical revascularization.
Conclusions
Moyamoya disease and moyamoya syndrome carry a unique risk for misdiagnosis as an affective or psychotic disorder. Transient ischemic events may be easily mistaken for anxiety and panic disorder, emphasizing the need for thorough screening of precipitating triggers and characterization of symptoms. In the case presented here, the proximity of the patient’s father’s death confounded diagnosis. The absence of a family history, particularly of psychotic illness, in combination with atypical illness features (e.g., age at onset, visual hallucinations) should prompt neurological investigation, with a strong argument in favor of MRI or MR angiography rather than screening CT. In cases of new psychiatric symptoms in patients with known moyamoya disease, repeat neuroimaging is justified to rule out new ischemia.
The pathogenesis of psychiatric symptoms in moyamoya disease appears to be multifactorial, with both genetic and environmental factors influencing specific phenotypes. Cellular destruction of internal carotid artery distribution territories from ischemic stroke, repeated transient ischemic attacks, or hemorrhage of aneurysms and collateral vascular networks may manifest immediately or progress with time and new vascular insults. However, patients without ischemic or hemorrhagic stroke also suffer from depression, anxiety, and other symptoms, indicating a possible linkage with the disease pathogenesis, likely related to chronic hemodynamic insufficiency of small cortical vessels. Finally, psychological precipitants, related to prognosis, decrements in quality of life, and frustration from cognitive impairment, likely contribute (34). Early engagement in psychotherapy is highly recommended. One study (8) suggested that the regions of the brain that are susceptible to hemodynamic insufficiency in moyamoya disease change as the disease progresses, shifting from the inner to the outer cortical structures and from the anterior to the posterior territories. Thus, even in stable moyamoya disease, new psychiatric symptoms may develop over time.
Psychiatric comorbidity in moyamoya disease appears to be a heterogeneous and dynamic phenomenon that affects at least one-quarter of patients, although the prevalence is likely significantly underestimated. Treatment choice should be guided by the patient’s symptoms and neuroimaging of affected brain territories. Neuropsychological testing may be considered, to fully characterize a patient’s psychiatric and cognitive deficits. Classical psychotropic medications appear to be effective but underutilized. Dopamine precursors, dopamine agonists, and acetylcholinesterase inhibitors are virtually unexplored in treating the illness. Because untreated or poorly treated psychiatric symptoms may have a negative impact on quality of life, disability, and prognosis, a team-based multidisciplinary approach is highly recommended, with collaboration between psychiatry and neurology early in diagnosis.
1 : Cerebrovascular “moyamoya” disease: disease showing abnormal net-like vessels in base of brain. Arch Neurol 1969; 20:288–299Crossref, Medline, Google Scholar
2 : Diagnosis of moyamoya disease: international standard and regional differences. Neurol Med Chir (Tokyo) 2015; 55:189–193Crossref, Medline, Google Scholar
3 : Epidemiological features of moyamoya disease in Japan. Neurol Med Chir (Tokyo) 2012; 52:295–298Crossref, Medline, Google Scholar
4 : Clinical features of familial moyamoya disease. Childs Nerv Syst 2006; 22:258–262Crossref, Medline, Google Scholar
5 : Moyamoya disease: epidemiology, clinical features, and diagnosis. J Stroke 2016; 18:2–11Crossref, Medline, Google Scholar
6 : Clinical course, surgical management, and long-term outcome of moyamoya patients with rebleeding after an episode of intracerebral hemorrhage: an extensive follow-up study. Stroke 1999; 30:2272–2276Crossref, Medline, Google Scholar
7 : Headache in pediatric moyamoya disease: review of 204 consecutive cases. J Neurosurg 2005; 103(Suppl):439–442Medline, Google Scholar
8 : Changing ischaemic lesion patterns in adult moyamoya disease. J Neurol Neurosurg Psychiatry 2009; 80:36–40Crossref, Medline, Google Scholar
9 : Neurocognitive dysfunction in adult moyamoya disease. J Neurol 2010; 257:806–815Crossref, Medline, Google Scholar
10 : Neurocognitive impairment in adults with moyamoya disease without stroke. Neurosurgery 2012; 70:634–638Crossref, Medline, Google Scholar
11 : Moyamoya disease in adults: characteristics of clinical presentation and outcome after encephalo-duro-arterio-synangiosis. Clin Neurol Neurosurg 1997; 99(suppl 2):S151–S155Crossref, Medline, Google Scholar
12 : Long-term outcome in children with moyamoya syndrome after cranial revascularization by pial synangiosis. J Neurosurg 2004; 100(2 suppl pediatrics):142–149Medline, Google Scholar
13 : Clinical features and outcome in North American adults with moyamoya phenomenon. Stroke 2006; 37:1490–1496Crossref, Medline, Google Scholar
14 : Moyamoya disease in Washington State and California. Neurology 2005; 65:956–958Crossref, Medline, Google Scholar
15 : Moyamoya disorder in the United States. Neurosurgery 2012; 71:93–99Crossref, Medline, Google Scholar
