Reproductive Safety of Second-Generation Antipsychotics: Current Data From the Massachusetts General Hospital National Pregnancy Registry for Atypical Antipsychotics
Abstract
Objective:
Second-generation antipsychotics are used to treat a spectrum of psychiatric illnesses in reproductive-age women. The National Pregnancy Registry for Atypical Antipsychotics was established to determine the risk of major malformations among infants exposed to second-generation antipsychotics during pregnancy relative to a comparison group of unexposed infants of mothers with histories of psychiatric morbidity.
Method:
Women were prospectively followed during pregnancy and the postpartum period; obstetric, labor, delivery, and pediatric medical records were obtained. Eligible enrollees were pregnant women ages 18–45. The Registry is based at the Center for Women’s Mental Health at Massachusetts General Hospital. Women were recruited through provider referral, self-referral, and the Center’s web site.
Results:
As of December 2014, 487 women were enrolled: 353 who used second-generation antipsychotics and 134 comparison women. Medical records were obtained for 82% of participants. A total of 303 women had completed the study and were eligible for inclusion in the analysis. Of 214 live births with first-trimester exposure to second-generation antipsychotics, three major malformations were confirmed. In the control group (N=89), one major malformation was confirmed. The absolute risk of major malformations was 1.4% for exposed infants and 1.1% for unexposed infants. The odds ratio for major malformations comparing exposed infants with unexposed infants was 1.25 (95% CI=0.13–12.19).
Conclusions:
The results suggest that it would be unlikely for second-generation antipsychotics to raise the risk of major malformations more than 10-fold beyond that observed in the general population or among control groups using other psychotropic medications. If the estimate stabilizes around the null with ongoing data collection, findings may be reassuring for both clinicians and women trying to make risk-benefit treatment decisions about using atypical antipsychotics during pregnancy. These findings are timely given the renewed focus of regulatory agencies on reproductive safety.
Although second-generation antipsychotics have been available since the mid-1990s and are increasingly used as primary or adjunctive therapy across a wide range of psychiatric disorders, including bipolar disorder, schizophrenia, unipolar depression, and anxiety disorders (1–3), reliable data regarding the reproductive safety of these compounds remain sparse (4). The existing safety data on second-generation antipsychotics derive largely from observational case studies, manufacturer reports, and, more recently, from a select number of larger cohort studies (5–14). Thus far, cumulative data suggest that second-generation antipsychotics are not major teratogens (15).
Human reproductive safety data are generally not required for the approval of new medications, and historically the accurate recognition of teratogenic effects of therapeutic agents has been challenging and slow. Over the past two decades, pregnancy registries have emerged as a rapid and systematic means of collecting important reproductive safety data on risk for major malformations following prenatal exposure to a particular medication or class of medications (16–18). Several registries have been established to evaluate the reproductive safety of a broad range of medications, including those used to treat HIV, cancers, epilepsy, and diabetes (http://www.fda.gov/ScienceResearch/SpecialTopics/WomensHealthResearch/ucm251314.htm). Other registries have been created specifically for psychiatric medications, including bupropion, fluoxetine, and lithium (19–21). In 2005, the National Register of Antipsychotic Medication in Pregnancy (NRAMP) was established as an ongoing prospective observational cohort study, enrolling women from Australia and New Zealand (17, 22).
In 2008, the National Pregnancy Registry for Atypical Antipsychotics was established in an ongoing effort to obtain reproductive safety data regarding fetal exposure to second-generation antipsychotics. The National Pregnancy Registry for Atypical Antipsychotics is modeled after the North American Antiepileptic Drug Pregnancy Registry (16, 23). Based at Massachusetts General Hospital in Boston, the National Pregnancy Registry for Atypical Antipsychotics is the first hospital-based pregnancy registry for second-generation antipsychotics in North America to systematically and prospectively evaluate risk of malformations among infants exposed in utero to second-generation antipsychotics. The objective of this Registry is to obtain reproductive safety data, such as teratogenicity and risk for adverse obstetrical and neonatal outcomes associated with in utero exposure to second-generation antipsychotics.
