Developmental Delay, Treatment-Resistant Psychosis, and Early-Onset Dementia in a Man With 22q11 Deletion Syndrome and Huntington’s Disease
This case provides an example of genetic conditions associated with “copy number variation,” where portions of the genome are either excessively repeated as “copies” (i.e., duplications) or where normal “copies” are absent (i.e., deletions). The early-life developmental abnormalities in this patient were associated with what?
A trinucleotide repeat on the huntingtin gene.
Chromosome 21 trisomy.
A deletion on chromosome 22.
Multiple deletions on chromosome 4.
“Mr. E,” a 41-year-old man, participated in a precision medicine study of highly treatment-resistant schizophrenia. Study procedures were ethically approved, Mr. E’s legal guardian provided written informed consent, and the protocol allowed for return of results and subject recontact.
Table 1 lists milestones for Mr. E’s life events, development, and course of illness. Mr. E was born at term as a breech presentation, with hernia, cleft palate, and anteverted auricles. He was delayed in some developmental milestones, and he needed extra support in school. At age 21, he developed psychotic symptoms, and he resided in a psychiatric hospital or in a supported living facility nearly continuously for the next 20 years.
The initial psychiatric presentation at age 21 included elaborate delusion of strangers surrounding his family home threatening to do harm. At one point, the police were called when he was striking relatives and destroying furniture. He experienced persistent auditory hallucinations of multiple distinct voices making derogatory comments. He was often observed talking to himself and laughing inappropriately. Given data available for the initial year of illness (ages 21–22), Mr. E met DSM-5 criteria for schizophrenia: he had continuously present delusions, hallucinations, thought disorder, and bizarre behavior; his functioning was markedly impaired; he had continuous illness for >6 months; he had no clinically significant manic or depressive episodes; he had no significant drug use or (at the time) any known potentially causal medical condition; and his psychotic symptoms were new and prominent despite a history of developmental delay.
Mr. E’s clinical response to conventional antipsychotics was inadequate because of persistent positive symptoms, so he received clozapine early in his illness (at age 21). Despite medication adherence, Mr. E’s response to clozapine was inadequate, and he was transferred to a state psychiatric hospital (ages 22–27). Augmentation of clozapine with risperidone led to an improved clinical response, indicated by discharge to a supervised community living program (ages 27–29). During this period, Mr. E was warmly sociable, consistently denied auditory hallucinations and delusions, usually made good eye contact, evidenced a sense of humor, was active in therapeutic groups, followed professional sports, and enjoyed his work in a sheltered workshop. Mr. E was considering vocational education or community college, and this was judged reasonable by his treatment team.
At age 29, Mr. E’s medications were abruptly discontinued, leading to marked deterioration, with aggression, paranoid delusions, and hallucinations. Despite resumption of a previously effective medication regimen, Mr. E required a lengthy inpatient stay before his clinical improvement was sufficient to allow him to be discharged back to the supervised community living program, although he never returned to his prior baseline. Beginning in his early 30s, Mr. E experienced cognitive decline, which was unequivocal by age 35. In the 5 years before he was examined by our group, his condition had deteriorated to the point where he had lost all capacity for meaningful communication and sat silently in a chair for hours, occasionally swinging his arms and grunting.
Given this unusual presentation and profound cognitive decline, we requested evaluation by a behavioral neurologist (M.L.).
Past Medical History
Mr. E’s past medical history included myopia, asthma, chronic obstructive pulmonary disease, resolved obesity, obstructive sleep apnea, idiopathic thrombocytopenia, hypocalcemia, psoriasis, seborrheic dermatitis, reflux esophagitis, dyslipidemia, kyphoscoliosis, and constipation. Surgeries included two herniorrhaphies, cleft palate correction, and reduction of protuberant ears.
Family History
The patient’s psychiatric and neurological family history was unclear, as Mr. E was adopted when he was 6 months old.
Investigations
Brain MRI performed when Mr. E was in his mid-20s was reported as normal. Another at age 40 was confounded by movement artifact but was interpreted to show general volume loss without lobar predominance, as well as left lens dislocation. Laboratory investigations were notable for thrombocytopenia and hypocalcemia.
