Discoveries on the Genetics of ADHD in the 21st Century: New Findings and Their Implications
Abstract
The 21st century has witnessed the discovery of multiple rare and common gene variants associated with attention deficit hyperactivity disorder (ADHD), and these discoveries have already provided a starting point for the investigation of the biology of the disorder and novel treatments. The purpose of this selective review is to examine genetic findings from the past 5 years and consider their implications for the conceptualization of ADHD and future clinical practice. Recent discoveries reveal the strong genetic overlaps between ADHD and autism spectrum disorder (ASD) as well as intellectual disability. Thus, the removal of the previous diagnostic exclusion criteria for ADHD in the presence of ASD is a welcome change in DSM-5. However, ADHD also shows substantial genetic correlations with a much broader group of neuropsychiatric disorders as well as with nonpsychiatric conditions (e.g., lung cancer). Investigating potential explanations for these links is an important next step. ADHD, while usefully conceptualized as a disorder in clinical practice, can be viewed as a trait. Recent genome-wide association study findings, consistent with twin studies, highlight that ADHD lies at the extreme end of a continuously distributed dimension, akin to hypertension along the continuum of blood pressure. Although ADHD levels typically decline with age, twin and molecular genetic studies suggest that a persistent trajectory is associated with higher genetic loading. Routine testing for rare mutations in ADHD is not yet recommended, although guidelines in many countries recommend testing individuals with mild intellectual disability or ASD, so practice could change. Common gene variants for ADHD are only weakly predictive and therefore have limited clinical value at present, as does pharmacogenomics.
[AJP at 175: Remembering Our Past As We Envision Our Future
November 1938: Electroencephalographic Analyses of Behavior Problem Children
The electroencephalogram was the first biological technique to be applied to childhood behavioral disorders. Jasper, Solomon, and Bradley reported that ”the electroencephalogram has succeeded in revealing a definite abnormality of brain function in over one half of a group of childhood behavior disorders which had been previously considered as largely psychogenic.” (Am J Psychiatry 1938; 95:641–658)]
The concept of attention deficit hyperactivity disorder (ADHD) as a clinical condition can be traced back to well before the 20th century (1). The view that the behaviors associated with ADHD arose from early “brain damage” remained highly influential through much of the 20th century until the first wave of family and twin studies (2) indirectly revealed the contribution of genetic risk factors. During the 21st century, technological advances and large-scale collaborative efforts have enabled direct and successful genetic investigations into neuropsychiatric disorders, including ADHD (3). ADHD, like all common medical conditions, is not explained by genes alone; environmental risks also contribute (for a full discussion on environmental risk factors and designs to test causality, see reference 2).
This article is a selective and conceptual review, the purpose of which is to consider genetic findings on ADHD from the past 5 years and their implications for the conceptualization of ADHD and future clinical practice. The rationale for this approach was that there are already published comprehensive and systematic reviews on the genetics of ADHD that cover the literature that predates this period (e.g., 2, 4).
The Neurodevelopmental Nature of ADHD
It has been known for decades that ADHD is familial and highly heritable (5), with heritability estimates in the range of 60%–90%. Twin studies have shown a strong genetic overlap with other child psychopathology, most prominently with behavioral problems, such as conduct disorder (6). Indeed, ADHD was long considered primarily an externalizing or behavioral problem (7). However, more recent genetic studies have highlighted its neurodevelopmental nature (8), and these findings have added to clinical arguments that ADHD behaves as a neurodevelopmental disorder. ADHD, like autism spectrum disorder (ASD) and intellectual disability as well as other childhood neurodevelopmental disorders, typically has an early onset, tends to show a steady clinical course rather than one that is remitting and relapsing, and is commonly accompanied by early neurocognitive deficits (8).
Familial Overlap With Autism and Intellectual Disability
Recent national registry-based family and twin studies have enabled the investigation of diagnoses rather than traits and have revealed a strong familial and genetic overlap between ADHD and ASD. In one Swedish study (9), the monozygotic co-twins of individuals with ASD were found to have an increased risk of ADHD (odds ratio=17.77, 95% CI=9.8–32.22) compared with dizygotic co-twins (odds ratio=4.33, 95% CI=3.21–5.86). These associations were most prominent for individuals with higher-functioning ASD rather than low-functioning ASD (with intellectual disability). Although the within-individual and within-family overlap of ADHD and ASD is now accepted, until the publication of DSM-5, a diagnosis of ADHD in the presence of autism was disallowed.
