The Bidirectional Associations Between Psychotic Experiences and DSM-IV Mental Disorders
Abstract
Objective:
While it is now recognized that psychotic experiences are associated with an increased risk of later mental disorders, we lack a detailed understanding of the reciprocal time-lagged relationships between first onsets of psychotic experiences and mental disorders. Using data from World Health Organization World Mental Health (WMH) Surveys, the authors assessed the bidirectional temporal associations between psychotic experiences and mental disorders.
Method:
The WMH Surveys assessed lifetime prevalence and age at onset of psychotic experiences and 21 common DSM-IV mental disorders among 31,261 adult respondents from 18 countries. Discrete-time survival models were used to examine bivariate and multivariate associations between psychotic experiences and mental disorders.
Results:
Temporally primary psychotic experiences were significantly associated with subsequent first onset of eight of the 21 mental disorders (major depressive disorder, bipolar disorder, generalized anxiety disorder, social phobia, posttraumatic stress disorder, adult separation anxiety disorder, bulimia nervosa, and alcohol abuse), with odds ratios ranging from 1.3 (95% CI=1.2–1.5) for major depressive disorder to 2.0 (95% CI=1.5–2.6) for bipolar disorder. In contrast, 18 of 21 primary mental disorders were significantly associated with subsequent first onset of psychotic experiences, with odds ratios ranging from 1.5 (95% CI=1.0–2.1) for childhood separation anxiety disorder to 2.8 (95% CI=1.0–7.8) for anorexia nervosa.
Conclusions:
While temporally primary psychotic experiences are associated with an elevated risk of several subsequent mental disorders, these data show that most mental disorders are associated with an elevated risk of subsequent psychotic experiences. Further investigation of the underlying factors accounting for these time-order relationships may shed light on the etiology of psychotic experiences.
A recent study (1) based on data from 18 countries participating in the World Health Organization (WHO) World Mental Health (WMH) Surveys found that the lifetime prevalence of any psychotic experience among adults was 5.8% (SE=0.2). The WMH estimates were broadly consistent with prevalence estimates in other population-based studies (2, 3). The relatively high lifetime prevalence of psychotic experiences is much higher than the 0.7% mean morbid risk for schizophrenia (4), and it raises important questions about the clinical significance of psychotic experiences with respect to the risk of psychosis and mental disorders in general.
There is solid evidence from prospective studies that psychotic experiences are associated with an increased risk of later psychotic disorders (5–7). In addition, there is some evidence that psychotic experiences are associated with an increased risk of subsequent nonpsychotic mental disorders (8), but this field of research has for the most part examined associations between lifetime psychotic experiences and lifetime common mental disorders regardless of temporal priority. For example, there is evidence linking the lifetime prevalence of psychotic experience with high prevalence of depression and anxiety disorders (9–12), substance use disorders (13), and behavioral disorders (14). Despite these clues, there is a lack of empirical data on whether temporally primary common mental disorders are associated with an increased risk of subsequent first onset of psychotic experiences, and conversely, which mental disorders are predicted by pre-existing psychotic experiences.
We had the opportunity to carry out a preliminary exploration of the bidirectional time-lagged associations between psychotic experiences and mental disorders using retrospective age-at-onset reports within the WMH Surveys. The aims of this study were to explore the associations of temporally primary psychotic experiences with the subsequent onset of mental disorders and of temporally primary mental disorders with the subsequent onset of psychotic experiences.
Method
Samples
The WMH Surveys are a coordinated set of community epidemiological surveys administered in probability samples of the household population in countries throughout the world (15). Eighteen of the 29 WMH Surveys completed up to now included a psychosis module. These 18 countries are distributed across North and South America (the United States, Colombia, Mexico, Peru, São Paulo in Brazil), Africa (Nigeria), the Middle East (Iraq, Lebanon), Asia (Shenzhen in the People’s Republic of China), the South Pacific (New Zealand), and Europe (Belgium, France, Germany, Italy, the Netherlands, Portugal, Romania, Spain). All 18 surveys were based on multistage clustered area probability household sampling designs (see Table S1 in the data supplement that accompanies the online edition of this article). Individual country sample sizes ranged from 301 in France to 7,263 in New Zealand. The weighted average response rate across all 18 surveys was 72.1%.
In keeping with previous epidemiological studies of psychotic experiences (1, 13, 16, 17), we made the a priori decision to exclude individuals who had psychotic experiences but who also screened positive for possible schizophrenia/psychosis or manic-depression/mania (i.e., respondents who reported schizophrenia/psychosis or manic-depression/mania in response to the question “What did the doctor say was causing (this/these) experiences?” as well as those who ever took any antipsychotic medication for these symptoms). This resulted in the exclusion of 140 respondents (0.4% of all respondents), leaving 31,261 respondents for this study (see Table S1).
Procedures
All surveys were conducted in respondents’ homes by trained lay interviewers. Informed consent was obtained before beginning interviews in all countries. Procedures for obtaining informed consent, ethical approvals, and protection of individuals were monitored for compliance by the institutional review boards of the collaborating organizations in each country (18). Standardized interviewer training and quality control procedures were used consistently in the surveys. Full details of these procedures have been provided elsewhere (18, 19).
All WMH interviews had two parts. Part I, administered to all respondents, contained assessments related to core mental disorders. Part II included additional information relevant to a wide range of survey aims, including assessment of psychotic experiences. Part II was administered to all Part I respondents who met criteria for any DSM-IV mental disorder as well as a probability sample of other respondents. Within the different sites, items related to psychotic experiences were either administered to all respondents or to a random sample of Part II respondents. Part II respondents were weighted by the inverse of their probability of selection for Part II to adjust for differential sampling. Additional weights were used to adjust for differential probabilities of selection within households and nonresponse and to match the samples to population sociodemographic distributions. The weighted data are analyzed here.