The objective of this report is to present current results from the National Pregnancy Registry for Atypical Antipsychotics. Its timing is noteworthy as it coincides with a renewed focus on reproductive safety on the part of federal regulatory agencies. The older system of reproductive safety classification, a pregnancy category label, will soon be replaced with a new system that includes available data regarding known risk of fetal exposure to medication and that places an emphasis on data collected from well-designed pregnancy registries (http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/Labeling/ucm093307.htm).
Method
A detailed description of the methodology for the Registry has been described elsewhere (24–26). Briefly, this ongoing prospective cohort study follows pregnant women ages 18–45 who are exposed and unexposed to second-generation antipsychotics during pregnancy. Second-generation antipsychotics included in the Registry are aripiprazole, asenapine, clozapine, iloperidone, lurasidone, olanzapine, paliperidone, quetiapine, risperidone, and ziprasidone. As new second-generation antipsychotic medications become available, reproductive safety regarding these agents will be included in the Registry. Participants are recruited primarily through health care provider referral, consultations at the Massachusetts General Hospital Center for Women’s Mental Health, and the Center’s web site (www.womensmentalhealth.org). The exposed group consists of women who have used one or more second-generation antipsychotics during pregnancy. The comparison group consists mostly of women with a history of psychiatric illness being treated with a variety of psychotropic medications other than second-generation antipsychotics.
Participants are interviewed at three time points across pregnancy: at enrollment, at 7 months, and at 3 months postpartum. The initial interview ascertains information regarding demographic characteristics, medication use and dosage changes (if any, before and during pregnancy), social habits (i.e., smoking, alcohol consumption, and illicit drug use), medical and psychiatric history, and family history of birth defects. The 7-month interview collects data on changes in medication or dosage (if any) and intervening medical problems across pregnancy. During the final postpartum interview, information is gathered from maternal reports regarding pharmacotherapy, labor, delivery, and neonatal health outcomes.
Outcome data are also obtained through systematic review of obstetric, labor and delivery, and newborn pediatric medical records. Information regarding primary and secondary outcomes is abstracted from medical records by a trained research coordinator and a senior study physician-investigator (A.C.V.). If a major malformation is noted, pediatric medical records are redacted and sent to a trained dysmorphologist blind to medication exposure to confirm presence of a malformation. Final adjudication of the records is ultimately the responsibility of the dysmorphologist.
All participants in the Registry provide verbal informed consent, and all study procedures were approved by the Massachusetts General Hospital Institutional Review Board. Participants initially received a stipend; as of 2012, this practice was discontinued. Participants who enrolled in the study between Nov. 14, 2008, and Dec. 10, 2014, were eligible for inclusion in this analysis (N=487).
Major policy decisions, including release of findings, are made by a scientific advisory board, which consists of experts on teratology, epidemiology, pharmacology, and pediatrics. Funding for the Registry derives from multiple manufacturers of second-generation antipsychotics who agree to support the reproductive safety initiative with a fixed proportion of Registry operating costs. Since the Registry’s inception, manufacturers have chosen either to participate or to decline, and several manufacturers have renewed participation while others have deferred the option to renew after an initial 24-month period. Study sponsors have no role in data collection, analysis, interpretation, or manuscript preparation and review.
The primary outcome is the presence of a major malformation identified within 6 months of birth. A major malformation is defined as a structural abnormality with surgical, medical, or cosmetic importance (16, 27). Exclusions include: minor anomalies; deformations; physiological features due to prematurity, such as undescended testes; birthmarks; genetic disorders and chromosomal abnormalities; and any finding by prenatal sonography, such as absence of one kidney, or at surgery (or autopsy) that was not identified by an examining pediatrician.
Statistical Analyses
All analyses were conducted using SAS, version 9.3 (SAS Institute, Cary, N.C.). The primary exposure in this study was second-generation antipsychotic use during the first trimester of pregnancy (<13 weeks gestational age). This exposure was operationalized into a binary variable: use of a second-generation antipsychotic during the first trimester (exposed) or no use during the entire pregnancy (unexposed). Women who used second-generation antipsychotics during only the second and/or third trimester of pregnancy were excluded from the analysis.
