Impact of SSRI Therapy on Risk of Conversion From Mild Cognitive Impairment to Alzheimer’s Dementia in Individuals With Previous Depression
Abstract
Objective:
Depression is associated with an increased risk of Alzheimer’s disease. Research has shown that the selective serotonin reuptake inhibitor (SSRI) citalopram decreases amyloid-β generation and plaque load. The authors evaluated the impact of SSRI treatment on CSF biomarkers and progression from mild cognitive impairment (MCI) to Alzheimer’s dementia.
Method:
Data sets from 755 currently nondepressed participants from the longitudinal Alzheimer’s Disease Neuroimaging Initiative were evaluated by Kaplan-Meier analysis and analyses of variance and covariance with ApoE4 status and age as covariates.
Results:
In MCI patients with a history of depression, long-term SSRI treatment (>4 years) was significantly associated with a delayed progression to Alzheimer’s dementia by approximately 3 years, compared with short-term SSRI treatment, treatment with other antidepressants, or no treatment and compared with MCI patients without a history of depression. No differences in CSF biomarker levels were observed between treatment groups.
Conclusions:
Long-term SSRI treatment may delay progression from MCI to Alzheimer’s dementia.
Mild cognitive impairment (MCI) is defined by impaired cognitive performance that does not meet the criteria for dementia (1). MCI bears a high risk of progression to Alzheimer’s dementia. Notably, an intervention that could delay this process by 5 years could result in a 57% reduction in Alzheimer’s dementia prevalence and health insurance costs (2). In this respect, the repurposing of approved medications has been recognized as a promising strategy; for example, antihypertensive drugs that act on the renin-angiotensin system have been shown to be associated with slower conversion from MCI to Alzheimer’s dementia (3, 4).
Late-life depression is associated with an increased risk of Alzheimer’s dementia (5–9). The underlying reasons are still a matter of debate, but possibilities include depression as a disease prodrome, an early symptom, a bona fide risk factor, or a confounder associated with comorbidities, such as vascular disease, that themselves predispose to Alzheimer’s dementia (10, 11).
Experiments in animal models have suggested that selective serotonin reuptake inhibitors (SSRIs) may reduce amyloid plaque burden and cognitive impairment, presumably by shifting the balance from pro- toward non-amyloidogenic processing of the amyloid precursor protein (APP) (12–14). In cognitively normal humans, long-term medication with the SSRI citalopram has been found to be associated with lower amyloid plaque load (12, 15), and acute treatment with citalopram was found to reduce the rate of newly generated amyloid-β (15).
Although these results suggest a favorable effect of SSRIs on Alzheimer’s disease pathology, evidence from clinical studies is less conclusive. Several randomized and placebo-controlled studies have evaluated the effect of SSRIs on cognition in Alzheimer’s dementia, finding either favorable effects (16, 17), no effects (18–20), or even disadvantageous effects of SSRI treatment (21, 22). With one exception (18), these studies covered only short time spans, ranging from 8 to 24 weeks. Whether treatment with an SSRI affects the progression from MCI to Alzheimer’s dementia is even less well studied, especially in prospective cohorts with longitudinal cognitive assessments. One retrospective analysis of cognitively healthy patients discharged with a diagnosis of depression (23) reported a lower rate of subsequent Alzheimer’s dementia in patients receiving first-generation antidepressants compared with no treatment, SSRIs, or serotonin-norepinephrine reuptake inhibitors. In contrast, another retrospective study (24) found that antidepressant treatment (SSRIs in 98% of the sample) was associated with delayed dementia onset and increased longevity in patients with Down’s syndrome, who have a high risk of Alzheimer’s dementia because of location of the APP gene on the triplicated chromosome 21.
Based on the promising effects of SSRIs in animal models and the lack of prospective and large clinical studies, we aimed to test the hypothesis that SSRI treatment may be associated with 1) a lower risk of Alzheimer’s dementia in patients with MCI and a history of depression, 2) a delayed progression of MCI to Alzheimer’s dementia, and 3) altered concentrations of the CSF biomarker amyloid-β42, as suggested by preclinical studies. To that end, we analyzed data from the multicenter, prospective, longitudinal Alzheimer’s Disease Neuroimaging Initiative (ADNI) cohort. This cohort has so far enrolled more than 1,500 participants, predominantly patients with amnestic MCI or Alzheimer’s dementia, as well as cognitively normal control subjects, all of whom undergo extensive neuropsychological assessments annually. The ADNI cohort is exceptional as it provides a unique data set of expertly diagnosed participants, including MRI data, genetic data, and data on CSF and blood biomarkers.
Method
ADNI Sample
Individuals with amnestic MCI or early Alzheimer’s dementia and age-matched cognitively normal subjects were recruited to ADNI-1, ADNI-2, and ADNI-GO from over 50 sites across the United States and Canada. All sites had approval by their respective institutional review boards, and written informed consent was obtained from all participants. Inclusion criteria relevant to our analysis were age between 55 and 90 and absence of major depressive disorder within the year prior to screening or current significant symptoms of depression (defined as a score ≥6 on the Geriatric Depression Scale). During the screening process, the exclusion criterion “major depressive disorder according to DSM-IV within the past year prior to screening” was controlled by reviewing the participant’s self-reported medical history for a previous or current diagnosis of depression. Participants were asked at screening for a lifetime history of psychiatric diseases, including symptoms or a diagnosis of depression and, if applicable, date of onset. (For more information on inclusion and exclusion criteria, see reference 25 and http://www.adni-info.org.)