Data Collection and Data Items
The diagnostic instrument used in the WMH Surveys was the WHO Composite International Diagnostic Interview (CIDI) (20), a validated fully structured diagnostic interview designed to assess the prevalence and correlates of a wide range of mental disorders according to the definitions and criteria of both the DSM-IV and ICD-10 diagnostic systems. WHO translation, back-translation, and harmonization protocols were used to adapt the CIDI for use in each participating country (21).
Psychotic experiences.
The CIDI psychosis module included questions about six types of psychotic experience—two related to hallucinations (visual hallucinations, auditory hallucinations) and four related to delusions (thought insertion/withdrawal, mind control/passivity, ideas of reference, plot to harm/follow) (see Tables S2A,B in the online data supplement). The module began by asking respondents if they ever had the psychotic experiences (e.g., “Have you ever seen something that wasn’t there that other people could not see?” “Have you ever heard any voices that other people said did not exist?”). Positive responses were then probed to determine whether the reported psychotic experiences ever occurred when the person was “not dreaming, not half asleep, or not under the influence of alcohol or drugs” (this probe question was included in the main question in the European Study of the Epidemiology of Mental Disorders countries). Respondents who reported psychotic experiences were then asked a further probe question about their age at the onset of the psychotic experiences (i.e., “How old were you the very first time [this/either of these things/any of these things] happened to you?”). In the 8.0% of cases where age at onset was missing, we used imputation to assign predicted values based on a set of predictors that included all the variables in the substantive models (22). Key summary statistics for the observed data (without imputation) and the entire data set after imputation are provided in Table S3 in the data supplement.
DSM-IV mental disorders.
The WMH Survey version of the CIDI assessed lifetime history of 21 mental disorders, including mood disorders (major depressive disorder and bipolar disorder); anxiety disorders (panic disorder, generalized anxiety disorder, specific phobia, social phobia, agoraphobia without panic, posttraumatic stress disorder [PTSD], and separation anxiety disorder, further divided into childhood and adult separation anxiety disorder); impulse control disorders (intermittent explosive disorder, attention deficit hyperactivity disorder [ADHD], oppositional defiant disorder, and conduct disorder); eating disorders (anorexia nervosa, bulimia nervosa, and binge eating disorder); and substance use disorders (alcohol abuse, alcohol dependence, drug abuse, and drug dependence). The disorders that require childhood onset (ADHD, oppositional defiant disorder, conduct disorder, separation anxiety disorder) were included in Part II and are limited to respondents in the age range 18–39 or 18–44 years (depending on site) because of concerns about recall bias among older respondents (23). All other disorders were assessed for the full sample age range. Clinical reappraisal studies indicate that lifetime diagnoses based on the CIDI have good concordance with diagnoses based on blind clinical interviews (24).
In keeping with previous research, standardized diagnostic hierarchy rules among the disorders assessed were applied where appropriate (25). In this approach, individuals are excluded from diagnoses for which they have sufficient symptoms to meet the criteria if they have another disorder that is thought to account for those symptoms. For example, someone who has alcohol dependence cannot be diagnosed with alcohol abuse, because dependence is a more severe diagnosis. Therefore, alcohol abuse with hierarchy means it cannot occur exclusively during episodes of alcohol dependence. However, given that the analysis was based on a person-year data array (see below), these diagnoses can change within persons over time. For example, a respondent with an onset of alcohol abuse at age 18 and alcohol dependence at age 22 would be coded as having alcohol abuse from ages 18 to 21 and as having alcohol dependence beginning at age 22.
Statistical Analysis
Discrete-time survival analyses (26) with person-year as the unit of analysis and time-varying measures for prior onset of other mental disorders were used to examine the predictive associations of temporally prior disorders with the subsequent onset of each mental disorder considered in the analysis. A person-year data set was created such that each year in the life of each respondent (up to and including the age at onset of the outcome disorder or age at interview, whichever came first) was treated as a separate observational record. We estimated survival models that examined bivariate associations between psychotic experiences and only one common mental disorder at a time (with adjustment for age cohort, sex, person-year, education, marital and employment status, and country) as well as multivariate models that included information on all temporally primary common mental disorders to predict the outcome disorder. The latter models included measures for both type and number of prior mental disorders. In the case of multivariate models of the associations between temporally primary psychotic experiences and later disorders, we controlled for the association of other temporally primary disorders, again including measures for both type and number of these disorders, in order to determine whether significant net associations existed between temporally primary psychotic experiences and subsequent onsets of the outcome disorders. Measures of number of temporally primary disorders (e.g., dummy predictors for exactly two, exactly three, and more than three such disorders) provide a coarse characterization of the nonadditive associations among the predictor disorders. Previous research has shown that the coefficients associated with these predictors are for the most part negative and significant (27), indicating the presence of “sub-additive” interactions among comorbid disorders. The latter suggests that the predictive associations of having multiple temporally prior disorders increase at a decreasing rate as the number of such disorders increases. A more detailed discussion of the logic of these models has been presented elsewhere (27).
All survival coefficients and standard errors were exponentiated to create odds ratios with 95% confidence intervals. Because the WMH Survey data were based on geographically clustered and weighted data, standard errors were estimated with the Taylor series linearization method (28) using the SUDAAN software package (29) to adjust for weighting and clustering. Statistical significance was evaluated using F tests or Wald chi-square tests based on design-corrected coefficient variance-covariance matrices. Statistical significance was evaluated consistently using two-tailed 0.05-level tests.