A number of binary and continuous predictors were also examined. All predictors were provided by maternal report and were measured before or concurrently with the second-generation antipsychotic use and before the outcome of interest occurred (Table 1). Chronicity of illness, determined at enrollment, was calculated as the difference between a participant’s current age and age at onset of first symptoms, divided by the participant’s current age.
| Overall (N=303) | First-Trimester Exposure to Second-Generation Antipsychotic (N=214) | Unexposed to Second-Generation Antipsychotic (N=89) | |||||
|---|---|---|---|---|---|---|---|
| Measure | Valid N | N | % | N | % | N | % |
| Demographic characteristics | |||||||
| College educated | 292 | 188 | 64.4 | 117 | 57.1 | 71 | 81.6 |
| Married | 303 | 236 | 77.9 | 155 | 72.4 | 81 | 91.0 |
| White | 303 | 276 | 91.1 | 193 | 90.2 | 83 | 93.3 |
| Pregnancy history | |||||||
| Planned pregnancy | 299 | 213 | 71.2 | 142 | 66.7 | 71 | 82.6 |
| >1 prior pregnancies | 303 | 187 | 61.7 | 140 | 65.4 | 47 | 52.8 |
| Prior miscarriageb | 188 | 97 | 51.6 | 74 | 52.1 | 23 | 50.0 |
| First trimester use | |||||||
| Cigarettes | 303 | 60 | 19.8 | 51 | 23.8 | 9 | 10.1 |
| Alcohol | 301 | 72 | 23.9 | 45 | 21.0 | 27 | 31.0 |
| Illicit drugs | 299 | 21 | 7.0 | 17 | 8.1 | 4 | 4.6 |
| Prenatal vitamins | 303 | 216 | 71.3 | 142 | 66.4 | 74 | 83.2 |
| Psychiatric illness | |||||||
| Primary diagnosis | |||||||
| Bipolar | 303 | 177 | 58.4 | 142 | 66.4 | 35 | 39.3 |
| Schizophrenia | 303 | 5 | 1.7 | 5 | 2.3 | 0 | 0.0 |
| Depression | 303 | 50 | 16.5 | 30 | 14.0 | 20 | 22.5 |
| Anxiety | 303 | 21 | 6.9 | 9 | 4.2 | 12 | 13.5 |
| History of postpartum depression or psychosisb | 172 | 65 | 37.8 | 51 | 40.2 | 14 | 31.1 |
| First trimester psychotropic medication use | |||||||
| First-generation antipsychotic | 303 | 8 | 2.6 | 7 | 3.3 | 1 | 1.1 |
| Selective serotonin reuptake inhibitor | 303 | 91 | 30.0 | 56 | 26.2 | 35 | 39.3 |
| Serotonin-norepinephrine reuptake inhibitor | 303 | 26 | 8.6 | 20 | 9.4 | 6 | 6.7 |
| Tricyclic antidepressant | 303 | 8 | 2.6 | 7 | 3.3 | 1 | 1.1 |
| Atypical antidepressant | 303 | 25 | 8.3 | 20 | 9.4 | 5 | 5.6 |
| Lithium | 303 | 24 | 7.9 | 11 | 5.1 | 13 | 14.6 |
| Anticonvulsant | 303 | 103 | 34.0 | 86 | 40.2 | 17 | 19.1 |
| Antianxiety medication | 303 | 60 | 19.8 | 42 | 16.6 | 18 | 20.2 |
| Sedative | 303 | 16 | 5.3 | 16 | 7.5 | 0 | 0.0 |
| Stimulant | 303 | 8 | 2.6 | 7 | 3.3 | 1 | 1.1 |
| Polytherapyc | 303 | 208 | 68.7 | 169 | 79.0 | 39 | 43.8 |
| Valid N | Mean | SD | Mean | SD | Mean | SD | |
| Demographic characteristics | |||||||
| Age | 303 | 32.3 | 5.1 | 31.9 | 5.4 | 33.2 | 4.2 |
| Baseline BMI | 297 | 27.1 | 6.1 | 27.9 | 6.4 | 25.7 | 5.0 |
| Psychiatric illness severity | |||||||
| Age at onset of primary disorder | 271 | 17.6 | 6.9 | 18.1 | 6.8 | 16.2 | 6.9 |
| Lifetime number of psychiatric hospitalizations | 242 | 1.7 | 3.4 | 2.1 | 3.7 | 0.9 | 2.6 |
| Chronicity (proportion of life)d | 271 | 45.0 | 20.5 | 13.8 | 19.7 | 51.0 | 21.3 |
TABLE 1. Characteristics of Study Populationa
To compare the odds of a major malformation between infants exposed and unexposed to second-generation antipsychotics, an unadjusted logistic regression model was used (PROC LOGISTIC). Because of the rare outcome, we will interpret only the crude analysis; however, a sensitivity analysis was conducted to examine confounding by a number of factors. Because the majority of women included in the comparison group also had psychiatric conditions and used psychotropic medications, confounding by factors associated with both psychiatric illness and risk of malformations is reduced. Furthermore, the comparison population allows us to examine treatment options within this high-risk population of women.