Neurological Examination
Functional status.
Mr. E’s caretakers had noted a marked decline in cognition and function beginning when he was in his mid-30s, to the point where he required continual one-to-one assistance. He insidiously became nonverbal except for occasional grunting. He became disengaged from external stimuli, sat in a chair for hours, and did not spontaneously initiate any purposeful activities. He appeared unable to recognize family. He was unable to use utensils and ate with his hands. He needed assistance in dressing and bathing, and he was incontinent of stool and urine.
Physical examination.
The patient’s height was 173 cm and his weight 71.7 kg (body mass index 24.0). He had mild scoliosis and long, thin hands and feet. Facial features included a long, tubular nose with bulbous tip, narrow palpebral fissures, and anteverted auricles with extra cartilage. He was edentulous (as a result of dental caries), and he had a small mouth with a crowded oral cavity. He had dermatitis and dry skin on his cheeks and forehead. The examination was otherwise unremarkable.
Mental status examination.
The patient was mostly awake, but he dozed briefly several times during the interview and examination. His engagement with the environment was limited. He was nonverbal and did not respond to commands or to his name, but he did orient to voice. He did not appear to be responding to visual or auditory hallucinations.
Neurological examination.
Mr. E’s right pupil was round and reactive to light. The left cornea was opacified, with an unreactive pupil, and he did not blink to threat on the left. He did not pursue moving objects in his visual field. There was no gaze deviation and no obvious facial asymmetry or ptosis. He showed very brief orienting responses to loud noises.
A hyperkinetic movement disorder was evidenced by multiple components: oro-lingual-buccal dyskinesia; nearly continuous right upper extremity choreoathetosis mingled with repetitive semipurposeful grabbing movements; and posturing of the left upper extremity. Hyperkinetic movements ceased when he was asleep. There were no adventitious movements of the lower extremities. Muscle tone was increased in the right upper extremity (likely because it was constantly in motion). Tone was normal in the other extremities. The patient did not participate in strength testing, but there was no obvious focal weakness. Plantar responses were flexor, and deep tendon reflexes were unremarkable. The patient withdrew to touch in all extremities. He was unable to stand independently from a seated position. His gait was wide-based and ataxic, with a choreiform component.
Genetic Analyses
A peripheral venous blood sample was collected, and genomic DNA was extracted using standard methods. Assays included genotyping (Illumina InfiniumOmniExpressExome-8 array, version 1.3) and whole-genome sequencing (Illumina HiSeq X Ten, PCR-free library preparation, 150 bp paired-end reads, 30× coverage). All genetic assays were done according to the manufacturer’s protocols. Sequence alignment and variant calling were performed using the GenomeAnalysis Toolkit. Copy number variation in the SNP array data was analyzed using PennCNV (http://penncnv.openbioinformatics.org/).
Using the SNP array data, we identified a large one-copy deletion on chromosome 22 from 18.66 to 21.70 Mb (hg19) that was also present in the whole genome sequencing data. This finding was consistent with 22q11 deletion syndrome, and its presence was confirmed with array comparative genome hybridization and fluorescent in situ hybridization on an independent blood sample in a laboratory certified in accordance with the Clinical Laboratory Improvement Amendments (CLIA).
Because the neurological examination showed profound early-onset dementia with choreoathetoid movements, we carefully examined the sequence data aligning to exon 1 of the huntingtin gene (HTT). There was a large “insertion” adjacent to the HTT exon 1 CAG repeat known to be causal for Huntington’s disease, suggesting misalignment of sequencing reads due to the presence of many CAG repeats over that in the hg19 genome reference. A triplet-primed polymerase chain reaction assay followed by fragment analysis in a CLIA-certified laboratory revealed a 48-copy CAG allele (pathogenic range ≥40 copies). An HTT CAG repeat of this size is “full penetrance” for Huntington’s disease.