Recent genetic findings have also challenged the historical reticence about diagnosing ADHD in individuals with intellectual disability and about including those with lower IQ in genetic studies. It has always been recognized that ADHD is strongly associated with lower IQ and intellectual disability (10). Population-based twin studies of IQ and learning ability (e.g., reading ability) have consistently found that most of the correlation with ADHD symptoms was explained by shared heritability (10). Interestingly, even though the genetic overlaps between intellectual disability and autism (11) and between intellectual disability and schizophrenia are well recognized (12), investigations into the genetic links between intellectual disability and ADHD have been sparse. One recent registry-based family study addressed this gap by examining ADHD diagnosis and intellectual disability (13). The authors found that most of the correlation between ADHD and intellectual disability liabilities was explained by genetic factors (estimated at 91%) except in the case of profound intellectual disability. Again these findings point to the neurodevelopmental nature of ADHD, and they are important for scientists and clinicians. Historically, although individuals with lower IQ or intellectual disability have been excluded from studies of ADHD, this has not been the case for studies of schizophrenia and autism. This means that ADHD research studies, including gene discovery investigations, with some exceptions (14), have not included the full IQ spectrum. If individuals with ADHD who have comorbid intellectual disability or ASD are excluded from genetic studies, this is a potential problem, because it means that such investigations will not have been fully representative of the clinical population or included the most severely affected individuals.
Gene Discoveries and Their Implications for the Conceptualization of ADHD
With technological advances, reductions in cost, and large-scale collaborations, such that very large sample sizes are available, researchers have been able to investigate different classes of genetic variation across the whole genome. Early linkage and association studies revealed that ADHD, like other neuropsychiatric disorders, is in most instances a multifactorial disorder. It is not explained by Mendelian inheritance or common gene variants of large effect size. Recent molecular genetic studies of ADHD have shown that it is highly polygenic; that is, its genetic architecture is explained by thousands of common gene variants, each of small effect size (typical odds ratio, <1.1) (15), as well as by rare mutations, some of which have a much larger effect size (e.g., chromosome 16p13.11 duplication, odds ratio=13.88, 95% CI=2.3–82.2) (14).
Rare Genetic Mutations of Strong Effect Sizes That Influence ADHD and Other Neurodevelopmental Disorders
Established syndromes.
A number of rare Mendelian disorders and chromosomal anomalies are accompanied by ADHD as well as other neurodevelopmental and neuropsychiatric disorders. For example, ADHD is the most common neuropsychiatric disorder observed in 22q11.2 deletion syndrome (16), even though this syndrome is typically considered primarily a risk factor for psychosis. ADHD is also associated with tuberous sclerosis, Smith-Magenis syndrome, fragile X syndrome, and Prader-Willi syndrome. These disorders, however, lead to a wide range of neurodevelopmental and neuropsychiatric phenotypes beyond ADHD, including ASD, anxiety, and depression, and they are often accompanied by intellectual disability and physical anomalies; none of these known mutations is ADHD specific.
Copy number variants.
In the past decade, there have been growing efforts, through systematic investigations across the whole genome, to identify novel rare gene mutations that are associated with ADHD and other neuropsychiatric disorders (3). One class of rare mutations, known as copy number variants (CNVs), which are segments of chromosome that are duplicated or deleted, has been implicated in disorders that might be considered more neurodevelopmental in nature than, for example, major depressive disorder or many anxiety disorders. These include intellectual disability, autism, schizophrenia, and, more recently, Tourette’s syndrome as well as ADHD (3, 14, 17–21). The genomic regions spanned by the ADHD-associated CNVs have been found to show significant overlap with CNVs involved in autism and schizophrenia, highlighting the highly pleiotropic effect of CNVs. Puzzlingly, no one has yet investigated the overlap with CNVs implicated in intellectual disability yet.
Sequence variants.
The largest and only systematic investigation of another class of rare mutations in ADHD, rare exome sequence variants, is an as-yet unpublished analysis of 3,536 individuals with ADHD from the Danish iPSYCH register-based study (22). The investigators observed a burden of rare protein-truncating variants (PTVs) in ADHD similar to that found in ASD, and both groups were enriched for PTVs above the rate observed in the unaffected control group (odds ratios, 1.24 for ADHD and 1.23 for ASD). Although substantially higher rates of PTVs were observed in those with comorbid intellectual disability, in keeping with results from a previous study of CNV burden (19), a significant burden of rare PTVs was found in individuals with ADHD who had no comorbid intellectual disability. Intriguingly, these authors found that the genes affected by ADHD and ASD rare variants were indistinguishable.
Common Gene Variants: Small Effect Sizes That Have Shared Effects on ADHD and Other Neurodevelopmental Disorders and Traits
ADHD GWAS discoveries to date.
Very large sample sizes are required to detect individual common gene variants using genome-wide association study (GWAS) designs, and until recently these have been lacking for ADHD. However, the most recent ADHD GWAS, which included 20,183 ADHD cases and 35,191 controls, robustly implicated 12 independent genomic loci (15), including one containing the gene FOXP2, which has previously been implicated in severe speech and language problems (23). Given that none of the genome-wide significant loci contained any of the candidate genes previously implicated in ADHD (e.g., dopaminergic genes), this earlier literature is not discussed further. It is possible that evidence implicating some of these genes could emerge with larger GWAS samples, because effect sizes are small and ADHD GWAS remain underpowered, so it is too soon to dismiss these findings. However, it is important in the meantime to remain wary of previous candidate gene findings and studies that utilize them.
