A Geriatrics Perspective on Dementia Prevention and Treatment
With the aging of the population worldwide, the “silver tsunami” of a growing number of older adults with dementia predicted a few years ago is rapidly approaching (1). The health and financial cost burdens of dementia to individuals, families, and societies are daunting. Yet, as noted in a recent comprehensive Lancet review (2), the incidence of dementia in several developed countries is actually decreasing, possibly as a result of lifetime exposure to health and lifestyle factors that promote healthy cognitive aging. That we may already possess the means to prevent or delay onset of dementia is a promising development. Two articles in this issue of the Journal are consistent with such a hopeful note.
Bartels et al. (3) examined results from 755 non–currently depressed participants in the Alzheimer’s Disease Neuroimaging Initiative and found that for patients with mild cognitive impairment who had a history of depression, long-term treatment (more than 4 years) with a selective serotonin reuptake inhibitor (SSRI) was associated with a delay of approximately 3 years in progression to Alzheimer’s dementia. Comparison groups were individuals with a history of depression who received shorter-term SSRI treatment, those taking other antidepressants, those who received no treatment, and participants without a depression history.
The second article, by Rutherford et al. (4), represents an excellent review and synthesis of data linking age-related hearing loss with depression and dementia. Underlying mechanisms include both social-behavioral and neurobiological factors. For example, hearing loss may lead to social withdrawal, isolation, loneliness, and ultimately to cognitive decline and depression. From a neural standpoint, chronic hearing loss may lead to a compensatory increase in cognitive control network activation, dysfunctional auditory-limbic connectivity, and deafferentation-induced atrophy in frontal brain regions, all of which contribute to subsequent executive dysfunction and emotion dysregulation. The authors provide helpful figures modeling not only these psychosocial and biological mechanisms but also how the underlying pathophysiology may incorporate targets for different biologically informed treatments.
While research is ongoing for a long-hoped-for gene-based immunological treatment targeting pathological changes of Alzheimer’s disease, the process of bringing promising compounds to clinical use is a decades-long proposition. In the meantime, clinicians should find it encouraging that there are practical steps that can be taken to reduce the development of clinical symptoms and the accompanying functional impairment associated with dementia. In this vein, the perspective of geriatric medicine may be particularly instructive. Geriatricians are trained to adopt an approach to patient care that moves beyond focusing on a list of individual medical problems toward addressing broader health constructs such as cognition, mobility, mood, physical function, and sensory function, in which deficits can lead to geriatric syndromes such as immobility and cognitive and functional impairment (5). Geriatric physicians, whether their background is internal medicine, family medicine, psychiatry, or a subspecialty, recognize that multidisciplinary approaches that account for multiple comorbidities are vital in the overall care of older adults (6).
Conceptualizing cognitive impairment as a geriatric syndrome rather than a set of clinical or preclinical diagnoses broadens options for prevention and intervention. In contrast to a conventional medical approach comprising diagnostic precision and clinical trial–proven, evidenced-based treatment, the geriatric syndromal approach acknowledges that there are many medical, lifestyle, and aging-related factors that affect cognitive performance in older adults and can inform a comprehensive treatment plan.
Consistent with a geriatrics perspective, the two studies presented in this issue support the notion that evaluation and management of cognitive decline in older adults should include assessment and treatment of depression as well as screening for hearing impairment and appropriate referral if it is present. Yet mood and hearing are but two elements of a geriatric-informed strategy. As shown in Figure 1, several other components can be incorporated. The traditional medical approach begins with characterization of cognitive impairment to inform diagnosis and tailoring of pharmacological management, if appropriate. Beyond this focus on cognitive decline, other medical approaches include addressing cerebrovascular risk factors, as worsening cerebrovascular disease, especially white-matter disease, has been associated with cognitive decline and development of dementia (7, 8). Here, management of hypertension, diabetes, and atrial fibrillation may be particularly important. Similarly, screening for depression, diagnosing depressive disorder, and providing depression treatment are key steps that clinicians can take to prevent or delay cognitive decline and dementia (9).
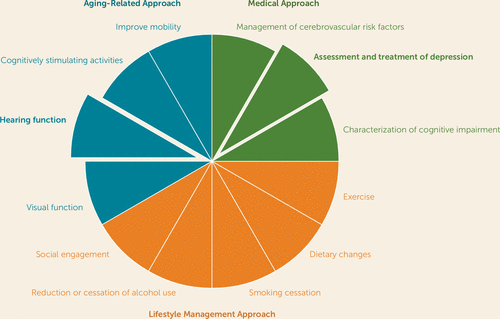
FIGURE 1. Management of Cognitive Impairment as a Geriatric Syndrome
Beyond a medical model, clinicians can incorporate health behavioral strategies to prevent and treat dementia (10). Active treatment of midlife obesity that may involve exercise and dietary modifications is a key component of prevention and management of cognitive decline (2). Exercise in later life is also important for healthy cognitive aging (11). Adhering to a Mediterranean diet with a low proinflammatory potential has been associated with decreased cognitive decline and dementia (12). Smoking cessation has been shown to prevent cognitive decline (9). Assessment of alcohol intake is also key, with moderation being the focus in midlife and cessation being important if cognitive impairment is present in later life (13). Finally, it is clear that social engagement is important to prevent cognitive decline. Mechanistically, engaging in social activities may prevent loneliness, which has been associated with cognitive decline (14). It may also prevent depression.