Each potential confounding factor was added individually to the crude logistic regression model to examine the changes in the odds ratio estimate from the unadjusted model. A hypothetical propensity score–adjusted model was also examined in the sensitivity analysis. The propensity score predicting exposure was calculated using first-trimester exposure to 10 medication classes and a primary diagnosis of bipolar disorder. Each odds ratio estimate for the effect of second-generation antipsychotic exposure on malformations after adjustment is presented in Figure 1.
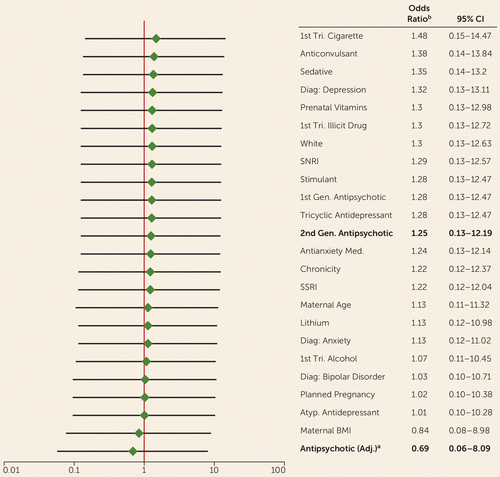
FIGURE 1. Odds of Major Malformations Comparing Infants Exposed and Unexposed to Second-Generation Antipsychotics, After Adjustment for Potential Confounders (N=303)
a The propensity score was created using a logistic regression model to predict exposure to second-generation antipsychotics. The score was created using a primary diagnosis of bipolar disorder and first-trimester use of first-generation antipsychotics, selective serotonin reuptake inhibitors, serotonin-norepinephrine reuptake inhibitors, tricyclic antidepressants, atypical antidepressants (bupropion), antianxiety medications, sedatives, and stimulants.
b The odds ratios listed are the odds of a major malformation comparing exposed with unexposed infants, after adjusting for the characteristic indicated on the y-axis. For example, after adjusting for a diagnosis of depression, infants exposed to an atypical antidepressant had 1.32 times the odds of a malformation compared with unexposed infants (95% CI=0.13–13.11).
An additional sensitivity analysis examined the relationship between major malformations and second-generation antipsychotic use excluding all participants exposed to known teratogens: valproic acid, isotretinoin, lithium, and first-trimester illicit drug use.
Results
From Nov. 14, 2008, to Dec. 10, 2014, a total of 487 women were enrolled in the Registry; 107 (22.0%) women were ineligible for this analysis because they had not reached the postpartum interview at the time of data extraction; 27 (5.5%) dropped out of the study, often because of time constraints; 35 (7.2%) women were lost to follow-up; and 15 (3.1%) had a therapeutic or spontaneous abortion. For this analysis, there were 303 women in the final sample: 214 with first-trimester exposure to a second-generation antipsychotic and a comparison group of 89 who remained unexposed to a second-generation antipsychotic throughout pregnancy. Medical records were obtained and reviewed for 82% of participants.