Death and Pathological Examination
Mr. E died about 3 weeks after the neurological examination. He appeared to staff to be in his usual state of health, but he died suddenly during routine daily care. An autopsy was performed by a county medical examiner, and the cause of death was recorded as lobar pneumonia. There was no report of a large embolism in an artery to the lung, heart, or brain.
Mr. E’s brain was removed, sectioned, fixed in formalin, and sent to clinical neuropathologists for evaluation (Figure 1). Gross inspection of the brain revealed mild leptomeningeal fibrosis and mild cortical atrophy in the parietal region with focal thinning of the corpus callosum. There were no obvious abnormalities of the gyral pattern and no gray matter heterotopias. The deep gray nuclei showed mild flattening of the head of the caudate nucleus and atrophy and discoloration of the globus pallidus. Mild dilation of the ventricular system was also seen. The putamen, amygdala, left hippocampus, posterior thalamus, and cerebellum were normal. The brainstem was not available for evaluation. On histological examination, moderate chronic neurodegenerative changes were seen in the basal ganglia, which were most marked in the caudate nucleus. Small, round ubiquitin-immunoreactive neuronal intranuclear inclusions were present in the basal ganglia and neocortex, consistent with abnormal accumulation of expanded huntingtin protein. Tau, TDP-43, and alpha-synuclein immunohistochemistry were unremarkable. Thorough evaluation of multiple tissue blocks for developmental abnormalities revealed only subtle changes of uncertain significance, including mildly ectatic vessels in the cerebral and cerebellar white matter, focal thinning of the cerebellar granular cell layer, and focal periventricular nodular ependymitis.
The neuropathological diagnoses were Huntington’s disease and minor vascular and structural abnormalities of uncertain significance.
| Age | Life Event | Clinical Notes |
|---|---|---|
| 0 | Birth | Breech birth, hernia, cleft palate, protuberant ears |
| 3 months | Surgery | Two herniorrhaphies |
| 6 months | Adopted | |
| 4 years | Delayed milestones | Preschool staff did not view him as ready for kindergarten compared with peers |
| Surgery | Surgery to correct cleft palate | |
| Grades K–5 | Elementary school | Learning disability; “warm, sociable” |
| Grades 6–8 | Middle school | Special education classes. IQ measured at 80, dyslexia, auditory processing problems. Surgery to correct protuberant ears |
| 14–19 years | High school | Graduated 2 years behind grade level; angry, possible substance misuse |
| 20 years | College | Brief college attendance. Few friends, abused by peers, possible substance misuse |
| 21 years | Psychotic episodes resulting in two admissions to community psychiatric hospital (14 days each) and day treatment | Delusions of strangers surrounding house with intent to do physical harm, auditory hallucinations, hitting mother, throwing objects. GAF score, 20–25. Medications: perphenazine, lithium |
| 21–22 years | Three inpatient admissions in university psychiatry unit for psychosis (1 month each) | Intense paranoia, auditory hallucinations, aggressive threats, suicidal ideation. GAF score, 10–20. Brain MRI was unremarkable. Thrombocytopenia. Medications: perphenazine, olanzapine, clozapine |
| 22–27 years | Long-term state inpatient hospitalization | Admission GAF score, 20, due to severe paranoia and auditory hallucinations. Poor initial response to clozapine, but good response with adequate dose and duration. Obesity. No extrapyramidal symptoms and normal gait noted on annual medical/neurological examination. Thrombocytopenia. Medications: clozapine, risperidone, fluoxetine |
| 27–29 years | 24-hour supervised community living program | Few delusions or auditory hallucinations; the patient was actively involved in discharge processing; vocational training recommended as part of therapy program. GAF score, ∼60. Medications: clozapine, ziprasidone |
| 29 years | Acute psychotic episode, medication abruptly stopped; 7-month inpatient admission | Extensive bruising, fetal position, incontinent, incoherent, randomly swinging fists. Thrombocytopenia. Medications: clozapine |