Results
Lifetime Prevalence of Mental Disorders Among Respondents With and Without Psychotic Experiences
We first examined lifetime prevalence of mental disorders among respondents with and without psychotic experiences regardless of the temporal order of psychotic experience and mental disorders (Table 1). Compared with those with no psychotic experiences, those with psychotic experiences had significantly higher odds of having 20 of the 21 mental disorders examined (based on lifetime prevalence). Odds ratios ranged from 1.6 (95% CI=1.2–2.1) for drug abuse to 3.6 (95% CI=2.6–5.0) for bulimia nervosa.
| Total Sample (N=31,261) | Respondents With Lifetime Psychotic Experiences (N=2,385) | Respondents Without Lifetime Psychotic Experiences (N=28,876) | Odds Ratio Between Lifetime Psychotic Experiences and Lifetime Mental Disorder | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mental Disorder | Na | %b | SE | Na | %b | SE | Na | %b | SE | Odds Ratioc | 95% CI |
| Mood disorders | |||||||||||
| Major depressive disorder with hierarchy | 6,824 | 11.8 | 0.2 | 897 | 25.4 | 1.2 | 5,927 | 10.9 | 0.2 | 2.3* | 2.0–2.6 |
| Bipolar disorder (broad) | 1,212 | 2.3 | 0.1 | 196 | 6.5 | 0.6 | 1,016 | 2.1 | 0.1 | 2.8* | 2.2–3.5 |
| Anxiety disorders | |||||||||||
| Panic disorder | 1,019 | 1.8 | 0.1 | 181 | 4.8 | 0.5 | 838 | 1.6 | 0.1 | 2.7* | 2.2–3.4 |
| Generalized anxiety disorder with hierarchy | 1,832 | 3.4 | 0.1 | 276 | 8.0 | 0.6 | 1,556 | 3.1 | 0.1 | 2.4* | 2.0–3.0 |
| Social phobia | 2,495 | 4.7 | 0.1 | 382 | 12.1 | 0.8 | 2,113 | 4.3 | 0.1 | 2.4* | 2.0–2.8 |
| Specific phobia | 4,108 | 8.4 | 0.2 | 630 | 20.5 | 1.1 | 3,478 | 7.6 | 0.2 | 2.6* | 2.3–3.0 |
| Agoraphobia without panic | 557 | 1.0 | 0.1 | 102 | 3.1 | 0.4 | 455 | 0.9 | 0.1 | 2.8* | 2.0–3.9 |
| Posttraumatic stress disorder | 1,811 | 3.7 | 0.1 | 328 | 10.2 | 0.7 | 1,483 | 3.3 | 0.1 | 3.0* | 2.5–3.6 |
| Separation anxiety disorder (child) | 449 | 2.2 | 0.1 | 90 | 5.7 | 0.8 | 359 | 2.0 | 0.1 | 2.3* | 1.6–3.1 |
| Separation anxiety disorder (adult) | 877 | 3.7 | 0.2 | 186 | 11.1 | 1.0 | 691 | 3.2 | 0.2 | 3.1* | 2.4–4.0 |
| Impulse control disorders | |||||||||||
| Intermittent explosive disorder with hierarchy | 1,023 | 3.3 | 0.1 | 153 | 8.4 | 0.7 | 870 | 3.0 | 0.1 | 2.1* | 1.7–2.6 |
| Attention deficit hyperactivity disorder | 368 | 1.5 | 0.1 | 79 | 5.1 | 0.8 | 289 | 1.3 | 0.1 | 2.6* | 1.8–3.9 |
| Oppositional defiant disorder with hierarchy | 311 | 2.2 | 0.2 | 68 | 5.8 | 0.9 | 243 | 1.9 | 0.2 | 2.7* | 1.8–4.1 |
| Conduct disorder | 329 | 2.1 | 0.2 | 64 | 5.4 | 0.8 | 265 | 1.8 | 0.2 | 2.3* | 1.6–3.4 |
| Eating disorders | |||||||||||
| Anorexia nervosa | 69 | 0.4 | 0.1 | 13 | 0.7 | 0.3 | 56 | 0.3 | 0.1 | 1.8 | 0.7–4.4 |
| Binge eating disorder with hierarchy | 563 | 2.1 | 0.1 | 128 | 5.0 | 0.5 | 435 | 1.9 | 0.1 | 2.0* | 1.5–2.6 |
| Bulimia nervosa with hierarchy | 364 | 1.1 | 0.1 | 89 | 3.8 | 0.5 | 275 | 0.9 | 0.1 | 3.6* | 2.6–5.0 |
| Substance use disorders | |||||||||||
| Alcohol abuse with hierarchy | 1,908 | 5.1 | 0.2 | 232 | 9.8 | 0.9 | 1,676 | 4.8 | 0.2 | 1.7* | 1.4–2.0 |
| Alcohol dependence | 1,170 | 2.4 | 0.1 | 205 | 6.3 | 0.6 | 965 | 2.1 | 0.1 | 2.3* | 1.8–2.8 |
| Drug abuse with hierarchy | 644 | 1.8 | 0.1 | 83 | 3.6 | 0.5 | 561 | 1.7 | 0.1 | 1.6* | 1.2–2.1 |
| Drug dependence | 481 | 1.2 | 0.1 | 104 | 3.8 | 0.5 | 377 | 1.0 | 0.1 | 2.7* | 1.9–3.6 |
TABLE 1. Lifetime Prevalence of DSM-IV Mental Disorders Among Respondents With and Without Psychotic Experiences Across Samples From 18 Countries
Temporal Priorities Between Onset Ages for Psychotic Experiences and Mental Disorders
Table 2 summarizes the temporal sequence between ages at onset of psychotic experiences compared with mental disorders within the subset of respondents who had both psychotic experiences and particular mental disorders. We present the proportion of respondents with 1) psychotic experience onset prior to particular mental disorder onset, 2) psychotic experience onset in the same year as the mental disorder, and 3) psychotic experience onset after the mental disorder onset, and then test whether the proportions with psychotic experience onset before or after mental disorders differ significantly (those with onset in the same year were excluded from this comparison). While the overall findings indicate that most psychotic experiences have their onset after mental disorders, this pattern varies between diagnoses. For example, the proportion of respondents with onset of psychotic experience prior to the onset of bipolar disorders was significantly higher (54%) than the proportion with onset of psychotic experience after the onset of bipolar disorder (39%) (χ2=6.0, p<0.02), while there was no difference of psychotic experience onset with respect to the onset of depressive disorders. By contrast, for many disorders (e.g., four of eight anxiety disorders and all of the impulse control disorders), higher proportions of respondents reported onset of psychotic experience after the onset of the mental disorder.