As indicated in Figure 1, it is not surprising that a focus on cognition as a geriatric syndrome would incorporate aging processes. Recognition that sensory changes occur with aging and that impairment in vision and hearing have a negative impact on cognition should inform screening for such changes among patients who are at risk for cognitive decline or are experiencing cognitive impairment (15). In addition, participation in cognitively stimulating leisure activities is associated with higher cognitive performance (16). Finally, attending to mobility issues is a significant component of the geriatric approach. Impairments in mobility and gait have been shown to worsen outcomes for a variety of disorders and are associated with cognitive decline (17).
In sum, conceptualizing cognitive decline as a geriatric syndrome has clear advantages in informing here-and-now prevention and active treatment efforts. Despite advances in the biological understanding of the major neurodegenerative disorders, these neuroscientific discoveries have not led to translational research that has a profound impact on patients struggling with dementia. Yet, Livingston et al. (2) calculated that about 35% of dementia is attributable to nine modifiable factors occurring across the lifespan: education to a maximum of age 11–12 years, midlife hypertension, midlife obesity, hearing loss, late-life depression, diabetes, physical inactivity, smoking, and social isolation. These factors fit in well with a geriatrics perspective, and the fact that they can be modified offers hope that we might be able to bend the curve of dementia burden until that happy day when we have a cure.
1 : Alzheimer’s disease: the silver tsunami of the 21st century. Neural Regen Res 2016; 11:693–697Crossref, Medline, Google Scholar
2 : Dementia intervention, prevention, and care. Lancet (Epub ahead of print, July 19, 2017)Google Scholar
3 : Impact of SSRI therapy on risk of conversion from mild cognitive impairment to Alzheimer’s dementia in individuals with previous depression. Am J Psychiatry 2018; 175:232–241Link, Google Scholar
4 : Sensation and psychiatry: linking age-related hearing loss to late-life depression and cognitive decline. Am J Psychiatry 2018; 175:215–224Link, Google Scholar
5 : Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc 2007; 55:780–791Crossref, Medline, Google Scholar
6
7 : Subcortical hyperintensities are associated with cognitive decline in patients with mild cognitive impairment. Stroke 2007; 38:2924–2930Crossref, Medline, Google Scholar
8 : Longitudinal magnetic resonance imaging vascular changes, apolipoprotein E genotype, and development of dementia in the neurocognitive outcomes of depression in the elderly study. Am J Geriatr Psychiatry 2007; 15:839–849Crossref, Medline, Google Scholar
9 : Potential for primary prevention of Alzheimer’s disease: an analysis of population-based data. Lancet Neurol 2014; 13:788–794Crossref, Medline, Google Scholar
10 : Strategies for dementia prevention: latest evidence and implications. Ther Adv Chronic Dis 2017; 8:121–136Crossref, Medline, Google Scholar
11 : Timing of physical activity, apolipoprotein E ε4 genotype, and risk of incident mild cognitive impairment. J Am Geriatr Soc 2016; 64:2479–2486Crossref, Medline, Google Scholar
12 : The association between an inflammatory diet and global cognitive function and incident dementia in older women: the Women’s Health Initiative Memory Study. Alzheimers Dement 2017; 13:1187–1196Crossref, Medline, Google Scholar
13 : Alcohol consumption and risk of dementia up to 27 years later in a large, population-based sample: the HUNT study, Norway. Eur J Epidemiol 2015; 30:1049–1056Crossref, Medline, Google Scholar
14 : Loneliness, depression, and cognitive function in older US adults. Int J Geriatr Psychiatry 2017; 32:564–573Crossref, Medline, Google Scholar
15 : The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. J Am Geriatr Soc 2000; 48:1697–1706Crossref, Medline, Google Scholar
16 : Association between mentally stimulating activities in late life and the outcome of incident mild cognitive impairment, with an analysis of the APOE ε4 genotype. JAMA Neurol 2017; 74:332–338Crossref, Medline, Google Scholar
17 : Slowing gait and risk for cognitive impairment: the hippocampus as a shared neural substrate. Neurology 2017; 89:336–342Crossref, Medline, Google Scholar