| 29–41 years | 24-hour supervised community living program | Initially relatively functional; able to do most self-care, worked in sheltered environment, participated in group outings, could play video games. Decline in function beginning in early 30s. By his late 30s, marked cognitive impairment, impaired gait, incontinence, incoherence, randomly swinging fists, language limited to grunting, required 24-hour one-to-one care. GAF score, 10–20. Loss of speech prominent at age 37. Thrombocytopenia (workup unrevealing). Medications: clozapine, lorazepam (for agitation), p.r.n. temazepam, melatonin (for sleep) |
| 37 years | Psychiatric consultation (second opinion) | Prompted by decline in function compared with early 30s: decline in activities of daily living (had done most self-care independently, e.g., personal hygiene, making meals); worsening performance in sheltered work environment; lessened interest in activities that had previously been of interest. Staff had initiated plan to try to correct lessened verbal output (e.g., from lengthy responses to queries to very brief responses). Examination: halting/listing gait; inconsistent responses to questions; bizarre behavior (e.g., standing in center of room in “almost catatonic state”). Occasionally incontinent of stool and urine. Brain MRI: poor quality due to motion, read as unremarkable. Thrombocytopenia, hypocalcemia |
| 39 years | Admission to inpatient neurology unit at a major academic center | Cognitive decline now severe. No expressive language, minimal or no comprehension. Evaluated for frontotemporal dementia. Brain MRI without contrast: poor quality due to motion, and terminated prematurely because the patient was unable to cooperate, but read as no gross intracranial mass or disproportionate atrophy. No movement disorder noted. Discharged back to community living program |
| 41 years | Enrolled in precision medicine study | Review of all records, genetic testing, examination by behavioral neurologist |
| 41 years | Death | Autopsy (cause of death, lobar pneumonia), neuropathology |
TABLE 1. Life Course Chart for the Patienta
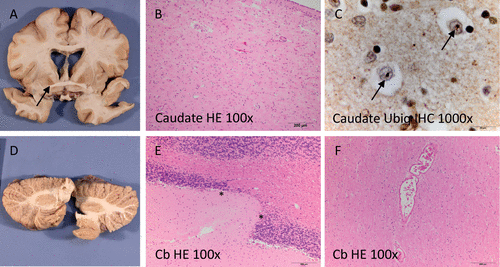
FIGURE 1. Postmortem Neuropathological Findingsa
a The basal ganglia showed mild atrophy of the caudate nucleus and brown discoloration of the globus pallidus (panel A). On microscopy, mild chronic neurodegenerative changes (panel B) and ubiquitin-immunoreactive intranuclear inclusions (arrows in panel C) were observed. The cerebellum was subtly globular in shape (panel D) and showed focal thinning of the cerebellar cortical granular cell layer (between asterisks in panel E). Ectatic vessels as shown in panel F were seen focally in the deep white matter. Cb=cerebellum; HE=hematoxylin and eosin; Ubiq=ubiquitin; IHC=immunohistochemistry.
Schizophrenia usually emerges in late adolescence or early adulthood (1, 2). If cognitive deterioration occurs, it generally appears early and with relatively stable impairment over the next 5–10 years. Later in the illness, psychotic symptoms may become less intense, and there can be modest improvement in function later in life (3–5). Mr. E’s early development was abnormal and showed multiple indications of a genetic syndrome such as 22q11 deletion syndrome (22q11DS) (e.g., cleft palate, dysmorphic features, and developmental delay). Mr. E’s initial psychiatric presentation was consistent with idiopathic schizophrenia, but treatment resistance was notable. He subsequently developed profound dementia with a hyperkinetic movement disorder. Genetic analysis, clinical evaluation, and neuropathology provided definite diagnoses of 22q11 deletion syndrome and Huntington’s disease.
To our knowledge, this is the only reported co-occurrence of 22q11DS and Huntington’s disease. The prevalence of the 22q11DS is around 1 in 4,000 live births (6–9), and a pathogenic trinucleotide expansion in HTT occurs in approximately 1 in 10,000 live births (10). These genetic variants are on different chromosomes, and we are not aware of a mutational mechanism that could predispose to both events. If these were independent events, the probability of co-occurrence would be around 1/40 million, or fewer than 10 similar cases in the United States. The co-occurrence of multiple genetic syndromes (referred to as “blended” phenotypes) has been reported in ∼4% of individuals referred for clinical genetic testing (11, 12), particularly in people like Mr. E, with multisystem disease.