Participants were categorized at baseline as cognitively normal control subjects, patients with MCI, and patients with Alzheimer’s dementia and were comprehensively reassessed every 6 months (in ADNI-1) or annually (in ADNI-2 and ADNI-GO) for progression from cognitively normal to MCI or Alzheimer’s dementia, or from MCI to Alzheimer’s dementia.
Assessments
Clinical and demographic variables.
Any clinically significant history of past and current health issues, including history of depression, was documented as qualitative and quantitative self-reported medical history data and pursued at follow-up.
Medications received within 3 months prior to screening, including antidepressants, were recorded retrospectively and prospectively via self-report, with dosage, frequency, and the approximate start and (if applicable) stop dates. Information on antidepressant dosage was not taken into account for our analysis, as doses between different antidepressants are difficult to compare and group sizes would have become too small if subgroups of high- and low-dose treatment were made within classes of antidepressants. All concomitant medications were entered into the medication log at every study visit. Duration of antidepressant intake (in days) was calculated from start to stop date, date of conversion, or end of observation.
The Geriatric Depression Scale (15-item short form; maximum score, 15) was used to assess depression symptoms; scores of 0–5 were considered normal and scores ≥6 were considered to indicate depression.
Among the cognitive parameters, scores on the Mini-Mental State Examination (MMSE) and the Rey Auditory Verbal Learning Test (sum of trials 1 to 5, and forgetting, indicative of verbal learning and long-term memory) were available for almost all participants in our study sample.
Apolipoprotein E (ApoE) genotyping.
ApoE genotyping was performed at screening as described in the ADNI study procedure manuals (http://adni.loni.usc.edu/methods/documents/).
CSF biomarker analysis.
Acquisition, processing, and storage of CSF in ADNI have been previously described (26; http://adni.loni.usc.edu/methods/documents/). Levels of amyloid-β1–42, total tau, and tau phosphorylated at threonine 181 (p-tau181) were determined using the xMAP Luminex platform (Luminex Corporation, Austin, Tex.) and Innogenetics/Fujirebio AlzBio3 immunoassay kits (Innogenetics, Ghent, Belgium). Baseline values of CSF amyloid-β1–42, total tau, p-tau181, and soluble APPβ (sAPPβ) (27) were downloaded from the ADNI data repository.
Study Sample and Group Design
ADNI data for the present study were downloaded from the ADNI data repository on July 15, 2013. From the 1,674 ADNI participants, we excluded those without baseline CSF biomarker results (N=832) or insufficient or inconsistent data on sociodemographic characteristics, cognition, or prior medication (N=2). The remaining participants were classified into groups with and without a history of depression, based on their medical history. Information about previous depression was obtained as part of the participants’ self-reported medical history. Participants and their informants or caretakers were asked by trained research assistants at screening for a lifetime history of psychiatric diseases, including depression, but not specifically about recurrent depressive episodes or numbers of episodes. With the exception of two participants with Geriatric Depression Scale scores of 6 and 7, respectively, only currently nondepressed participants with a score <6 were included in our analysis. Five participants with acute depressive symptoms (baseline Geriatric Depression Scale score ≥6) but no treatment were not included to the final analysis because of insufficient information on depression diagnosis in the database. From the final data set of 789 individuals, data for 46 participants were excluded because multiple antidepressant use or switch between antidepressant classes during the observation period prohibited clear assignment to treatment subgroups. A small number of participants (N=34) in the “no history of depression” group received antidepressant treatment (SSRIs or non-SSRIs) for indications other than depression, including anxiety (N=16), insomnia (N=10), dizziness (N=1), behavioral and psychological symptoms of dementia (N=2), irritability (N=1), agitation (N=1), pain (N=2), and low blood pressure (N=1). Because of small sample sizes, these subgroups were not considered for further analysis.
The “history of depression” group was subdivided based on antidepressant medication into three groups: patients without antidepressant treatment at baseline (prior depression–no antidepressants), patients who received SSRIs (prior depression–SSRI), and patients who received antidepressants with a different mode of action (prior depression–other antidepressants) (see Table S1 in the data supplement that accompanies the online edition of this article). Antidepressant medication had to be continued at least until baseline or beyond.
Thus, of the final 755 participants, 532 were allocated at baseline to the “no history of depression–no antidepressants” group and 223 to the “history of depression” group. Of the latter group, 60 were untreated (prior depression–no antidepressants), 116 had received SSRIs (prior depression–SSRI), and 47 had received antidepressants other than SSRIs (prior depression–other antidepressants) (Figure 1).
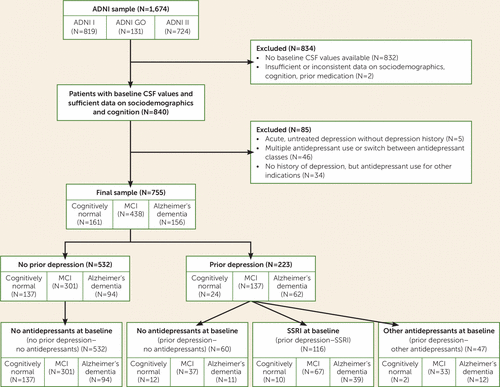
FIGURE 1. Patient Flow in a Study of the Impact of SSRI Therapy on Risk of Conversion From Mild Cognitive Impairment (MCI) to Alzheimer’s Dementia
Statistical Analysis
Baseline variables for the participants with MCI were compared across the different treatment groups using univariate analyses of variance (ANOVAs) for continuous variables and chi-square tests for categorical variables.