Sex differences.
Males are more commonly affected by ADHD, and this is a characteristic feature of child neurodevelopmental disorders. Previous studies based on siblings suggested that females with ADHD may carry a higher burden of ADHD genetic risk, and this would be a potential explanation for why males are more commonly affected (i.e., because females have to carry a greater burden of risk to manifest disorder). However, such findings do not seem to be explained by common genetic factors yielded by the latest GWAS (24).
ADHD overlap with autism.
GWAS data can also be used to investigate genetic correlations between two different disorders or traits. Puzzlingly, and in contrast to family and twin study findings, the international Psychiatric Genomics Consortium cross-disorder study published in 2013 (25) found no genetic overlap between ADHD and ASD. In contrast, in the as-yet unpublished and much larger Danish nationwide iPSYCH GWAS of ASD, the authors observed a significant genetic correlation of 0.36 between ADHD and ASD even when individuals with both disorders were excluded (26). The likely explanation is that the new iPSYCH GWAS is very much larger and thus better powered.
From the perspective of recent genetic discoveries, ADHD behaves as a typical neurodevelopmental disorder, with a higher burden of rare mutations compared with controls and with genetic overlap with ASD and intellectual disability. However, as discussed in the next section, genetic overlap is extensive among different neuropsychiatric disorders, and the genetic links of ADHD are not restricted to a neurodevelopmental grouping.
Genetic Overlap Between ADHD and Other Neuropsychiatric Disorders
One of the most striking findings to emerge from GWAS is the extensive genetic overlap for biomedical traits and disorders (27). ADHD GWAS findings are no exception; ADHD has shown significant positive genetic correlations with a wide range of neuropsychiatric disorders, including schizophrenia, bipolar disorder, Tourette’s syndrome, anxiety disorder, and major depressive disorder, and a significant negative genetic correlation with anorexia nervosa (15). Neuropsychiatric disorders in general show strong pleiotropy with each other but little overlap with neurological disorders (28). ADHD also shows genetic overlap with some physical conditions and traits (e.g., lung cancer, insomnia, migraine, body mass index) as well as some social and environmental phenotypes (e.g., educational attainment, smoking behaviors) (15). It is clear, therefore, that ADHD does not show genetic overlap with neurodevelopmental disorders alone and that risk factors (e.g., gene variant or environmental risk, such as smoking for lung cancer or heart disease) for complex disorders, including ADHD, do not necessarily provide a good means of defining diagnostic boundaries or meaningful groupings or making treatment decisions. For example, cigarette smoking is a risk factor for lung cancer, heart disease, and hypertension—diseases that we would not class as a single disease or treat in the same way. ADHD shows pleiotropy with schizophrenia, but it is not clinically indicated to treat schizophrenia with stimulant medication or atomoxetine.
These recent GWAS findings of pleiotropy are not enormously surprising given that recent family and twin studies have also observed familial and genetic overlaps between ADHD and later-onset neuropsychiatric problems, including schizophrenia, bipolar disorder (29), and major depressive disorder (30), as well as deliberate self-harm (31), completed suicide (31), and alcohol misuse (32). The observation of genetic overlap in itself is no longer a novel observation. Rather, we should be asking, What are the reasons that underlie these genetic correlations? For example, GWAS findings reveal that ADHD, typically an early-onset disorder, shows the strongest genetic correlation with major depressive disorder (rg=0.42). Is that because ADHD has an especially close biological relationship with depression? There is limited evidence to date to support this hypothesis. Does pleiotropy represent a causal risk effect of ADHD on major depression? Consistent with this providing a partial explanation, some studies suggest that treatment of ADHD reduces the risk of future depression (33). However, first-line ADHD treatments are not effective for major depression. Another hypothesis is that a proportion of individuals with recurrent major depression who are recruited into GWAS are misclassified and have undiagnosed ADHD or a history of ADHD. This requires investigation. These are all hypotheses that can be tested via epidemiological designs and novel genomic methods, among other designs, to disaggregate pleiotropy (34) (e.g., Mendelian randomization, a method that uses identified genetic variants to strengthen causal inference, because observational epidemiological designs are prone to various biases [35]). Another example of testing potential hypotheses relates to the observed genetic correlation between ADHD and lung cancer. That link is presumably mediated by cigarette smoking or other risk behaviors rather than a result of pleiotropy or of ADHD treatment. Shared biological processes seem unlikely, although they are not completely implausible (e.g., a broader predisposition to broader inflammatory processes). However, these alternative hypotheses require explicit testing.