22q11DS is caused by a hemizygous deletion (i.e., the presence of one instead of two copies) of a region on chromosome 22 (13). The deleted region is usually ∼3 million bases (chr22:18.6–21.7 Mb, hg19), contains over 50 genes, and is a de novo event in ∼90% of cases (7, 14). 22q11DS has been observed worldwide because the fine structure of this region increases the chances of its occurrence (the deleted region is flanked by low copy repeat regions that predispose to nonallelic homologous recombination). The clinical presentation of 22q11DS is variable and can include developmental delay, intellectual disability, cardiac anomalies, palatal defects, immunodeficiency, thrombocytopenia, and hypocalcemia (15). The neuropsychiatric concomitants of 22q11DS are diverse. It is the strongest known genetic risk factor for schizophrenia (with a prevalence of 0.3% in cases, and a genotypic relative risk >20) (16, 17). While 25%−30% of individuals with 22q11DS will develop psychosis, it is also associated with autism, attention deficit hyperactivity disorder, pervasive developmental delay, and early-onset Parkinson’s disease (18).
Huntington’s disease is a dominantly inherited neurodegenerative disorder (19). Most cases (∼90%) are inherited (20). It is caused by a mutation in the first exon of the huntingtin gene (HTT), where three DNA bases (cytosine-adenine-guanine, or CAG) are repeated multiple times (21, 22). The number of CAG repeats is normally in the range of 11–35, but if there are ≥40 CAG repeats, Huntington’s disease virtually always occurs, as the resulting protein becomes selectively toxic to neurons in the caudate, putamen, and deep layers of frontal and parietal cortex (23, 24). Key manifestations of Huntington’s disease include chorea, cognitive decline or dementia, and psychiatric symptoms (25). Although choreiform movements are its diagnostic hallmark, cognitive and psychiatric manifestations often predate motor signs (26, 27). Although mood symptoms are common, psychotic symptoms occur in 6%−25% of cases of Huntington’s disease (25, 28–30).
With the benefit of hindsight and a genotype-driven review of all available medical records, it is apparent that Mr. E manifested multiple physical signs (e.g., hernia, cleft palate, facial dysmorphism, thrombocytopenia, and hypocalcemia), developmental signs (developmental delay, lowered IQ), and psychosis, collectively strongly suggestive of 22q11DS (31). Mr. E subsequently experienced an early-onset progressive dementia due to Huntington’s disease. The onset of cognitive decline in Huntington’s disease usually occurs in the mid-40s, but it can be earlier in individuals with longer CAG repeats (32), as was the case with Mr. E. Mr. E had dyskinetic as well as choreiform movements in the context of prolonged antipsychotic use, which may be why Huntington’s disease was not suspected earlier. Mr. E’s chronic psychotic symptoms during most of his third decade support a diagnosis of schizophrenia. Informed clinicians disagree as to whether the presence of 22q11DS is sufficient to negate DSM-5’s criterion E (“not attributable to … another medical condition”), as it is a probabilistic but not deterministic risk factor. In either case, Huntington’s disease led to a profound neurocognitive disorder, which had an overwhelming impact during Mr. E’s last decade of life.
We speculate that Mr. E was affected both independently and interactively by this extremely rare combination of genetic insults. Mr. E’s abnormal development and predisposition to schizophrenia can be reasonably attributed to 22q11DS. The early onset and rapidly progressive dementia were certainly due to pathologically confirmed Huntington’s disease. These two features of Mr. E’s clinical course were probably independent, given their distinctive ages at onset. We speculate that these two genetic conditions may have interacted, particularly later in his illness. Brain regions jointly affected by Huntington’s disease, schizophrenia, and antipsychotic treatment converge on a limited set of neuronal cell types (e.g., medium spiny neurons of the ventral striatum and cortical pyramidal neurons) (23). This could have been most evident in Mr. E’s atypical chorea, where an overt movement disorder may have been masked. The combination of a hypokinetic movement disorder (22q11DS increases the risk of early-onset Parkinson’s disease, associated with overactivity in the indirect striato-pallido-thalamo-cortical pathway, which predisposes to hypokinesia) (33) and a hyperkinetic movement disorder (due to Huntington’s disease, associated with underactivity of this pathway) as well as the extrapyramidal side effects of antipsychotic treatment may have resulted in the observed atypical motoric presentation.