The relationship between previous depression and Alzheimer’s dementia or MCI at baseline was assessed by estimating odds ratios with 95% confidence intervals.
We performed Kaplan-Meier survival analysis to determine the impact of a history of depression and assignment to the different antidepressant treatment groups on time to dementia conversion in MCI. Changes in diagnosis from MCI to cognitively normal (N=14) or from Alzheimer’s dementia to MCI (N=1) were defined as nonconversions. Time to event is expressed as days from baseline to conversion to Alzheimer’s dementia or to the patient’s last study visit in the absence of progression (i.e., censored data).
In the MCI group, differences between treatment groups in CSF biomarker levels at baseline were compared using t tests, ANOVA, and analysis of covariance (ANCOVA). All statistical analyses were two-sided with the significance threshold set at 0.05 and were carried out using SPSS Statistics, version 21.0 (IBM, Armonk, N.Y.).
Results
Between-Group Comparisons of Baseline Characteristics
A total of 755 ADNI participants with CSF baseline data were included in this analysis. Participants who had no history of depression but who received antidepressant medication for indications other than depression were excluded (see Figure 1). This subsample was representative of the full ADNI sample with respect to gender, number of ApoE4 alleles, education, ethnicity, baseline MMSE score, and Rey Auditory Verbal Learning Test sum trials 1–5 and forgetting scores. Minor differences in mean age (74.1 years [SD=7.1] compared with 73.4 years [SD=7.4]; p=0.039) were considered clinically not relevant.
The mean follow-up period was 691 days (SD=646). The mean duration of antidepressant treatment was 2,256 days (SD=2,474, range=4–16,051), with 22.8% being treated for up to 1 year before baseline, 52.1% between 1 and 4 years, and 25.1% for more than 4 years. (Further information on type and duration of antidepressant treatment is provided in Table S1 in the online data supplement.) Baseline demographic, clinical, and cognitive characteristics for the participants with MCI, categorized into the different treatment groups, are summarized in Table 1.
| Prior Depression (N=137) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | No Prior Depression (N=301) | Total (N=137) | No Antidepressants (N=37) | SSRIs (N=67) | Other Antidepressants (N=33) | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | p | |
| Age (years) | 73.3 | 7.5 | 71.1 | 8.0 | 72.4 | 8.4 | 70.7 | 8.0 | 70.3 | 7.6 | 0.02 |
| Education (years) | 15.9 | 2.9 | 16.5 | 2.7 | 16.9 | 2.4 | 15.9 | 2.8 | 17.2 | 2.4 | 0.01 |
| Mini-Mental Status Examination score | 27.5 | 1.9 | 27.8 | 1.7 | 27.5 | 1.9 | 28.2 | 1.6 | 27.5 | 1.5 | 0.06 |
| Rey Auditory Verbal Learning Test | |||||||||||
| Sum trials 1–5 | 34.0 | 10.3 | 35.3 | 11.3 | 36.3 | 12.4 | 36.0 | 10.7 | 32.8 | 11.2 | 0.26 |
| Forgetting score | 4.1 | 2.6 | 4.3 | 2.6 | 4.1 | 2.4 | 4.6 | 2.7 | 3.8 | 2.7 | 0.39 |
| Geriatric Depression Scale score | 1.5 | 1.4 | 1.9 | 1.4 | 1.6 | 1.3 | 1.9 | 1.3 | 2.1 | 1.5 | 0.03 |
| N | % | N | % | N | % | N | % | N | % | p | |
| Geriatric Depression Scale classification | 0.14 | ||||||||||
| Normal | 301 | 100 | 136 | 99 | 37 | 100 | 66 | 98 | 33 | 100 | |
| Mild to moderate depression | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | |
| Severe depression | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Gender | 0.003 | ||||||||||
| Male | 201 | 67 | 68 | 50 | 18 | 49 | 30 | 45 | 20 | 61 | |
| Female | 100 | 33 | 69 | 50 | 19 | 51 | 37 | 55 | 13 | 39 | |
| ApoE statusb | 0.03 | ||||||||||
| No E4 allele | 150 | 50 | 63 | 46 | 17 | 46 | 37 | 56 | 09 | 27 | |
| 1 E4 allele | 119 | 40 | 54 | 40 | 15 | 40 | 24 | 36 | 15 | 46 | |
| 2 E4 alleles | 29 | 10 | 19 | 14 | 05 | 14 | 05 | 08 | 09 | 27 | |
| Ethnicity | 0.72 | ||||||||||
| Not Hispanic or Latino | 287 | 95 | 132 | 96 | 36 | 97 | 65 | 97 | 31 | 94 | |
| Hispanic or Latino | 13 | 4 | 4 | 3 | 1 | 3 | 1 | 2 | 2 | 6 | |
| Unknown | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 0 | |
| Race | 0.92 | ||||||||||
| White | 280 | 93 | 135 | 98 | 36 | 97 | 66 | 98 | 33 | 100 | |
| Black | 6 | 2 | 2 | 2 | 1 | 3 | 1 | 2 | 0 | 0 | |
| Asian | 8 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Other or unknown | 7 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
TABLE 1. Baseline Characteristics of Participants With Mild Cognitive Impairment (MCI) (N=438) in a Study of the Impact of SSRI Therapy on Risk of Conversion From MCI to Alzheimer’s Dementiaa
Baseline Diagnosis of MCI or Alzheimer’s Dementia and History of Depression
As shown in Table 2, a baseline diagnosis of MCI was associated with a 2.60-fold higher likelihood of a history of depression compared with the cognitively normal group, and a baseline diagnosis of Alzheimer’s dementia was associated with a 3.77-fold higher likelihood.