Conceptualizing ADHD as a Trait and Category
Twin and Epidemiological Studies
From an epidemiological perspective, ADHD can be viewed as a trait as well as a category; higher scores are associated with adverse outcomes, with no discontinuity at a specific cut-point (8). Although a diagnostic category for ADHD is helpful in clinical practice because many clinical decisions are categorical in nature (e.g., using or not using medication), an understanding of its underlying structure can be informative for clinicians and patients as well as researchers.
Twin studies suggested many years ago that ADHD appears to lie at the end of a continuum of genetic risk, with the same genetic risks contributing across the ADHD continuum in the population (2), with the possible exception of those scoring at the extreme low end of the continuum (36). These findings, coupled with epidemiological studies, suggested that ADHD could be viewed as something akin to hypertension, where the disorder lies at the extreme of a continuously distributed phenotype (i.e., blood pressure) that is present in the whole population.
Common Gene Variants
GWAS findings have further added to this evidence. Two studies used an independent ADHD GWAS discovery data set to generate ADHD polygenic risk scores (PRS)—the relative burden of ADHD common gene variant risk alleles carried by an individual—in population cohorts in the United Kingdom (37) and the Netherlands (38). Both found that ADHD PRS predicted ADHD trait levels in the general population. A third U.K. study (39) further observed that when PRS were derived for an ADHD trait measure from a population-based cohort, they predicted ADHD diagnosis in an independent patient sample. The largest and most recent ADHD GWAS (15) was able to go further by testing the genetic correlation between ADHD diagnosis and a large GWAS meta-analysis of ADHD trait measures in 17,666 European individuals from the EAGLE consortium (40). The findings are striking in that the investigators observed a genetic correlation of 0.94 between ADHD diagnosis in patients and ADHD traits in the population-based cohorts. Overall genetic findings converge in showing that ADHD diagnosis lies at the extreme of a quantitative trait.
However, genetic risk for ADHD diagnosis is not exclusively related to a single continuously distributed trait measure of ADHD. For example, one population-based cohort study (41) found that ADHD genetic risk scores predicted multiple childhood neurodevelopmental traits, including social communication, cognitive ability or IQ, language, and working memory, although not emotion recognition. The findings on working memory have recently been replicated (42). Another study found that ADHD genetic risk scores also predicted childhood irritability (43). These findings could simply reflect pleiotropy once again, with the same genes contributing to biological mechanisms that in turn contribute to different childhood traits in the population. An alternative interpretation for the clinician and scientist is that a diagnosis of ADHD could be conceptualized as the extreme of multiple liabilities or dimensions. A dimensional view of ADHD that is underpinned by genetic liabilities that also contribute to cognitive characteristics would be in keeping with the thinking of the Research Domain Criteria project. This framework encourages us to view ADHD and other mental disorders as dimensions that span the range from normal to abnormal and encompass multiple levels of information (e.g., genes, circuits). If ADHD behaves as a dimension, then that has implications for how clinicians view individuals with subthreshold ADHD symptoms, which will be discussed later.
Development and Adult Life
ADHD Trajectories
Although ADHD symptom levels typically decline with age, a proportion of individuals show persistently elevated symptoms or a continued diagnosis into adolescence or adult life (8). Longitudinal twin studies (44) have all observed that ADHD persistence is associated with higher genetic loading; one study that used latent growth curve analysis to examine individual trajectories further suggested that ADHD trajectories through adolescence may be explained by genetic liabilities that are independent of those that contribute to baseline symptom levels in childhood (45). A recent population-based cohort investigation used ADHD GWAS findings to assess the contribution of ADHD common genetic variants to ADHD symptom trajectories between ages 4 and 17 (46). ADHD PRS were significantly higher in children in the persistent trajectory than in those in a low-symptom group (odds ratio=1.31, 95% CI=1.13–1.51), an intermediate group (odds ratio=1.22, 95% CI=1.01–1.47), and a childhood-limited ADHD group (odds ratio=1.27, 95% CI=1.05–1.53). Interestingly, although the schizophrenia GWAS discovery sample is very much larger and more powerful than the ADHD GWAS, schizophrenia PRS and other neuropsychiatric PRS did not predict ADHD symptom persistence. This highlights the fact that there is a degree of specificity to genetic findings.
Adult ADHD
Other genetic studies that have assessed adult ADHD in individuals over age 18 using cross-sectional designs also support the suggestion that persistent ADHD has higher genetic loading. For example, a recent Swedish registry-based family study observed a substantially higher relative risk of clinically recognized ADHD in siblings of those who had ADHD at age 18 or older (hazard ratio=11.49) than in siblings of those who had ADHD before age 18 (hazard ratio=4.68) (5).