Perspective From Mr. E’s Mother
I lost my son to mental illness. According to staff members who were with him, he awoke that morning and was being dressed when he collapsed to the floor. First responders who arrived at the facility were unable to rouse him, and on the way to the hospital there were no measurable vital signs. He was formally pronounced dead on arrival.
But that was not the first time I lost him. That had happened two decades earlier when he came home from his first year at college and experienced a psychotic break. He had auditory hallucinations, was talking, laughing, and shouting at imaginary people, constantly rocking back and forth, and had a deeply held belief that strangers were stalking our home with the intent of doing extreme harm to everyone. The voices in his head were unbearable to him. When he starting destroying furniture in his room, the police were called and they took him for the first of many psychiatric admissions. I had no frame of reference for what was happening, but clearly his life was unraveling and none of us were ever going to be the same.
After nearly a decade of inpatient treatment, including several years in a large state psychiatric hospital with clozapine treatment, his psychotic symptoms became less intense and it seemed that there was a modest improvement in his condition. I began to hope that this diminished quality of his life was not how he would live out his days.
Then the trajectory of his illness took a devastating turn. It was as if his personhood began to be slowly erased. Although he was alive, there seemed to be nothing inside him. His eyes were vacant and he no longer recognized his name or any of his family. He sat in a chair swatting at the air with his right arm and grunting. It was not like my son was trapped within the haze of schizophrenia—rather, it seemed like he was literally not there anymore.
I have always been one who believes that there is something sacred in every human life. The Quakers call it “the light that is within.” But there was no light left inside my son—a living body without a living person. We tried every test known in modern psychiatry, but there was no explanation. My son’s treatment team and I reached out to the best academic minds, and yet when he died, there were no answers.
Many mornings after he died I would lie in bed a few extra minutes, trying to conjure up specific images of my son. He was once a boy, gentle and kind, playing with his friends, knees stained green and grass clippings in his hair. But even in those mornings, the haunt of unknowing was always present.
When I learned about the genomic findings of 22q11DS and Huntington’s disease, my deepest feelings were sadness and palpable relief. Finally I knew what had gone wrong. There was nothing more I could have done.
I have now been able to let my son go, knowing that although his life ended too soon, in this tragedy he has made a vibrant contribution to science that will live into the future. And for that, I am deeply grateful.
Recommendations and Conclusions
Given that the diagnostic yield of genetic testing for copy number variants (like 22q11DS) in people with schizophrenia is on the order of 2%−3% (34), we advocate broader and even routine testing. Many psychiatrists evaluate people with a new-onset psychotic disorder with tests to exclude rare causes of psychosis (e.g., brain imaging, endocrine, metabolic, viral exposure, and autoimmune studies), although the diagnostic yield is considerably lower than evaluating genomic structural variants. Psychiatrists need not become experts in genetic testing but should have sufficient familiarity with and working knowledge of medical genetics to identify patients with signs and symptoms suggestive of a genetic condition that would benefit from referral. Psychiatrists can work closely with specialists in medical genetics to identify which patients should be tested, what clinical circumstances warrant closer evaluation, how to interpret results and incorporate them into clinical care, and how to convey important results to patients in a way that augments and informs the clinical process.