| Prior Depression | No Prior Depression | ||||||
|---|---|---|---|---|---|---|---|
| Baseline Diagnosis | N | % | N | % | Odds Ratio | 95% CI | p |
| Cognitively normal (N=161) | 24 | 15 | 137 | 85 | |||
| MCI (N=438) | 137 | 31 | 301 | 69 | 2.60 | 1.61–4.20 | <0.001 |
| Alzheimer’s dementia (N=156) | 62 | 40 | 94 | 60 | 3.77 | 2.20–6.46 | <0.001 |
TABLE 2. History of Depression as a Risk Factor for Baseline Diagnosis of Mild Cognitive Impairment (MCI) or Alzheimer’s Dementia
History of Depression and Rate of MCI Conversion to Alzheimer’s Dementia
A history of depression was not associated with an elevated rate of conversion of MCI to Alzheimer’s dementia. Survival analysis in the MCI group revealed comparable cumulative probabilities for progression to Alzheimer’s dementia in patients with MCI and a history of depression compared with patients with MCI and no previous depression (log-rank χ2=0.77, p=0.38; Δ mean survival time=−172 days) (Figure 2A,D).
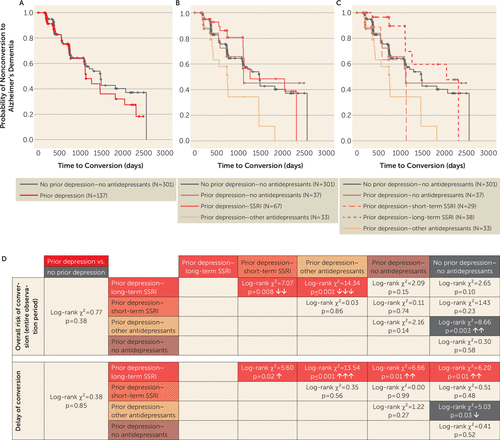
FIGURE 2. Kaplan-Meier Survival Analyses for Conversion of Mild Cognitive Impairment (MCI) to Alzheimer’s Dementia in Patients With and Without Histories of Depression and Antidepressant Treatmenta
a In panel A, the depression and no-depression groups show comparable probabilities for MCI progression to Alzheimer’s dementia. SSRI treatment (panel B) and long-term SSRI treatment (panel C) are advantageous compared with treatment with other antidepressants in MCI patients with a history of depression. In panel C, in the first 3 years of observation, the probability of progression is lower with long-term SSRI treatment (prior depression–long-term SSRI) compared with all other groups. Treatment with other antidepressants also results in unfavorable progression rates compared with the no depression group. Panel D presents a statistical summary; arrows indicate decreased conversion probabilities (upper panel) and increased time interval to conversion (lower panel) for the prior depression–long-term SSRI group compared with other groups.
History of Depression, Long-Term SSRI Treatment, and MCI Progression to Alzheimer’s Dementia
To address the impact of different antidepressant classes on the associated risk of progression from MCI to Alzheimer’s dementia, we performed survival analysis in individuals with MCI, separated into treatment subgroups of prior depression–SSRI, prior depression–other antidepressants, and prior depression–no antidepressants. The cumulative probability of progression was lower in the SSRI-treated subgroup compared with the other-antidepressants subgroup (log-rank χ2=9.16, p=0.002; Δ mean survival time=691 days) (Figure 2B). The prior depression–other antidepressants group was also associated with higher conversion rates compared with the no prior depression–no antidepressants group (log-rank χ2=8.66, p=0.003; Δ mean survival time=−654 days). No significant differences were observed between any of the other groups.
We next investigated whether the duration of SSRI treatment would be associated with different conversion rates from MCI to Alzheimer’s dementia. To this end, we divided the prior depression–SSRI group by median split into short-term (≤1,610 days) and long-term (>1,610 days) treatment groups (Figure 2C).
The probability of MCI progression to Alzheimer’s dementia was lower in patients with a history of depression and long-term SSRI treatment compared with treatment with other antidepressants (log-rank χ2=14.34, p≤0.001; Δ mean survival time=891 days) and short-term SSRI treatment (log-rank χ2=7.07, p=0.008; Δ mean survival time=884 days). All other group comparisons did not show significant differences (Figure 2D), except for the higher conversion rates of the prior depression–other antidepressants group in comparison to the no prior depression–no antidepressants group (log-rank χ2=8.66, p=0.003; Δ mean survival time=−654 days).
Kaplan-Meier analysis restricted to the first 3 years of the observation period showed a significantly decreased probability of conversion to Alzheimer’s dementia in MCI patients with a history of depression and long-term SSRI treatment compared with all other groups (prior depression–long-term SSRI versus prior depression–short-term SSRI: log-rank χ2=5.60, p=0.02; Δ mean survival time=188 days; prior depression–other antidepressants: log-rank χ2=13.54, p≤0.001; Δ mean survival time=343 days; prior depression–no antidepressants: log-rank χ2=6.66, p=0.01; Δ mean survival time=177 days; no prior depression–no antidepressants: log-rank χ2=6.20, p=0.01; Δ mean survival time=162 days) (Figure 2C,D). The risk of conversion was increased in MCI patients with a history of depression and other antidepressant treatment compared with the no prior depression–no antidepressants group (log-rank χ2=5.03, p=0.03; Δ mean survival time=−181 days). All other group comparisons revealed no significant differences.