| Mental Disorder | Respondents With Psychotic Experience and Mental Disorder | Respondents With Both Lifetime Mental Disorder of Interest and Lifetime Psychotic Experiences | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Psychotic Experience Onset Prior to Disorder Onset | Psychotic Experience Onset and Disorder Onset in the Same Year | Psychotic Experience Onset After Disorder Onset | Goodness-of-Fit Test for Equal Proportiona | ||||||
| N | %b | SE | %b | SE | %b | SE | χ2 | p | |
| Mood disorders | |||||||||
| Major depressive disorder with hierarchy | 897 | 44.5 | 2.0 | 10.2 | 1.2 | 45.4 | 2.2 | 0.1 | 0.767 |
| Bipolar disorder (broad) | 196 | 53.7 | 5.0 | 7.2 | 2.5 | 39.1 | 5.0 | 6.0 | 0.024 |
| Anxiety disorders | |||||||||
| Panic disorder | 181 | 42.2 | 5.3 | 10.9 | 3.9 | 46.9 | 5.3 | 1.0 | 0.343 |
| Generalized anxiety disorder with hierarchy | 276 | 46.1 | 4.2 | 6.8 | 1.9 | 47.1 | 4.3 | 0.0 | 0.839 |
| Social phobia | 382 | 22.6 | 2.8 | 6.7 | 2.2 | 70.7 | 3.3 | 113.0 | <0.001 |
| Specific phobia | 630 | 6.7 | 1.2 | 4.5 | 0.9 | 88.9 | 1.5 | 549.8 | <0.001 |
| Agoraphobia without panic | 102 | 28.4 | 5.8 | 3.7 | 1.9 | 68.0 | 5.9 | 38.2 | <0.001 |
| Posttraumatic stress disorder | 328 | 40.0 | 3.9 | 11.2 | 2.3 | 48.8 | 3.6 | 4.2 | 0.047 |
| Separation anxiety disorder (child) | 90 | 9.1 | 2.8 | 3.8 | 2.0 | 87.1 | 3.4 | 163.5 | <0.001 |
| Separation anxiety disorder (adult) | 186 | 46.7 | 5.0 | 7.5 | 1.9 | 45.8 | 5.0 | 0.0 | 0.893 |
| Impulse control disorders | |||||||||
| Intermittent explosive disorder with hierarchy | 153 | 25.4 | 3.3 | 5.3 | 2.3 | 69.4 | 3.9 | 105.0 | <0.001 |
| Attention deficit hyperactivity disorder | 79 | 9.4 | 4.9 | 2.9 | 2.7 | 87.6 | 6.1 | 3,145.0 | <0.001 |
| Oppositional defiant disorder with hierarchy | 68 | 17.6 | 5.8 | 1.1 | 1.1 | 81.3 | 5.9 | 401.3 | <0.001 |
| Conduct disorder | 64 | 17.7 | 5.9 | 4.9 | 3.4 | 77.5 | 7.2 | 54.0 | <0.001 |
| Eating disorders | |||||||||
| Anorexia nervosa | 13 | 23.6 | 13.6 | — | — | 76.4 | 13.6 | —c | — |
| Binge eating disorder with hierarchy | 128 | 44.7 | 5.9 | 6.9 | 2.9 | 48.3 | 5.9 | 0.2 | 0.656 |
| Bulimia nervosa with hierarchy | 89 | 49.8 | 6.3 | 8.4 | 4.0 | 41.8 | 6.4 | 1.4 | 0.266 |
| Substance use disorders | |||||||||
| Alcohol abuse with hierarchy | 232 | 50.1 | 4.6 | 2.6 | 1.2 | 47.3 | 4.6 | 0.5 | 0.494 |
| Alcohol dependence | 205 | 43.3 | 4.4 | 6.3 | 2.6 | 50.4 | 4.8 | 2.8 | 0.109 |
| Drug abuse with hierarchy | 83 | 55.0 | 6.9 | 3.2 | 2.0 | 41.8 | 6.8 | 36.6 | 0.026 |
| Drug dependence | 104 | 48.2 | 6.3 | 7.6 | 3.8 | 44.2 | 6.2 | 2.5 | 0.192 |
TABLE 2. Lifetime Prevalence of Psychotic Experience Onset Before and After Onset of DSM-IV Mental Disorder
Time-Lagged Associations Between Lifetime Psychotic Experiences and Subsequent Onset of Mental Disorders
A limitation of the results in Table 2 is that they do not take into consideration differences in the age-at-onset distributions of the different disorders in relation to the age-at-onset distribution of psychotic experiences. This issue is addressed in Table 3 (a more detailed presentation of these analyses can be found in Table S4 in the data supplement), where we present the results from bivariate and multivariate models of the associations between psychotic experiences and subsequent mental disorders (i.e., the temporal order requires the onset of psychotic experience to precede that of the mental disorder of interest). In the bivariate analysis, the associations are adjusted for age cohort, gender, person-year, employment status, and country as well as time-varying education and marital status (without mental disorder comorbidity). In these models, preceding psychotic experiences were significantly associated with increased odds for 16 of the 21 mental disorders. It is noteworthy that three of the five remaining disorders have childhood onsets by definition (ADHD, conduct disorder, childhood separation anxiety disorder). These three disorders also had elevated odds ratios with temporally prior psychotic experiences, in the range of 1.7–2.5, but none of these elevated odds ratios was statistically significant because of the rarity of psychotic experiences occurring prior to the onsets of these disorders. In