However, twin studies of adult ADHD have repeatedly yielded very low heritability estimates, in contrast to the consistently high heritability estimates observed in child twin studies. The most likely explanation for this is the change in informant from parent to self- report in adult life, because self-reported ADHD symptoms have been found to show low heritability even in children (47). A Swedish registry study (48), however, found substantial heritability for ADHD across the life span, including in adult life (h2=0.72, 95% CI=0.56–0.84). Here, ADHD was defined on the basis of either an ICD diagnosis or prescribed ADHD medication. These findings suggest that with a clinician’s diagnosis, where perhaps detailed clinical assessments and impairment criteria are used rather than self-report questionnaire measures, the developmental differences in genetic architecture are not as pronounced, at least in terms of twin study findings.
Although there are international efforts to assemble large adult ADHD data sets, so far GWAS have yet to yield genome-wide significant loci, and rare variant studies (49, 50) will also require much larger sample sizes (51) before they can be used to further assess developmental differences in ADHD genetic architecture at a molecular level (see reference 52 for an extensive review of adult ADHD genetics).
Future Directions
Further Gene Discovery, Biology, and Environment
The 21st century has witnessed the discovery of ADHD-associated rare and common genetic variants and resolved that its genetic architecture involves a spectrum of genetic variation in terms of frequencies and effect sizes. These discoveries are already providing a starting point to gaining insights into the underlying biology of ADHD (e.g., 18, 53) and for identifying and assessing novel treatments. Some clues about treatment come from rare mutations—for example, in a recent preliminary open-label, single-blind, placebo-controlled trial of the metabotropic glutamate receptor activator fasoracetam in 30 adolescents carrying mGluR mutations (54). There is also growing interest in using genome-wide association data to highlight candidates for drug repositioning in psychiatry, but so far this has not been applied to ADHD (55). However, further work still will be needed to investigate the functional impacts of associated genetic variants at the level of molecules, cells, neural systems, and circuits, as well as their impacts on brain development, using bioinformatics as well as experimental designs. Another observation is that, so far, the identified common genetic variants explain only a relatively small fraction of the heritability of ADHD (h2=0.22) (15) that has been inferred from traditional twin study designs. This suggests that continued collaborative and larger ADHD genetic discovery studies will be required to detect additional genetic contributions, together with robust epidemiological designs to identify causal environmental risk factors. The so-called single-nucleotide polymorphism heritability will always be an underestimate of true heritability, as it only encompasses common genetic loci detected by GWAS that are not necessarily causal, and it does not include the contributions of rare mutations, environmental risk factors, gene-environment correlation, or interactions. Novel strategies may be needed to consider alternative plausible risk mechanisms that might manifest as heritability—for example, parentally provided early environments (56), including the prenatal environment. The literature of the past 5 years on ADHD genetics has shown the enormous advantage of genetically informative and genotyped nationwide patient registries, and these will remain an invaluable resource for investigating many of the questions raised in this review.
Implications of Genetic Overlap
Recent genetic discoveries highlight the strong overlap between ADHD, ASD, and intellectual disability. Indeed, in terms of rare mutations, thus far, it has not been possible to distinguish between ADHD and ASD (22), so the removal of the previous diagnostic exclusion criteria for ADHD in the presence of ASD is a welcome change in DSM-5. Yet the clinical features and treatments of these disorders are very different. What determines these differences is another crucial next research question (Figure 1). Typically these are early-onset disorders. One possible contribution to differences is variation in prenatal environmental exposures; another is background common genetic variants. For example, ADHD common genetic variant risk scores predict lower cognitive ability and educational attainment, whereas ASD common genetic variants predict higher cognition and educational attainment (57). Lessons from genetic discoveries on strong diagnostic overlaps are timely for clinicians and especially for service providers who tend to be diagnosis specific (e.g., ASD only), especially given the historical reticence about diagnosing ADHD in the context of ASD or intellectual disability. This is beginning to be recognized. For example, in 2017 in Wales, a nation of 3 million, multidisciplinary child neurodevelopmental clinics informed by recent findings have been established and have replaced ASD- and ADHD-specific assessment pathways.
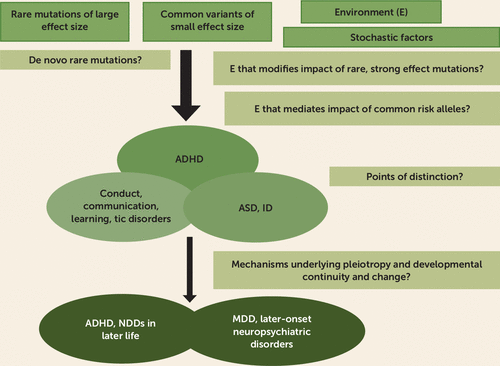
FIGURE 1. Neurodevelopmental Overlaps and Pleiotropy in ADHD: Questions for the Futurea
a ADHD=attention deficit hyperactivity disorder; ASD=autism spectrum disorder; ID=intellectual disability; MDD=major depressive disorder; NDD=neurodevelopmental disorder.