At later time points, i.e., after more than 3 years of observation, the advantage associated with long-term SSRI treatment in previously depressed participants dissolved and the probability of subsequent Alzheimer’s dementia became similar to that of all other groups (data not shown). The cutoff of 3 years was determined by the least difference in estimates of cumulative survival in the observation period for the no prior depression–no antidepressants and prior depression–long-term SSRI groups and resulted in an interval of 1,095 to 1,106 days (i.e., approximately 3 years).
Correction for ApoE4 status as well as restriction to MCI patients with pathological baseline CSF amyloid-β1–42 levels yielded similar results in all survival analyses (data not shown).
SSRI Treatment and CSF Amyloid-β1–42 Levels in MCI Patients
We next analyzed whether, in the MCI group, a previous depression might be associated with lower CSF amyloid-β1–42 or higher tau, p-tau181, and sAPPβ concentrations, reflecting increased disease pathology. However, CSF biomarker levels did not differ between those with and without a history of depression in the MCI group (N=438) (see Figure S1A–D, left panels, in the online data supplement).
If SSRI treatment conferred its beneficial effect by inhibiting amyloid-β1–42 generation, as has been proposed (12, 15), CSF levels of amyloidogenic APP cleavage products, such as soluble APP sAPP-β (28) and CSF amyloid-β1–42, should be lower in the SSRI treatment group. To unravel a potential effect of SSRI treatment on CSF amyloid-β1–42, we divided the MCI group into the antidepressant treatment subgroups prior depression–SSRI, prior depression–other antidepressants, and prior depression–no antidepressants. One-way ANOVA with Bonferroni-adjusted post hoc tests revealed no main effects of treatment on CSF amyloid-β1–42, tau, p-tau181, or sAPP-β levels (see Figure S1A–D, right panels, in the data supplement), or any significant between–treatment group differences in the MCI group (left panels).
From all potential covariates that differed significantly at baseline (Table 1) and fulfilled statistical requirements to enter ANCOVA, number of ApoE4 alleles had the highest impact on CSF amyloid-β1–42 levels (F=32.24, p≤0.001; partial eta2=0.13, i.e., moderate to large effect), followed by age (F=42.57, p≤0.001; partial eta2=0.09, i.e., moderate to large effect). Correction for these covariates by ANCOVA uncovered no significant main effect of different antidepressants or no treatment on CSF amyloid-β1–42 concentrations in participants with MCI (F=1.78, p=0.32; partial eta2=0.008).
Additionally, MCI participants were categorized into amyloid-β1–42 positive (CSF baseline amyloid-β1–42 <192 pg/mL) and negative (amyloid-β1–42 ≥192 pg/mL) subgroups. The distribution of patients into these groups did not differ significantly between MCI patients with and without a history of depression or between different antidepressant treatment regimens (see Figure S2 in the data supplement). Likewise, ANOVA with Bonferroni-adjusted post hoc tests revealed no main effect of depression or antidepressant treatment status (see Figure S3 in the data supplement).
Discussion
In this study of the ADNI cohort we found, in accord with previous findings, that MCI and Alzheimer’s dementia are associated with a history of depression. Notably, we found a delay of approximately 3 years in MCI progression to Alzheimer’s dementia in patients with a previous depression who received long-term SSRI treatment. In contrast, treatment with non-SSRI antidepressants was associated with a higher risk of conversion of MCI to Alzheimer’s dementia and a shorter interval to progression compared with the long-term SSRI group. CSF levels of amyloid-β1–42, tau, and p-tau were unaffected by history of prior depression and antidepressant treatment.
Similar to previous findings (29), we did not observe a higher rate of conversion of MCI to Alzheimer’s dementia in participants with a history of depression compared with those without a history of depression. This is in contrast to another prospective cohort (30) in which a higher rate of MCI progression to Alzheimer’s dementia was found to be associated with depression. In contrast to our study, participants in that cohort were acutely depressed at baseline, which may have influenced the outcome.
Strengths and Limitations
ADNI is a multicenter, prospective, longitudinal cohort with highly standardized, research-based definitions of MCI and Alzheimer’s dementia. Extensive and frequent follow-ups of cognitive state allow the timely detection of deterioration from MCI to Alzheimer’s dementia, in contrast to previous retrospective studies on antidepressants and dementia risk, which relied on patient registry data. The large number of individual data sets that also included CSF biomarker results further improves the quality of our study. Another strength of the ADNI cohort is the exclusion of patients with major depressive symptoms at baseline, since these may interfere with cognitive performance and result in false positive MCI or dementia diagnoses. Notably, we only included data sets of patients without multiple antidepressant use or switch between antidepressant classes during the observation period.
To our knowledge, this is the first study to investigate the relationship between a history of depression and antidepressant treatment with the overall probability and time interval of MCI conversion to Alzheimer’s dementia.