Table 1 summarizes demographic characteristics; clinical information, such as illness history; alcohol and tobacco use during pregnancy; and spectrum of medication use (psychotropic and nonpsychotropic) in participants from both the exposed and comparison groups. A number of differences were observed between the exposed and unexposed participants. Women who used a second-generation antipsychotic during the first trimester had a mean age of 31.9 years (SD=5.4) and had a mean prepregnancy body mass index (BMI) of 27.9 (SD=6.4). Women in the comparison group were slightly older on average, with a mean age of 33.2 years (SD=4.2) and had a lower BMI (mean=25.7, SD=5.0). Women in the exposed group were less likely to have a college education (57.1% compared with 81.6%) and were less likely to be married (72.4% compared with 91.0%). Cigarette use was also more prevalent in the exposed group, with 23.8% reporting smoking during the first trimester, compared with 10.1% of those in the comparison group.
The psychiatric history of participants in both groups was highly variable. Overall, women who were exposed to a second-generation antipsychotic had more psychiatric hospitalizations but were ill for a smaller proportion of their lifetime than women who were not exposed to a second-generation antipsychotic. Second-generation antipsychotics were also most likely to be used by women with a diagnosis of bipolar disorder. The use of other psychotropic medications varied by group, as well. Selective serotonin reuptake inhibitors were used more often by the women in the comparison group and were most commonly used to treat major depression. Exposure to psychotropic polytherapy was reported in 68.7% of the total cohort, 79.0% of the exposed group, and 43.8% of the comparison group. Anticonvulsants were the class of medication used most frequently in conjunction with a second-generation antipsychotic (40.2%) (Table 1).
The most commonly used second-generation antipsychotics in the exposed group were quetiapine, aripiprazole, and olanzapine, and 8.9% of the exposed group used more than one second-generation antipsychotic during the first trimester.
A total of 214 first-trimester exposed pregnancies and 89 comparison pregnancies were evaluated for the presence of a major malformation. Three major malformations were identified among exposed infants. The first infant had a transposition of the great arteries after first-trimester exposure to aripiprazole, quetiapine, bupropion, and labetalol. The second infant had a ventricular septal defect with surgical repair and had been exposed to ziprasidone, sertraline, and lamotrigine. Lastly, an imperforate hymen was identified in an infant exposed to aripiprazole, bupropion, and trihexyphenidyl.
One major malformation was identified in the comparison group: a midshaft hypospadias that required surgical repair, which was identified in an infant with no exposure to psychiatric medications during the first trimester.
Qualification of risk estimate was 1.4% (95% CI=0.29−4.04) in the exposed group and 1.1% (95% CI=0.0−6.10) in the unexposed group. The risk of a major malformation was not significantly different between the two groups. The unadjusted odds of a major malformation among infants exposed to a second-generation antipsychotic during the first trimester were 1.25 times the risk among unexposed infants (95% CI=0.13–12.19) (Table 2).
| Group | N | Prevalence | 95% CI | Odds Ratio | 95% CI |
|---|---|---|---|---|---|
| First-trimester exposure to second-generation antipsychotic (N=214) | 3 | 1.4 | 0.29–4.04 | 1.25 | 0.13–12.19 |
| Unexposed to second-generation antipsychotic (N=89) | 1 | 1.1 | 0.0–6.10 | Reference | Reference |
TABLE 2. Odds Ratio of a Major Malformation Comparing Exposure Status With Second-Generation Antipsychotic (N=303)
A sensitivity analysis was also conducted to identify potential confounding factors, to examine possible direction of bias, and to provide additional context for interpreting the results. As noted previously, a separate logistic regression model examining the exposure-outcome relationship was used for each potential confounder. Figure 1 lists the odds ratios of major malformations comparing infants exposed and unexposed to a second-generation antipsychotic, after adjusting for potential confounders. After adjusting for the characteristics noted, some notable changes in the odds ratio estimate occur. A number of likely confounding variables were identified using the 10% change in the effect estimate as a guide (e.g., first-trimester cigarette, anticonvulsant, alcohol, and bupropion use; bipolar diagnosis; planned pregnancy; and maternal BMI). With the exception of cigarette and anticonvulsant use, adjusting for these factors moves the odds ratio estimate closer to the null, indicating that our odds ratio estimate of 1.25 may be biased and that the true value may be closer to 1. These factors will be examined further to provide more precise effect estimates once the size of the study population increases.