the multivariate models, we further adjusted for temporally prior comorbid mental disorders (i.e., adjusting for comorbid mental disorders that had onsets prior to the onset of the mental disorder of interest, but not necessarily prior to the onset of psychotic experiences). Respondents with psychotic experiences were significantly more likely to subsequently experience eight of 21 disorders (major depressive disorder, bipolar disorder, generalized anxiety disorder, social phobia, PTSD, adult separation anxiety disorder, bulimia nervosa, alcohol abuse), with odds ratios ranging from 1.3 (95% CI=1.2–1.5) for major depressive disorder to 2.0 (95% CI=1.5–2.6) for bipolar disorder. In the multivariate models presented in Table S4 in the data supplement, the odds ratios associated with number of temporally prior disorders were for the most part lower than 1.0, indicating a general pattern of sub-additive interactions, whereby the incremental association of each additional temporally primary disorder lessens in magnitude as the number of such disorders increases.
| Bivariate Modelsa | Multivariate Modelsb | |||
|---|---|---|---|---|
| Mental Disorder | Odds Ratio | 95% CI | Odds Ratio | 95% CI |
| Mood disorders | ||||
| Major depressive disorder with hierarchy | 1.6* | 1.4–1.9 | 1.3* | 1.2–1.5 |
| Bipolar disorder (broad) | 2.7* | 2.0–3.5 | 2.0* | 1.5–2.6 |
| Anxiety disorders | ||||
| Panic disorder | 2.0* | 1.5–2.8 | 1.3 | 0.9–1.8 |
| Generalized anxiety disorder with hierarchy | 1.9* | 1.5–2.4 | 1.4* | 1.1–1.8 |
| Social phobia | 2.0* | 1.5–2.7 | 1.4* | 1.0–1.8 |
| Specific phobia | 1.0 | 0.7–1.5 | 0.9 | 0.6–1.3 |
| Agoraphobia without panic | 2.0* | 1.2–3.4 | 1.2 | 0.7–2.1 |
| Posttraumatic stress disorder | 2.0* | 1.6–2.6 | 1.3* | 1.1–1.7 |
| Separation anxiety disorder (child) | 1.7 | 0.9–3.2 | 1.2 | 0.6–2.3 |
| Separation anxiety disorder (adult) | 2.7* | 1.9–3.6 | 1.6* | 1.2–2.2 |
| Impulse control disorders | ||||
| Intermittent explosive disorder with hierarchy | 1.5* | 1.1–2.1 | 1.2 | 0.9–1.6 |
| Attention deficit hyperactivity disorder | 2.5 | 0.8–7.4 | 1.8 | 0.6–5.6 |
| Oppositional defiant disorder with hierarchy | 2.6* | 1.2–5.7 | 2.1 | 0.9–5.0 |
| Conduct disorder | 1.9 | 0.9–4.1 | 1.2 | 0.5–3.0 |
| Eating disorders | ||||
| Anorexia nervosa | 0.9 | 0.3–2.8 | 0.7 | 0.2–2.0 |
| Binge eating disorder with hierarchy | 1.7* | 1.1–2.5 | 1.0 | 0.7–1.6 |
| Bulimia nervosa with hierarchy | 3.2* | 2.2–4.8 | 1.9* | 1.2–3.1 |
| Substance use disorders | ||||
| Alcohol abuse with hierarchy | 1.7* | 1.3–2.3 | 1.4* | 1.1–1.9 |
| Alcohol dependence | 1.9* | 1.4–2.7 | 1.1 | 0.8–1.7 |
| Drug abuse with hierarchy | 1.9* | 1.2–2.8 | 1.4 | 0.9–2.1 |
| Drug dependence | 2.3* | 1.5–3.4 | 1.4 | 0.9–2.0 |
TABLE 3. Associations Between Lifetime Psychotic Experiences and Subsequent Onset of DSM-IV Mental Disorders
Time-Lagged Associations Between Lifetime Mental Disorders and Subsequent Onset of Psychotic Experiences
Next we examined the associations between preceding mental disorders and the subsequent onset of psychotic experiences (Table 4). In bivariate models, 20 of the 21 mental disorders were significantly associated with elevated odds of subsequent psychotic experiences (drug abuse was the only disorder not associated with the later onset of psychotic experiences; note that our study excluded psychotic experiences that were experienced under the influence of alcohol or drugs). In multivariate models, we found that 18 of the 21 mental disorders were significantly associated with the later onset of psychotic experiences, with odds ratios ranging from 1.5 (95% CI=1.0–2.1) for childhood separation anxiety disorder to 2.8 (95% CI=1.0–7.8) for anorexia nervosa. As with the previous models predicting mental disorders after temporally primary psychotic experiences (see Table S4), the odds ratios associated with number of temporally primary mental disorders (2, 3, 4, 5+) were for the most part less than 1, confirming the existence of sub-additive interactions among mental disorders in predicting first onset of temporally secondary psychotic experiences. The joint associations of all the number-of-disorder coefficients were significant (χ2=31.1, df=4, p<0.001).