Based on the available information, it was not possible to distinguish early-onset depression with recurrent episodes in later years from late-life-onset depression. Distinct neurobiological mechanisms may underlie these two depression phenotypes, and they may be associated with different risks of subsequent Alzheimer’s dementia. Thus, it must be acknowledged that longer antidepressant exposure may reflect early-onset depression, whereas shorter treatment times may indicate late-onset depression, possibly associated with prodromal Alzheimer’s disease. Consequently, it may not be long-term exposure to SSRIs itself that delays progression to Alzheimer’s dementia but rather the underlying depression phenotype. In that case, one would expect that long-term treatment with other, non-SSRI antidepressants would show a rate of MCI progression to Alzheimer’s dementia comparable to that of the long-term SSRI group. However, in our ADNI data set, long-term SSRI treatment was superior to long-term treatment with other antidepressants. This suggests that SSRI treatment itself, rather than differences in the underlying pathology of depression subtypes, may be causative for the observed protective effects.
It must be taken into account, too, that the patients in our sample were not randomly assigned to antidepressant treatment conditions and that our findings may be confounded by initial treatment response. Thus, we cannot exclude the possibility that individuals in the non-SSRI group represent a more treatment-resistant patient population compared with the SSRI treatment group.
A recent study (7) demonstrated that a history of recurrent depressive episodes was strongly associated with a cumulatively increasing risk of subsequent Alzheimer’s dementia. Other studies have found that the severity of depression at baseline was correlated with risk of developing dementia (31). No information was available on time point, treatment regimen, antidepressant treatment, dosage, and treatment response of past depressive episodes, or on their frequency, severity, or duration. The impact of these factors could therefore not be analyzed and may bear bias against positive findings in this study. Also, because of the small numbers of participants receiving antidepressant treatment for indications other than depression, we could not determine whether SSRI treatment effects are limited to patients with a history of depression. As participants were depression-free at baseline (as defined by Geriatric Depression Scale score), a clinical assessment of depression and evaluation of the depression diagnosis was not possible. Another limitation of our study is that we had to rely on self-reported data on depression diagnosis and antidepressant use, and these data did not cover the period prior to the starting date of current antidepressant medication taken at baseline. Thus, we cannot exclude the possibility that patients in the non-SSRI group received SSRIs during previous depressive episodes or that patients in the SSRI group previously received non-SSRI antidepressants. Of note, changes in antidepressant treatment during the observation period were monitored, and patients whose treatment changed were excluded from the analysis, as were patients on multiple antidepressants.
SSRI Treatment Effects
Previous studies suggest that SSRIs enhance the α-secretase-dependent processing of APP, thus lowering amyloidogenic APP cleavage and amyloid-β1–42 production. Inhibition of amyloid-β1–42 production was also observed on treatment of astrocytes with fluoxetine in vitro, paralleled by decreased astrocytic activation (32). However, we detected no differences in CSF amyloid-β1–42 levels between the antidepressant treatment groups in our study. Lower production rates of CSF amyloid-β1–42 have been observed in healthy volunteers after acute administration of citalopram (15), although another study obtained conflicting results (33). In a trial with the α-secretase activator acitretin (34), no changes in steady-state CSF amyloid-β1–42 concentrations were observed, although the α-secretase cleavage product soluble APP-α (sAPP-α) was increased in CSF. Since CSF sAPP-α values were unavailable from ADNI, we analyzed CSF sAPP-β, which should decrease upon activation of a non-amyloidogenic cleavage pathway. However, no between–treatment group differences in sAPP-β levels were detected. These results could support an additional and potentially α-secretase-independent mechanism of continuous SSRI treatment, e.g., by modulation of neuroinflammation (35, 36), acetylcholine release (37, 38), or neurodegeneration or neurogenesis (39), although it must be taken into account that changes in α-secretase activity are not always paralleled by effects on β-secretase (BACE) cleavage products (40).
A growing body of evidence points toward a complex role of neuroinflammation in amyloidogenesis, neurodegeneration, and cognitive decline (41). Neuroinflammation has also been detected in patients during major depressive episodes (42, 43). SSRIs can modulate key inflammatory factors, such as tumor necrosis factor alpha (36), interleukins-1β and -6, and reactive oxygen stress. Interestingly, in a mouse model, SSRIs were found to ameliorate experimental allergic encephalitis–induced symptoms (44, 45). SSRI-mediated modulation of neuroinflammation might therefore explain the favorable outcomes of patients under long-term SSRI treatment.
Additionally, increased synaptic serotonin concentrations under SSRI treatment may activate 5-HT4 receptors. 5-HT4 receptor stimulation has been shown to increase acetylcholine release and to counteract the cognitive impairment induced by anticholinergic drugs (46, 47). Thus, SSRI treatment may counteract effects of pathologically decreased acetylcholine in Alzheimer’s disease.
Serotonergic effects are not restricted to SSRIs but are a common feature of most antidepressants. It is therefore unclear why SSRI treatment but not treatment with other antidepressants is associated with a delay of MCI progression to Alzheimer’s dementia. Recent findings suggest that SSRIs, in contrast to other antidepressants, may up-regulate serum brain-derived neurotrophic factor (BDNF) (48). Compelling evidence demonstrates that neurogenesis is impaired in depression, a phenomenon likely mediated by a decrease of BDNF and vascular endothelial growth factor (VEGF) (49). Decreased levels of BDNF have also been described in Alzheimer’s disease and, conversely, high levels of brain BDNF have been found to be associated with lower rates of cognitive decline (50).
On a more speculative basis, SSRIs may also ameliorate endothelial dysfunction, which may be relevant to small-vessel disease, a relevant cause of vascular dementia and an important comorbidity factor in Alzheimer’s dementia and old-age depression (51). Finally, although all groups in our study were sufficiently treated with antidepressants and scored below 6 on the Geriatric Depression Scale, we cannot exclude the possibility that SSRIs improve selected symptoms of depression better than other antidepressants, e.g., effects on sleep disturbances, appetite, or nutrition, which are associated with a higher risk of dementia (52).