16 : Moyamoya disease in a primarily white, midwestern US population: increased prevalence of autoimmune disease. Stroke 2013; 44:1997–1999Crossref, Medline, Google Scholar
17 : Incidence and clinical features of disease progression in adult moyamoya disease. Stroke 2005; 36:2148–2153Crossref, Medline, Google Scholar
18 : Moyamoya disease: treatment and outcomes. J Stroke 2016; 18:21–30Crossref, Medline, Google Scholar
19 : Revascularisation surgery for paediatric moyamoya: a review of the literature. Childs Nerv Syst 2005; 21:358–364Crossref, Medline, Google Scholar
20 : The current status of the treatment for hemorrhagic type moyamoya disease based on a 1995 nationwide survey in Japan. Clin Neurol Neurosurg 1997; 99(Suppl 2):S183–S186Crossref, Medline, Google Scholar
21 : Long-term natural history of hemorrhagic moyamoya disease in 42 patients. J Neurosurg 2000; 93:976–980Crossref, Medline, Google Scholar
22 : Clinical outcome after 450 revascularization procedures for moyamoya disease. J Neurosurg 2009; 111:927–935Crossref, Medline, Google Scholar
23 : Occlusive disease of intracranial main arteries with collateral networks (moyamoya disease) in adults. Acta Neurol Scand 1973; 49:307–322Crossref, Medline, Google Scholar
24 : Multiple progressive intracranial arterial occlusions (“moyamoya” disease). J Neurol Neurosurg Psychiatry 1977; 40:853–860Crossref, Medline, Google Scholar
25 : Neuropsychiatric manifestations in a child with moyamoya disease. J Neurosci Rural Pract 2016; 7:331–332Crossref, Medline, Google Scholar
26 : Mood disorder in association with moyamoya disease. Psychiatry Clin Neurosci 2012; 66:163–164Crossref, Medline, Google Scholar
27 : Moyamoya disease (presenting as schizophrenia). Indian J Psychiatry 1981; 23:188–189Medline, Google Scholar
28 : Moyamoya disease in a patient with schizophrenia. J Int Neuropsychol Soc 2003; 9:806–810Crossref, Medline, Google Scholar
29 : Moyamoya disease in a 12-year-old Caucasian boy presenting with acute transient psychosis. Eur Child Adolesc Psychiatry 1999; 8:149–153Crossref, Medline, Google Scholar
30 : Poststroke psychosis in an 8-year-old child with moyamoya disease. BMJ Case Rep 2013 (doi: 10.1136/bcr-2013-201771)Crossref, Google Scholar
31 : Unilateral moya moya disease in an adult: a case history. Angiology 1987; 38:342–346Crossref, Medline, Google Scholar
32 : Fluctuating frontal lobe dysfunction in a patient with moyamoya disease. Neurol Sci 2011; 32:743–746Crossref, Medline, Google Scholar
33 : A single case report of moyamoya disease presenting in a psychiatric setting. Appl Neuropsychol 2010; 17:73–77Crossref, Medline, Google Scholar
34 : Moyamoya disease, revascularisation surgery, and anaesthetic considerations. BMJ Case Rep 2014 (doi: 10.1136/bcr-2013-202784)Crossref, Medline, Google Scholar
35 : Cognitive deficits presenting as psychiatric symptoms in a patient with moyamoya disease. Psychol Rep 2010; 107:727–732Crossref, Medline, Google Scholar
36 : 30-year-old female with moyamoya disease and associated depression. Psychiatr Ann 2014; 44:549–551Crossref, Google Scholar
37 : Cognitive outcome of pediatric moyamoya disease. J Korean Neurosurg Soc 2015; 57:440–444Crossref, Medline, Google Scholar
38 : Effect of moyamoya disease on neuropsychological functioning in adults. Neurosurgery 2008; 62:1048–1051Crossref, Medline, Google Scholar
39 : Quality of life and psychological impact in adult patients with hemorrhagic moyamoya disease who received no surgical revascularization. J Neurol Sci 2013; 328:32–36Crossref, Medline, Google Scholar
40 : Influence of moyamoya disease on psychopathological abnormality in young males in Korea: analysis of multiphasic personal inventory test. Neurol Sci 2013; 34:949–953Crossref, Medline, Google Scholar
41 : Neurocognitive performance after cerebral revascularization in adult moyamoya disease. Stroke 2017; 48:1514–1517Crossref, Medline, Google Scholar
42 : Moyamoya disease: no need for anastomotic surgery? Acta Neurol Scand 1993; 88:32–34Crossref, Medline, Google Scholar
43 : Effectiveness of carbamazepine for benzodiazepine-resistant impulsive aggression in a patient with frontal infarctions. Psychiatry Clin Neurosci 2007; 61:695–697Crossref, Medline, Google Scholar
44 : Symptomatic schizophrenia with moya moya disease. Behav Neurol 1991; 4:25–28Crossref, Medline, Google Scholar
45 : [Athymhormic syndrome caused by bilateral striato-capsular infarction: moyamoya disease in adults]. Rev Neurol (Paris) 1995; 151:383–387 (French)Medline, Google Scholar
46 : Vascular parkinsonism in moyamoya: microvascular biopsy and imaging correlates. Ann Neurol 2003; 54:836–840Crossref, Medline, Google Scholar
47 : Athymhormia and disorders of motivation in basal ganglia disease. J Neuropsychiatry Clin Neurosci 2004; 16:509–524Crossref, Medline, Google Scholar
48 : Psychiatric and behavioral side effects of antiepileptic drugs in adults with epilepsy. Epilepsy Behav 2017; 76:24–31Crossref, Medline, Google Scholar
49 : Fluoxetine for motor recovery after acute ischaemic stroke (FLAME): a randomised placebo-controlled trial. Lancet Neurol 2011; 10:123–130Crossref, Medline, Google Scholar