| Mental Disorder | Bivariate Modelsa | Multivariate Modelb | ||
|---|---|---|---|---|
| Odds Ratio | 95% CI | Odds Ratio | 95% CI | |
| Mood disorders | ||||
| Major depressive disorder with hierarchy | 2.5* | 2.1–3.0 | 2.2* | 1.8–2.7 |
| Bipolar disorder (broad) | 2.6* | 1.9–3.7 | 1.9* | 1.3–2.9 |
| Anxiety disorders | ||||
| Panic disorder | 2.5* | 1.8–3.4 | 1.6* | 1.2–2.3 |
| Generalized anxiety disorder with hierarchy | 2.4* | 1.8–3.1 | 1.7* | 1.2–2.3 |
| Social phobia | 2.2* | 1.8–2.6 | 1.5* | 1.2–2.0 |
| Specific phobia | 2.6* | 2.2–2.9 | 2.2* | 1.8–2.7 |
| Agoraphobia without panic | 2.5* | 1.8–3.6 | 1.9* | 1.3–2.7 |
| Posttraumatic stress disorder | 2.5* | 1.9–3.2 | 1.8* | 1.3–2.4 |
| Separation anxiety disorder (child) | 2.1* | 1.5–3.0 | 1.5* | 1.0–2.1 |
| Separation anxiety disorder (adult) | 2.8* | 2.1–3.9 | 1.9* | 1.3–2.8 |
| Impulse control disorders | ||||
| Intermittent explosive disorder with hierarchy | 2.4* | 1.9–3.0 | 1.9* | 1.5–2.5 |
| Attention deficit hyperactivity disorder | 2.7* | 2.0–3.8 | 1.7* | 1.2–2.4 |
| Oppositional defiant disorder with hierarchy | 3.0* | 2.1–4.3 | 2.0* | 1.3–3.1 |
| Conduct disorder | 2.5* | 1.7–3.7 | 1.4 | 0.9–2.3 |
| Eating disorders | ||||
| Anorexia nervosa | 2.9* | 1.1–8.1 | 2.8* | 1.0–7.8 |
| Binge eating disorder with hierarchy | 2.2* | 1.6–3.2 | 1.8* | 1.2–2.6 |
| Bulimia nervosa with hierarchy | 2.7* | 1.8–4.2 | 1.8* | 1.2–3.0 |
| Substance use disorders | ||||
| Alcohol abuse with hierarchy | 1.8* | 1.4–2.5 | 1.8* | 1.3–2.5 |
| Alcohol dependence | 2.4* | 1.8–3.3 | 2.0* | 1.3–2.9 |
| Drug abuse with hierarchy | 1.5 | 1.0–2.3 | 1.1 | 0.7–1.8 |
| Drug dependence | 2.6* | 1.7–3.9 | 1.3 | 0.8–2.3 |
| χ2 | p | |||
| Joint effect of all types of disorders (df=21) | 130.4 | <0.001 | ||
| Difference between types of disorders (df=20) | 27.9 | 0.111 | ||
| Odds Ratio | 95% CI | |||
| Number of disorders | ||||
| 2 disorders | 1.0 | 0.7–1.3 | ||
| 3 disorders | 0.5* | 0.4–0.8 | ||
| 4 disorders | 0.4* | 0.2–0.8 | ||
| 5+ disorders | 0.2* | 0.1–0.4 | ||
| χ2 | p | |||
| Joint effect of all number of disorders (df=4) | 31.1 | <0.001 | ||
TABLE 4. Associations Between DSM-IV Mental Disorders and Subsequent Onset of Psychotic Experiences
The key findings from Tables 3 and 4 are summarized in Figure 1, which allows quick comparisons between 1) the odds ratios for the onset of mental disorders after the onset of psychotic experiences and 2) the odds ratios for the onset of psychotic experiences after the onset of mental disorders, when sorted by mental disorder type.
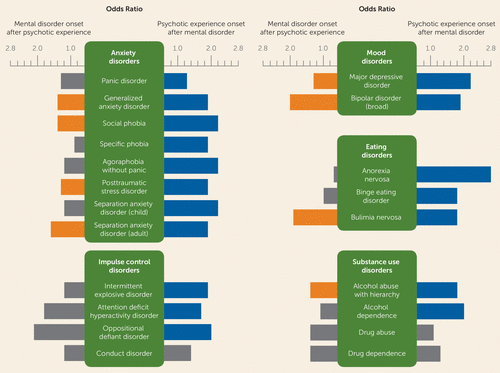
FIGURE 1. Summary of the Bidirectional Association Between Psychotic Experiences and Mental Disordersa
a The odds ratios are drawn from the multivariate analyses presented in Tables 3 and 4. Bars to the left (in orange if significant) represent the odds ratio of the onset of mental disorders after the onset of psychotic experiences. Bars to the right (in blue if significant) represent the odds ratio of the onset of mental disorders after the onset of psychotic experiences. Odds ratios for nonsignificant associations are shown in gray.