Despite the lack of changes in CSF amyloid-β1–42 levels in the SSRI group, there is compelling evidence that SSRI treatment reduces amyloid plaque burden in vivo (12, 15). Unfortunately, given the small numbers of individuals with available baseline amyloid positron emission tomography (PET) data in our data set (N=7), we were not able to correlate amyloid plaque load with different antidepressant treatments. Clearly, a prospective study with longitudinal amyloid PET imaging and CSF sampling is needed to investigate SSRI-mediated changes in amyloid metabolism.
Conclusions
Pending validation in an intervention trial, the data produced in this study may have important implications for clinical practice. They suggest that long-term SSRI maintenance treatment may be beneficial in elderly patients with MCI and a history of depression even after affective symptoms have resolved, as this was associated with a 3-year delay in conversion of MCI to Alzheimer’s dementia. A prospective study to confirm SSRI effects on MCI progression is now warranted, as an SSRI-mediated delay may contribute to an overall lower prevalence of Alzheimer’s dementia, with a major impact on affected individuals, caregivers, public health, and health costs.
1 : Mild cognitive impairment: beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med 2004; 256:240–246Crossref, Medline, Google Scholar
2 : Changing the Trajectory of Alzheimer’s Disease: How a Treatment by 2025 Saves Lives and Dollars. Washington, DC, Alzheimer’s Association, 2015 (http://www.alz.org/alzheimers_disease_trajectory.asp)Google Scholar
3 : Modulation of renin-angiotensin system may slow conversion from mild cognitive impairment to Alzheimer’s disease. J Am Geriatr Soc 2015; 63:1749–1756Crossref, Medline, Google Scholar
4 : Antihypertensive drugs decrease risk of Alzheimer disease: Ginkgo Evaluation of Memory study. Neurology 2013; 81:896–903Crossref, Medline, Google Scholar
5 : Midlife vs late-life depressive symptoms and risk of dementia: differential effects for Alzheimer disease and vascular dementia. Arch Gen Psychiatry 2012; 69:493–498Crossref, Medline, Google Scholar
6 : Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry 2013; 202:329–335Crossref, Medline, Google Scholar
7 : Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology 2010; 75:27–34Crossref, Medline, Google Scholar
8 : Depressive symptoms increase the risk of progression to dementia in subjects with mild cognitive impairment: systematic review and meta-analysis. Int J Geriatr Psychiatry 2016; 31:905–911Crossref, Medline, Google Scholar
9 : Depressive symptoms and risk of dementia: the Framingham Heart Study. Neurology 2010; 75:35–41Crossref, Medline, Google Scholar
10 : Clinical-pathologic study of depressive symptoms and cognitive decline in old age. Neurology 2014; 83:702–709Crossref, Medline, Google Scholar
11 : Vascular risk and Aβ interact to reduce cortical thickness in AD vulnerable brain regions. Neurology 2014; 83:40–47Crossref, Medline, Google Scholar
12 : Serotonin signaling is associated with lower amyloid-β levels and plaques in transgenic mice and humans. Proc Natl Acad Sci USA 2011; 108:14968–14973Crossref, Medline, Google Scholar
13 : 5-HT(4) receptor agonist mediated enhancement of cognitive function in vivo and amyloid precursor protein processing in vitro: a pharmacodynamic and pharmacokinetic assessment. Neuropharmacology 2011; 61:69–79Crossref, Medline, Google Scholar
14 : Redundant Gs-coupled serotonin receptors regulate amyloid-β metabolism in vivo. Mol Neurodegener 2016; 11:45Crossref, Medline, Google Scholar
15 : An antidepressant decreases CSF Aβ production in healthy individuals and in transgenic AD mice. Sci Transl Med 2014; 6:236re4Crossref, Medline, Google Scholar
16 : Does fluoxetine have any effect on the cognition of patients with mild cognitive impairment? A double-blind, placebo-controlled clinical trial. J Clin Psychopharmacol 2007; 27:67–70Crossref, Medline, Google Scholar
17 : Comparison of sertraline, venlafaxine, and desipramine effects on depression, cognition, and the daily living activities in Alzheimer patients. Pharmacopsychiatry 2014; 47:131–140Crossref, Medline, Google Scholar
18 : Multicenter, randomized, placebo-controlled, double-blind clinical trial of escitalopram on the progression-delaying effects in Alzheimer’s disease. Int J Geriatr Psychiatry 2016; 31:731–739Crossref, Medline, Google Scholar
19 : Cognitive outcomes after sertaline treatment in patients with depression of Alzheimer disease. Am J Geriatr Psychiatry 2012; 20:1036–1044Crossref, Medline, Google Scholar
20 : Treating depression in Alzheimer disease: efficacy and safety of sertraline therapy, and the benefits of depression reduction: the DIADS. Arch Gen Psychiatry 2003; 60:737–746Crossref, Medline, Google Scholar
21 : Sertraline or mirtazapine for depression in dementia (HTA-SADD): a randomised, multicentre, double-blind, placebo-controlled trial. Lancet 2011; 378:403–411Crossref, Medline, Google Scholar
22 : Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA 2014; 311:682–691Crossref, Medline, Google Scholar