In the past 5 years, GWAS have shown genetic overlap across multiple disorders. ADHD shows extensive pleiotropy that is especially prominent with major depressive disorder, but genetic risks also show some specificity, as only ADHD PRS appear to predict ADHD persistence (46). In the next 5 years, efforts need to focus on disaggregating this pleiotropy and testing alternative hypotheses using methods that are able to distinguish causal relationships, different ADHD subtypes, and misclassification from pleiotropy (e.g., using Mendelian randomization and other similar methods). This is important because the different explanations will have diverse clinical implications (Figure 1).
The Developmental Continuum of ADHD
Genetic findings have confirmed the epidemiological view of ADHD as lying at the extreme of a continuum (or several continua) and have started to highlight the importance of adopting a developmental perspective (45, 46). Funders and ethics bodies need to recognize that developmental studies require longitudinal follow-up and that this is expensive and time consuming yet scientifically invaluable. In this regard, longitudinal population cohort designs are attractive for developmentally informative genetic studies because, unlike patient registry data, they involve trait-based assessments; however, nonrandom attrition is a problem, and individuals with severe illness (at the extremes of dimensions) are underrepresented. If ADHD is a trait, akin to blood pressure, then public or population health-based samples and approaches can be integrated with traditional genetics research on patients. This is beginning to happen. The conceptualization of ADHD as both a trait and a disorder is relevant to clinicians in that most recognize the somewhat arbitrary nature of the diagnostic cut-point and frequently encounter subthreshold cases. The presence of an underlying continuum means that issues such as defining the boundary for impairment and treatment are not straightforward. For example, the blood pressure cut-point for defining hypertension has changed over time, and hypertension guidelines in some countries adjust the cut-point for comorbid disease (e.g., chronic renal disease).
Genetic Testing
Given recent genetic discoveries, what are the implications for genetic testing in ADHD? The implications for clinicians are twofold. First, relatives of individuals with ADHD, including their parents, are at elevated risk of a range of neurodevelopmental disorders (e.g., ADHD, ASD, learning difficulties) as well as other neuropsychiatric illnesses (most commonly major depression) that could have an impact on assessment, treatment delivery, or treatment effectiveness. At present, although the rare mutations detected have large effect sizes, routine testing in ADHD is not recommended, although guidelines for such testing in clinical settings have now expanded in many countries to include mild intellectual disability and, in the United States, ASD. It was not long ago that mild intellectual disability was considered primarily sociocultural in origin, so this represents a substantial shift in international clinical practice. There is little empirical evidence to guide us as to what should happen for ADHD. An older study showed that the rate of established syndromes was sufficiently low in ADHD that genetic testing is not warranted in individuals without intellectual disability (58). However, that study predates current methods for detecting genomic anomalies, and a crucial issue is how severely neurodevelopmentally impaired the target patient group is, especially given that ADHD is relatively common. Whether genetic testing in clinical settings extends to neuropsychiatric disorders such as ADHD and schizophrenia remains to be seen, but as costs diminish and more knowledge about the causality of different mutations is gained, in my view, clinical practice is likely to change within the next decade—and sooner if some of these rare mutations are found to have treatment or prognostic implications or are medically actionable.
The clinical utility of common genetic risk variants for predicting ADHD is less certain, because their predictive power is weak and risk effects are defined in relation to a population rather than an individual. It is possible in the future that combining information on common genetic risk scores and family history (59) will be helpful for stratifying patients for the purpose of treatment or prognosis—for example, in identifying those who are at elevated risk of future psychosis or in guiding treatment. At present, however, these ideas remain in the domain of research questions that need to be answered. Although there is much commercial interest in pharmacogenomics, currently there is no scientific evidence to support such testing in ADHD. No common or rare gene variants have been found to be robustly associated with treatment response in ADHD, and very large studies will be required to detect genome-wide significant loci.
In summary, many novel genetic findings on ADHD have emerged and will continue to do so in the 21st century. Such discoveries are, in my view, of interest and relevance to a broad scientific field, including neuroscientists, developmental scientists, and population epidemiologists as well as clinicians, because they contribute to our conceptualization of ADHD and can shape the next 5 years of research and clinical practice.