Certain clinical features raise the index of suspicion for the presence of a cryptic copy number variant in people with schizophrenia. Many copy number variants associated with schizophrenia also increase the risk for childhood psychiatric disorders, including intellectual disability, autism spectrum disorder, attention deficit hyperactivity disorder, and pervasive developmental delay. Because these copy number variants change the dosages of many genes in every cell, clinical features can include multisystem abnormalities. For example, as a group, copy number variants linked to schizophrenia are also associated with craniofacial dysmorphisms (e.g., cleft palate or atypical facial appearance), macro- or microcephaly, neurological disorders (e.g., epilepsy), congenital heart disease, immune and hematological dysfunction (e.g., thrombocytopenia and thymic aplasia), and renal disease. However, these additional somatic features can be subtle or absent, underscoring the importance of having a low threshold for screening for copy number variants.
In many health care systems, a clinician can order a “postnatal whole genome copy number variation by array comparative genomic hybridization.” A peripheral venous blood sample is the source of DNA for this test. The test can take several weeks. If abnormalities are found, the report will list the nature and significance of the finding and usually point to additional resources for the clinician and patient. Referral to clinical genetics may be indicated to ensure optimal care. Many copy number variants associated with schizophrenia have medical comorbidities. For instance, clinical management of people with 22q11DS requires care across medical specialties (15). In Mr. E’s case, there was extensive clinical investigation of long-standing thrombocytopenia (deemed “idiopathic” in life), and his hypocalcemia was untreated.
Identifying copy number variants may become therapeutically important. Multiple academic groups and companies are pursuing development of therapies for people with psychiatrically important structural variants. Although there are no approved medications, the situation could change in the next decade, and knowing which patients have a large structural variant could prove important therapeutically. This can also be important to families, to connect them to support networks that are available for many rare genetic syndromes. Knowledge of recurrence risk can be important for reproductive planning.
As genomic evaluation becomes more common clinically, phenotypes associated with “classic” genetic disorders will likely expand, and additional features may be recognized as part of a syndrome (including neuropsychiatric conditions). This may be particularly important for people with long-standing psychiatric disorders who develop new signs and symptoms (e.g., dementia and a movement disorder, as with Mr. E). Although individually rare, dozens of single gene disorders can initially present with a clinical portrait confusable with idiopathic psychosis (35), and many are detected only when new neurological or medical symptoms develop.
This is by no means a unique recommendation, but a strong case can be made for the rigorous and structured reevaluation of complex cases like Mr. E’s. In routine clinical care, important therapeutic and diagnostic decisions are often constrained by a lack of data. Systematic reassessment of medical records, psychiatric and medical reexamination, consideration of a broader differential diagnosis, and diagnostic testing can lead to new etiological insights.
In summary, this case report illustrates the value of genetic testing in psychiatry. What we call schizophrenia is a complex disorder caused by a heterogeneous disease process. The diagnosis and treatment of some proportion of people with schizophrenia will be influenced by the presence of rare, mechanistically potent variation.
C. A deletion on chromosome 22.
Want more? A CME course is available in the APA Learning Center at education.psychiatry.org
1 : Schizophrenia. N Engl J Med 2003; 349:1738–1749Crossref, Medline, Google Scholar
2 : Schizophrenia. N Engl J Med 1994; 330:681–690Crossref, Medline, Google Scholar
3 : Long-term trends of symptoms and disability in schizophrenia and related disorders. Soc Psychiatry Psychiatr Epidemiol 2000; 35:389–395Crossref, Medline, Google Scholar