23 : Do continued antidepressants protect against dementia in patients with severe depressive disorder? Int Clin Psychopharmacol 2011; 26:316–322Crossref, Medline, Google Scholar
24 : Effects of antidepressants on longevity and dementia onset among adults with Down syndrome: a retrospective study. J Clin Psychiatry 2014; 75:731–737Crossref, Medline, Google Scholar
25 : Ways toward an early diagnosis in Alzheimer’s disease: the Alzheimer’s Disease Neuroimaging Initiative (ADNI). Alzheimers Dement 2005; 1:55–66Crossref, Medline, Google Scholar
26 : Cerebrospinal fluid biomarker signature in Alzheimer’s disease neuroimaging initiative subjects. Ann Neurol 2009; 65:403–413Crossref, Medline, Google Scholar
27 : Decrease in brain soluble amyloid precursor protein β (sAPPβ) in Alzheimer’s disease cortex. J Neurosci Res 2011; 89:822–832Crossref, Medline, Google Scholar
28 : CNS amyloid-β, soluble APP-α and -β kinetics during BACE inhibition. J Neurosci 2014; 34:8336–8346Crossref, Medline, Google Scholar
29 : Neuropsychiatric predictors of progression from amnestic-mild cognitive impairment to Alzheimer’s disease: the role of depression and apathy. J Alzheimers Dis 2010; 20:175–183Crossref, Medline, Google Scholar
30 : Depression in patients with mild cognitive impairment increases the risk of developing dementia of Alzheimer type: a prospective cohort study. Arch Neurol 2004; 61:1290–1293Crossref, Medline, Google Scholar
31 : Depressive symptoms, cognitive decline, and risk of AD in older persons. Neurology 2002; 59:364–370Crossref, Medline, Google Scholar
32 : Regulation of astrocyte pathology by fluoxetine prevents the deterioration of Alzheimer phenotypes in an APP/PS1 mouse model. Glia 2016; 64:240–254Crossref, Medline, Google Scholar
33 : Comment on “An antidepressant decreases CSF Aβ production in healthy individuals and in transgenic AD mice”. Sci Transl Med 2014; 6:268le5Crossref, Medline, Google Scholar
34 : Increased CSF APPs-α levels in patients with Alzheimer disease treated with acitretin. Neurology 2014; 83:1930–1935Crossref, Medline, Google Scholar
35 : Fluoxetine and S-citalopram inhibit M1 activation and promote M2 activation of microglia in vitro. Neuroscience 2015; 294:60–68Crossref, Medline, Google Scholar
36 : A critical review of the mechanism of action for the selective serotonin reuptake inhibitors: do these drugs possess anti-inflammatory properties and how relevant is this in the treatment of depression? Neuropharmacology 2013; 67:304–317Crossref, Medline, Google Scholar
37 : Identification of multiple 5-HT4 partial agonist clinical candidates for the treatment of Alzheimer’s disease. J Med Chem 2012; 55:9240–9254Crossref, Medline, Google Scholar
38 : VRX-03011, a novel 5-HT4 agonist, enhances memory and hippocampal acetylcholine efflux. Neuropharmacology 2007; 53:563–573Crossref, Medline, Google Scholar
39 : Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol Psychiatry 2011; 16:738–750Crossref, Medline, Google Scholar
40 : Constitutive α- and β-secretase cleavages of the amyloid precursor protein are partially coupled in neurons, but not in frequently used cell lines. Neurobiol Dis 2013; 49:137–147Crossref, Medline, Google Scholar
41 : Inflammation in Alzheimer disease: a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med 2012; 2:a006346Crossref, Medline, Google Scholar
42 : Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 2015; 72:268–275Crossref, Medline, Google Scholar
43 : Altered expression of genes involved in inflammation and apoptosis in frontal cortex in major depression. Mol Psychiatry 2011; 16:751–762Crossref, Medline, Google Scholar
44 : The immunomodulatory effect of the antidepressant sertraline in an experimental autoimmune encephalomyelitis mouse model of multiple sclerosis. Neuroimmunomodulation 2011; 18:117–122Crossref, Medline, Google Scholar
45 : The antidepressant venlafaxine ameliorates murine experimental autoimmune encephalomyelitis by suppression of pro-inflammatory cytokines. Int J Neuropsychopharmacol 2009; 12:525–536Crossref, Medline, Google Scholar
46 : Stereoselective effects of (R)- and (S)-zacopride on cognitive performance in a spatial navigation task in rats. Neuropharmacology 1996; 35:321–327Crossref, Medline, Google Scholar
47 : Role of 5-HT4 receptors in the mouse passive avoidance test. J Pharmacol Exp Ther 1998; 286:1115–1121Medline, Google Scholar
48 : Serum levels of brain-derived neurotrophic factor in major depressive disorder: state-trait issues, clinical features, and pharmacological treatment. Mol Psychiatry 2011; 16:1088–1095Crossref, Medline, Google Scholar
49 : Novel therapeutic approaches beyond the serotonin receptor. Biol Psychiatry 1998; 44:324–335Crossref, Medline, Google Scholar
50 : Higher brain BDNF gene expression is associated with slower cognitive decline in older adults. Neurology 2016; 86:735–741Crossref, Medline, Google Scholar
51 : Chronic selective serotonin reuptake inhibition modulates endothelial dysfunction and oxidative state in rat chronic mild stress model of depression. Am J Physiol Regul Integr Comp Physiol 2015; 309:R814–R823Crossref, Medline, Google Scholar
52 : Sleep, cognitive impairment, and Alzheimer’s disease: a systematic review and meta-analysis. Sleep 2017; 40:zsw032Google Scholar