1 : Who says this is a modern disorder? The early history of attention deficit hyperactivity disorder. World J Psychiatry 2015; 5:379–386Crossref, Medline, Google Scholar
2 : What have we learnt about the causes of ADHD? J Child Psychol Psychiatry 2013; 54:3–16Crossref, Medline, Google Scholar
3 : Psychiatric genomics: an update and an agenda. Am J Psychiatry 2018; 175:15–27Link, Google Scholar
4 : Molecular genetics of attention deficit hyperactivity disorder. Psychiatr Clin North Am 2010; 33:159–180Crossref, Medline, Google Scholar
5 : Familial aggregation of attention-deficit/hyperactivity disorder. J Child Psychol Psychiatry 2017; 58:231–239Crossref, Medline, Google Scholar
6 : A common genetic factor explains the covariation among ADHD ODD and CD symptoms in 9–10 year old boys and girls. J Abnorm Child Psychol 2009; 37:153–167Crossref, Medline, Google Scholar
7 : Neurodevelopmental disorders: cluster 2 of the proposed meta-structure for DSM-V and ICD-11. Psychol Med 2009; 39:2013–2023Crossref, Medline, Google Scholar
8 : Neurodevelopmental disorders. Lancet Psychiatry 2017; 4:339–346Crossref, Medline, Google Scholar
9 : The familial co-aggregation of ASD and ADHD: a register-based cohort study. Mol Psychiatry 2018; 23:257–262Crossref, Medline, Google Scholar
10 : Neurodevelopmentmental disorders, in Rutter’s Child Adolescent Psychiatry, 6th ed. Edited by Thapar A, Pine DS, Leckman JF, . Chichester, John Wiley & Sons, 2015, pp 31–40Crossref, Google Scholar
11 : Targeted sequencing identifies 91 neurodevelopmental-disorder risk genes with autism and developmental-disability biases. Nat Genet 2017; 49:515–526Crossref, Medline, Google Scholar
12 : Analysis of intellectual disability copy number variants for association with schizophrenia. JAMA Psychiatry 2016; 73:963–969Crossref, Medline, Google Scholar
13 : The familial co-aggregation of attention-deficit/hyperactivity disorder and intellectual disability: a register-based family study. J Am Acad Child Adolesc Psychiatry 2017; 56:167–174.e1Crossref, Medline, Google Scholar
14 : Rare chromosomal deletions and duplications in attention-deficit hyperactivity disorder: a genome-wide analysis. Lancet 2010; 376:1401–1408Crossref, Medline, Google Scholar
15 : Discovery of the first genome-wide significant risk loci for ADHD. bioRxiv Jun 3, 2017 (
16 : Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. Am J Psychiatry 2014; 171:627–639Link, Google Scholar
17 : Rare copy number variants in NRXN1 and CNTN6 increase risk for Tourette syndrome. Neuron 2017; 94:1101–1111.e7Crossref, Medline, Google Scholar
18 : Genome-wide copy number variation study associates metabotropic glutamate receptor gene networks with attention deficit hyperactivity disorder. Nat Genet 2011; 44:78–84Crossref, Medline, Google Scholar
19 : Genome-wide analysis of copy number variants in attention deficit hyperactivity disorder: the role of rare variants and duplications at 15q13.3. Am J Psychiatry 2012; 169:195–204Link, Google Scholar
20 : Rare copy number variation discovery and cross-disorder comparisons identify risk genes for ADHD. Sci Transl Med 2011; 3:95ra75Crossref, Medline, Google Scholar
21 : Genome-wide analysis of rare copy number variations reveals PARK2 as a candidate gene for attention-deficit/hyperactivity disorder. Mol Psychiatry 2014; 19:115–121Crossref, Medline, Google Scholar
22 : ASD and ADHD have a similar burden of rare protein-truncating variants. bioRxiv March 6, 2018 (
23 : A functional genetic link between distinct developmental language disorders. N Engl J Med 2008; 359:2337–2345Crossref, Medline, Google Scholar
24 : A genetic investigation of sex bias in the prevalence of attention-deficit/hyperactivity disorder. Biol Psychiatry 2018; 83:1044–1053Crossref, Medline, Google Scholar
25 : Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. Lancet 2013; 381:1371–1379Crossref, Medline, Google Scholar
26 : Common risk variants identified in autism spectrum disorder. bioRxiv Nov 27, 2017 (
27 : Detection and interpretation of shared genetic influences on 42 human traits. Nat Genet 2016; 48:709–717Crossref, Medline, Google Scholar
28 : Analysis of shared heritability in common disorders of the brain. bioRxiv April 16, 2016 (
29 : Risk of bipolar disorder and schizophrenia in relatives of people with attention-deficit hyperactivity disorder. Br J Psychiatry 2013; 203:103–106Crossref, Medline, Google Scholar
30 : Genetic and environmental influences on the relationship between ADHD symptoms and internalizing problems: a Chinese twin study. Am J Med Genet B Neuropsychiatr Genet 2016; 171:931–937Crossref, Medline, Google Scholar
31 : Common etiological factors of attention-deficit/hyperactivity disorder and suicidal behavior: a population-based study in Sweden. JAMA Psychiatry 2014; 71:958–964Crossref, Medline, Google Scholar