Discussion
This large cross-national study demonstrates that individuals with psychotic experiences are at increased risk of experiencing a wide range of mental disorders at some stage in their life compared with other people in the population. The mental disorders include mood, anxiety, impulse control, eating, and substance use disorders. The lack of specificity in these associations, even in multivariate models, is consistent with the hypothesis that psychotic experiences may be nonspecific markers of a wide range of mental disorders (30).
Our temporally ordered analyses provide new insights into the bidirectional relationship between psychotic experiences and mental disorders. We found a strikingly consistent increased risk of psychotic experience onset after nearly all of the mental disorders we examined. That is, most mental disorders were associated with an increased risk of subsequent psychotic experiences, even in multivariate models. Of the 21 disorders examined in this study, only three externalizing disorders did not significantly predict subsequent psychotic experiences in the multivariate model (conduct disorder, drug abuse, and drug dependence).
We also found that the onset of psychotic experiences predicted significantly increased risk of subsequent major depressive disorder, bipolar disorder, generalized anxiety disorder, social phobia, PTSD, adult separation anxiety disorder, bulimia nervosa, and alcohol abuse. Thus, in addition to risk of psychotic disorders (8), an increased risk of subsequent mood disorders, some anxiety disorders, eating disorders, and substance use disorders is seen after onset of psychotic experiences. These findings call into the question the specificity of the association between psychotic experiences and psychotic disorders (31).
Curiously, in the analyses of psychotic experiences predicting subsequent onset of mental disorders, psychotic experiences predicted none of the four impulse control disorders and only one of the four substance use disorders (alcohol abuse), while psychotic experiences did predict a number of the mood and anxiety disorders. These findings reveal that the temporal relationships between psychotic experiences and mental disorders are bidirectional for some disorders. Importantly, this is not due to the different age-at-onset distributions of these disorders, as pervasively significant time-lagged bivariate associations were found across disorders. The differential was observed only when we estimated multivariate models in which we can see unique incremental associations of a single temporally primary disorder with a temporally secondary disorder net of the additive effects of comorbid disorders. Taking into account the temporal order of the variables of interest, sub-additive associations between comorbid mental disorders and psychotic experiences were identified in both analyses (that is, this feature is also bidirectional).
While this study has many strengths (e.g., large sample size, range of countries, uniform methodology for data collection), it also has several important limitations. We relied on lay interviewers to administer the questionnaire. We excluded individuals who were screen-positive for possible psychotic disorders (based on self-report of diagnoses or use of antipsychotic medications to treat psychotic experiences). However, we did not have access to valid measures of clinical psychotic disorders, an important consideration for understanding the utility of psychotic experiences as a predictor of later psychotic disorders. We relied on retrospective reports about age at onset, which may have led to recall bias despite the use of special age-at-onset probes in the CIDI that have been shown to improve the accuracy of retrospective age-at-onset reporting (32). And we estimated models that assumed that predictive associations were additive and independent of age at onset, time since onset, and, in the models for temporally primary psychotic experiences predicting later disorders, interactions of psychotic experiences with disorders occurring prior to the onset of the outcome disorders. More complex models that relax these simplifying assumptions might well shed light on the asymmetries found here in the time-lagged multivariate associations between psychotic experiences and other disorders. Large prospective surveys would be needed to confirm the temporal order between the variables examined in this study. An additional caution is that even in the presence of unequivocal information on temporal ordering of onsets, we cannot infer causality, as common unmeasured causes may influence associations between temporally primary and secondary disorders (see below).
Future Directions
Our findings provide a heuristic framework for the generation of new hypotheses related to psychotic experiences, which we will explore in future studies. For example, it will be of interest to see what proportion of early- versus late-onset psychotic experiences arise either de novo or after the onset of a mental disorder, as well as the extent to which early-onset psychotic experiences and psychotic experiences of different types (e.g., delusions versus hallucinations) predict the subsequent first onset of different types of mental disorders and do so differentially as a complex function of age at onset, time since onset, and existence of complex comorbidities. It is also feasible that an underlying diathesis may lead to both an increased risk of psychotic experiences and an increased risk of mental disorders (regardless of the temporal order of these variables). It is plausible that familial/genetic factors or adverse early life exposures (e.g., childhood adversities, cannabis use) could lead to both an increased risk of later mental disorders and an increased risk of psychotic experiences (33–35). The comprehensive nature of the WMH Survey will allow us to explore these and other related hypotheses in future analyses, albeit within the context of the inherent limitations of cross-sectional data assessed with fully structured diagnostic interviews.
Conclusions
Individuals with psychotic experiences were significantly more likely to have subsequent first onsets of eight of 21 common mental disorders (major depressive disorder, bipolar disorder, generalized anxiety disorder, social phobia, PTSD, separation anxiety disorder (adult), bulimia nervosa, alcohol abuse). Conversely, most temporally primary mental disorders were significantly associated with the subsequent first onset of psychotic experiences. Our findings have important implications for the understanding of how psychotic experiences fit into the structure of psychopathology and within psychiatric taxonomy. A better understanding of how psychotic experiences unfold across the lifespan and interact with mental disorders may provide clues to help guide clinical management.
