Personalized Human Cortical Spheroids
The recently discovered ability to generate human neurons from somatic cells that have been reprogrammed into pluripotent stem cells provides a novel tool for studying the pathophysiology of neuropsychiatric disorders. We have recently developed a relatively simple approach for generating from human pluripotent stem cells three-dimensional neural spherical structures resembling the human cerebral cortex, which we call human cortical spheroids. These floating spheroids grow up to 5 mm in diameter, and they include deep- and superficial-layer pyramidal neurons arranged concentrically in an inside-outside manner, as well as astrocytes. After developing in culture for ∼10 weeks, cortical spheroids display transcriptional characteristics of late midfetal human cortex. Neurons in these three-dimensional structures display spontaneous electrical activity and form functional synapses, and the emerging neural network activity can be probed in preparations similar to slice recordings of the animal brain. By more accurately reproducing the nervous system milieu, neural spheroids promise to bring novel insights into the unique features of the human cerebral cortex. Ultimately, personalized three-dimensional neural cultures derived from patients with specific psychiatric disorders promise to reveal disease-relevant cellular phenotypes and to provide an in vitro platform for drug screenings.
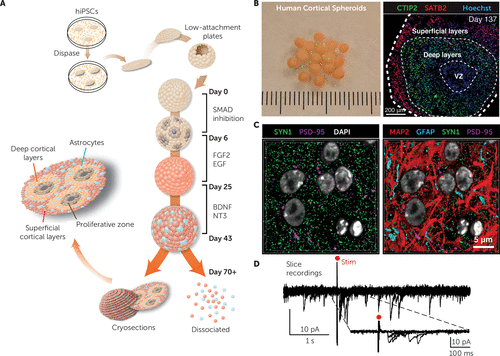
(A) The main stages for generating human cortical spheroids from human induced pluripotent stem cells (hiPSC). FGF2, fibroblast growth factor 2; EGF, epidermal growth factor; BDNF, brain-derived neurotrophic factor; NT3, neurotrophin-3. (B) Morphology of floating human cortical spheroids maintained in culture dishes (left) and cryosection of a human cortical spheroid demonstrating the organization of layer-specific neurons after ∼20 weeks of in vitro differentiation (right). VZ, ventricular zone; CTIP2, COUP-TF-interacting protein 2; SATB2, special AT-rich sequence-binding protein 2; Hoechst stain indicates nuclei. (C) Distribution of neurons (MAP2, microtubule-associated protein 2), astrocytes (GFAP, glial fibrillary acidic protein), and synaptic proteins (SYN1, synapsin-1; PSD-95, postsynaptic density protein 95) inside human cortical spheroids visualized with array tomography. (D) Voltage-clamp recordings showing excitatory postsynaptic currents after electrical stimulation (red dot) in an acute human cortical spheroid slice preparation. Figure adapted/modified with permission from A.M. Paşca et al. (Paşca AM, Sloan SA, Clarke LE, et al: Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods 2015; 12:671–678).