4 : Stability and course of neuropsychological deficits in schizophrenia. Arch Gen Psychiatry 2001; 58:24–32Crossref, Medline, Google Scholar
5 : New perspectives on schizophrenia in later life: implications for treatment, policy, and research. Lancet Psychiatry 2015; 2:340–350Crossref, Medline, Google Scholar
6 : A population-based study of the 22q11.2 deletion: phenotype, incidence, and contribution to major birth defects in the population. Pediatrics 2003; 112:101–107Crossref, Medline, Google Scholar
7 : Velo-cardio-facial syndrome: 30 years of study. Dev Disabil Res Rev 2008; 14:3–10Crossref, Medline, Google Scholar
8 : Incidence and prevalence of the 22q11 deletion syndrome: a population-based study in western Sweden. Arch Dis Child 2004; 89:148–151Crossref, Medline, Google Scholar
9 : Prevalence of 22q11 microdeletion. J Med Genet 1996; 33:719Crossref, Medline, Google Scholar
10 : HTT haplotypes contribute to differences in Huntington disease prevalence between Europe and East Asia. Eur J Hum Genet 2011; 19:561–566Crossref, Medline, Google Scholar
11 : Debunking Occam’s razor: diagnosing multiple genetic diseases in families by whole-exome sequencing. Clin Genet 2017; 92:281–289Crossref, Medline, Google Scholar
12 : Molecular findings among patients referred for clinical whole-exome sequencing. JAMA 2014; 312:1870–1879Crossref, Medline, Google Scholar
13 : Congenital absence of the thymus. Am J Roentgenol Radium Ther Nucl Med 1968; 103:32–37Crossref, Medline, Google Scholar
14 : 22q11 deletion syndrome: a genetic subtype of schizophrenia. Biol Psychiatry 1999; 46:882–891Crossref, Medline, Google Scholar
15 : Practical guidelines for managing patients with 22q11.2 deletion syndrome. J Pediatr 2011; 159:332–339.e1Crossref, Medline, Google Scholar
16
17 : Psychiatric genomics: an update and an agenda. Am J Psychiatry 2018; 175:15–27 Link, Google Scholar
18 : The 22q11.2 deletion syndrome as a window into complex neuropsychiatric disorders over the lifespan. Biol Psychiatry 2014; 75:351–360Crossref, Medline, Google Scholar
19 : On chorea. Medical and Surgical Reporter 1872; 26:317–321Google Scholar
20 : De novo Huntington disease caused by 26-44 CAG repeat expansion on a low-risk haplotype. Neurology 2013; 81:1099–1100Crossref, Medline, Google Scholar
21 : A DNA polymorphism for Huntington’s disease marks the future. Arch Neurol 1985; 42:20–24Crossref, Medline, Google Scholar
22
23 : A worldwide study of the Huntington’s disease mutation: the sensitivity and specificity of measuring CAG repeats. N Engl J Med 1994; 330:1401–1406Crossref, Medline, Google Scholar
24 : Huntington disease: natural history, biomarkers, and prospects for therapeutics. Nat Rev Neurol 2014; 10:204–216Crossref, Medline, Google Scholar
25 : Molecular analysis and clinical correlations of the Huntington’s disease mutation. Lancet 1993; 342:954–958Crossref, Medline, Google Scholar
26 : Huntington disease. J Neuropathol Exp Neurol 1998; 57:369–384Crossref, Medline, Google Scholar
27 : Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev 2000; 31:236–250Crossref, Medline, Google Scholar
28 : Early-onset Huntington’s chorea: diagnostic clues. Br J Psychiatry 1987; 151:850–852Crossref, Medline, Google Scholar
29 : Psychopathology in Huntington’s disease patients. Acta Psychiatr Scand 1994; 90:241–246Crossref, Medline, Google Scholar
30 : Psychiatric syndromes in Huntington’s disease. Am J Psychiatry 1983; 140:728–733Link, Google Scholar
31 : Thrombocytopenia in patients with chromosome 22q11.2 deletion syndrome. J Pediatr 2003; 143:277–278Crossref, Medline, Google Scholar
32 : CAG-repeat length and the age of onset in Huntington disease (HD): a review and validation study of statistical approaches. Am J Med Genet B Neuropsychiatr Genet 2010; 153B:397–408Crossref, Medline, Google Scholar
33 : Association between early-onset Parkinson disease and 22q11.2 deletion syndrome: identification of a novel genetic form of Parkinson disease and its clinical implications. JAMA Neurol 2013; 70:1359–1366Crossref, Medline, Google Scholar
34 : Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects. Nat Genet 2017; 49:27–35Crossref, Medline, Google Scholar
35 : Psychiatric manifestations of treatable hereditary metabolic disorders in adults. Ann Gen Psychiatry 2014; 13:27Crossref, Medline, Google Scholar