1 : Psychotic experiences in the general population: a cross-national analysis based on 31,261 respondents from 18 countries. JAMA Psychiatry 2015; 72:697–705Crossref, Medline, Google Scholar
2 : The continuum of psychotic symptoms in the general population: a cross-national study. Schizophr Bull 2012; 38:475–485Crossref, Medline, Google Scholar
3 : An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: on the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol Med 2013; 43:1133–1149Crossref, Medline, Google Scholar
4 : A systematic review of the prevalence of schizophrenia. Schizophr Res 2006; 81:182–183Google Scholar
5 : Children’s self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Arch Gen Psychiatry 2000; 57:1053–1058Crossref, Medline, Google Scholar
6 : Self-reported attenuated psychotic symptoms as forerunners of severe mental disorders later in life. Arch Gen Psychiatry 2012; 69:467–475Crossref, Medline, Google Scholar
7 : The incidence and outcome of subclinical psychotic experiences in the general population. Br J Clin Psychol 2005; 44:181–191Crossref, Medline, Google Scholar
8 : Do subthreshold psychotic experiences predict clinical outcomes in unselected non-help-seeking population-based samples? A systematic review and meta-analysis, enriched with new results. Psychol Med 2012; 42:2239–2253Crossref, Medline, Google Scholar
9 : Psychotic-like experiences and correlation with distress and depressive symptoms in a community sample of adolescents and young adults. Schizophr Res 2010; 119:258–265Crossref, Medline, Google Scholar
10 : Anxiety and depressive disorders are associated with delusional-like experiences: a replication study based on a National Survey of Mental Health and Wellbeing. BMJ Open 2012; 2:2Crossref, Google Scholar
11 : Mood, anxiety, and psychotic phenomena measure a common psychopathological factor. Psychol Med 2015; 45:1483–1493Crossref, Medline, Google Scholar
12 : Psychotic-like experiences in major depression and anxiety disorders: a population-based survey in young adults. Schizophr Bull 2011; 37:389–393Crossref, Medline, Google Scholar
13 : The association between delusional-like experiences, and tobacco, alcohol, or cannabis use: a nationwide population-based survey. BMC Psychiatry 2011; 11:202–210Crossref, Medline, Google Scholar
14 : Clinicopathological significance of psychotic experiences in non-psychotic young people: evidence from four population-based studies. Br J Psychiatry 2012; 201:26–32Crossref, Medline, Google Scholar
15 : The WHO World Mental Health Surveys: Global Perspectives on the Epidemiology of Mental Disorders. New York, Cambridge University Press, 2008Google Scholar
16 : The association between delusional-like experiences and suicidal thoughts and behaviour. Schizophr Res 2011; 132:197–202Crossref, Medline, Google Scholar
17 : Association between trauma exposure and delusional experiences in a large community-based sample. Br J Psychiatry 2007; 190:339–343Crossref, Medline, Google Scholar
18 : The World Health Organization Composite International Diagnostic Interview, in The WHO World Mental Health Surveys: Global Perspectives on the Epidemiology of Mental Disorders. Edited by Kessler RC, Üstün TB. New York, Cambridge University Press, 2008, pp 58–90Google Scholar
19 : The World Health Organization World Mental Health Survey Initiative. Epidemiol Psichiatr Soc 2006; 15:161–166Crossref, Medline, Google Scholar
20 : The World Mental Health (WMH) Survey Initiative Version of the World Health Organization (WHO) Composite International Diagnostic Interview (CIDI). Int J Methods Psychiatr Res 2004; 13:93–121Crossref, Medline, Google Scholar
21 : Translation procedures and translation assessment in the World Mental Health Survey Initiative, in The WHO World Mental Health Surveys: Global Perspectives on the Epidemiology of Mental Disorders. Edited by Kessler RC, Üstün TB. New York, Cambridge University Press, 2008Google Scholar
22 : Multiple Imputation for Nonresponse in Surveys. New York, John Wiley & Sons, 1987Crossref, Google Scholar
23 : Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry 2007; 6:168–176Medline, Google Scholar
24 : Concordance of the Composite International Diagnostic Interview Version 3.0 (CIDI 3.0) with standardized clinical assessments in the WHO World Mental Health surveys. Int J Methods Psychiatr Res 2006; 15:167–180Crossref, Medline, Google Scholar
25 : Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62:617–627Crossref, Medline, Google Scholar
26 : It’s about time: using discrete-time survival analysis to study duration and the timing of events. J Educ Stat 1993; 18:155–195Crossref, Google Scholar
27 : Development of lifetime comorbidity in the World Health Organization World Mental Health surveys. Arch Gen Psychiatry 2011; 68:90–100Crossref, Medline, Google Scholar
28 : Introduction to Variance Estimation. New York, Springer-Verlag, 1985Google Scholar
29 RTI International: SUDAAN: Software for the Statistical Analysis of Correlated Data. Research Triangle Park, NC, 1999Google Scholar
30 : The association between general psychological distress and delusional-like experiences: a large population-based study. Schizophr Res 2011; 127:246–251Crossref, Medline, Google Scholar
31 : Whither the psychosis-neurosis borderline. Schizophr Bull 2014; 40:266–268Crossref, Medline, Google Scholar
32 : Improving accuracy of major depression age-of-onset reports in the US National Comorbidity Survey. Int J Methods Psychiatr Res 1999; 8:39–48Crossref, Google Scholar
33 : Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective, and cross-sectional cohort studies. Schizophr Bull 2012; 38:661–671Crossref, Medline, Google Scholar
34 : Association between cannabis use and psychosis-related outcomes using sibling pair analysis in a cohort of young adults. Arch Gen Psychiatry 2010; 67:440–447Crossref, Medline, Google Scholar
35 : Does cannabis use predict the first incidence of mood and anxiety disorders in the adult population? Addiction 2007; 102:1251–1260Crossref, Medline, Google Scholar