32 : Genetic and environmental influences on the relationship between adult ADHD symptoms and self-reported problem drinking in 6024 Dutch twins. Psychol Med 2014; 44:2673–2683Crossref, Medline, Google Scholar
33 : Medication for attention-deficit/hyperactivity disorder and risk for depression: a nationwide longitudinal cohort study. Biol Psychiatry 2016; 80:916–922Crossref, Medline, Google Scholar
34 : A plethora of pleiotropy across complex traits. Nat Genet 2016; 48:707–708Crossref, Medline, Google Scholar
35 : Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet 2014; 23(R1):R89–R98Crossref, Medline, Google Scholar
36 : The opposite end of the attention deficit hyperactivity disorder continuum: genetic and environmental aetiologies of extremely low ADHD traits. J Child Psychol Psychiatry 2016; 57:523–531Crossref, Medline, Google Scholar
37 : Genetic risk for attention-deficit/hyperactivity disorder contributes to neurodevelopmental traits in the general population. Biol Psychiatry 2014; 76:664–671Crossref, Medline, Google Scholar
38 : Attention-deficit/hyperactivity disorder polygenic risk scores predict attention problems in a population-based sample of children. J Am Acad Child Adolesc Psychiatry 2014; 53:1123–9.e6Crossref, Medline, Google Scholar
39 : Shared genetic influences between attention-deficit/hyperactivity disorder (ADHD) traits in children and clinical ADHD. J Am Acad Child Adolesc Psychiatry 2015; 54:322–327Crossref, Medline, Google Scholar
40 : A genome-wide association meta-analysis of attention-deficit/hyperactivity disorder symptoms in population-based pediatric cohorts. J Am Acad Child Adolesc Psychiatry 2016; 55:896–905.e6Crossref, Medline, Google Scholar
41 : Neurocognitive abilities in the general population and composite genetic risk scores for attention-deficit hyperactivity disorder. J Child Psychol Psychiatry 2015; 56:648–656Crossref, Medline, Google Scholar
42 : Working memory and vigilance as multivariate endophenotypes related to common genetic risk for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2018; 57:175–182Crossref, Medline, Google Scholar
43 : Investigating the genetic underpinnings of early-life irritability. Transl Psychiatry 2017; 7:e1241Crossref, Medline, Google Scholar
44 . Developmental twin study of attention problems: high heritabilities throughout development. JAMA Psychiatry 2013; 70:311–318Crossref, Medline, Google Scholar
45 : Genetic and environmental influences on the developmental course of attention-deficit/hyperactivity disorder symptoms from childhood to adolescence. JAMA Psychiatry 2015; 72:651–658Crossref, Medline, Google Scholar
46 : Association of genetic risk variants with attention-deficit/hyperactivity disorder trajectories in the general population. JAMA Psychiatry 2016; 73:1285–1292Crossref, Medline, Google Scholar
47 : Developmental twin study of attention problems: high heritabilities throughout development. JAMA Psychiatry 2013; 70:311–318Crossref, Medline, Google Scholar
48 : The heritability of clinically diagnosed attention deficit hyperactivity disorder across the lifespan. Psychol Med 2014; 44:2223–2229Crossref, Medline, Google Scholar
49 : Genome-wide copy number variation analysis in adult attention-deficit and hyperactivity disorder. J Psychiatr Res 2014; 49:60–67Crossref, Medline, Google Scholar
50 : Exome chip analyses in adult attention deficit hyperactivity disorder. Transl Psychiatry 2016; 6:e923Crossref, Medline, Google Scholar
51 : Case-control genome-wide association study of persistent attention-deficit hyperactivity disorder identifies FBXO33 as a novel susceptibility gene for the disorder. Neuropsychopharmacology 2015; 40:915–926Crossref, Medline, Google Scholar
52 : The genetics of attention deficit/hyperactivity disorder in adults: a review. Mol Psychiatry 2012; 17:960–987Crossref, Medline, Google Scholar
53 : Psychiatric gene discoveries shape evidence on ADHD’s biology. Mol Psychiatry 2016; 21:1202–1207Crossref, Medline, Google Scholar
54 : Fasoracetam in adolescents with ADHD and glutamatergic gene network variants disrupting mGluR neurotransmitter signaling. Nat Commun 2018; 9:4Crossref, Medline, Google Scholar
55 : Analysis of genome-wide association data highlights candidates for drug repositioning in psychiatry. Nat Neurosci 2017; 20:1342–1349Crossref, Medline, Google Scholar
56 : The nature of nurture: effects of parental genotypes. Science 2018; 359:424–428Crossref, Medline, Google Scholar
57 : Polygenic transmission disequilibrium confirms that common and rare variation act additively to create risk for autism spectrum disorders. Nat Genet 2017; 49:978–985Crossref, Medline, Google Scholar
58 : Cytogenetic abnormalities in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2002; 41:806–810Crossref, Medline, Google Scholar
59 : Projecting the performance of risk prediction based on polygenic analyses of genome-wide association studies. Nat Genet 2013; 45:400–405Crossref, Medline, Google Scholar