When examining the population limited to participants unexposed to valproic acid, isotretinoin, lithium, and illicit drugs, the odds of a major malformation among infants exposed to a second-generation antipsychotic were still 1.25 times the odds of a malformation among the unexposed infants. None of the infants with a major malformation was exposed to these teratogenic substances.
Discussion
The National Pregnancy Registry for Atypical Antipsychotics was established with several aims, with the primary aim of quantifying the risk estimate for major congenital malformations following first-trimester exposure to second-generation antipsychotics. The clinical implications of such reproductive safety data are great, given the frequency with which these agents are used by women during the childbearing years across a range of psychiatric indications (1–3). Several studies have examined various outcomes following fetal exposure to second-generation antipsychotics, yielding inconsistent results. The Registry was established to enhance the rigor of the available cohort studies (11) and other analyses of reproductive safety data derived from large administrative databases (10). The effort is particularly timely given the recent revision by the Food and Drug Administration (FDA) of the long-standing system by which reproductive safety data were conveyed in product labels, the so-called pregnancy category (28).
This study provides current data regarding the primary outcome of the Registry: major malformations. Based on the 214 cases of first-trimester exposure to second-generation antipsychotics, it is reasonable to conclude that these agents as a class are not major teratogens. Although the confidence intervals around the odds ratio estimate remain very wide, with the probability for change over the course of the study, it is unlikely that the risk will rise to that of major teratogens such as valproate or thalidomide (29, 30).
These findings are consistent with some (11, 15) but not all studies regarding the reproductive safety of second-generation antipsychotics (10, 13, 17). The Australian NRAMP found a higher risk of congenital malformations (6%) among the 130 infants exposed to first- and second-generation antipsychotics compared with the expected rate in Australia (3.1%), particularly in congenital heart anomalies (17). A large prospective observational cohort study conducted by the Institute for Clinical Teratology and Drug Risk Assessment in Pregnancy in Germany found that the odds of a major malformation were about twofold greater, a significant difference, among those exposed to second-generation antipsychotics (5.1%) compared with an unexposed control group (adjusted odds ratio=2.17, 95% CI=1.20–3.91) (10). In the group exposed to second-generation antipsychotics, the most common major malformations were cardiovascular, and most (eight of 12) were atrial or ventricular septal defects. In another prospective cohort study, from the Motherisk program in Toronto, investigators assessed pregnancy outcomes in 133 women exposed to second-generation antipsychotics and other psychotropics and 133 matched unexposed comparison women (13). The risk of major malformations reported in the exposed group was approximately two and a half times higher than in the unexposed group (6.2% versus 2.6%), although this was not statistically significant. The types of malformations observed also included cardiovascular malformations (atrial septal defects), Chiari malformation, small bowel atresia, and hypospadias. While these findings suggest a possible increase in risk for major malformations above the baseline risk, these preliminary data need to be interpreted cautiously given the methodological differences among these studies. Moreover, cardiac septal defects are among the most common congenital malformations, and these findings could be due to a detection bias, as women exposed to a medication for which there are sparse reproductive safety data may be more likely to receive prenatal and postnatal diagnostic testing (10). Whether there are associations between first-trimester exposure to second-generation antipsychotics and specific malformations, such as cardiovascular malformations, will remain unclear until larger samples of both exposed and unexposed infants have been analyzed.
Strengths of the Registry include a rigorous prospective design, precise confirmation of outcomes, and the selection of appropriate comparison subjects. The rigor demonstrated by the Registry is lacking in some of the other available cohort studies (10, 13, 17). First, the inclusion of an internal comparison group consisting of women with psychiatric illnesses who are not exposed to second-generation antipsychotics limits confounding by indication, which is so often a problem in other studies using healthy comparison groups unaffected by a comparable underlying condition (10, 13). By using a psychiatric comparison group, behaviors associated with psychiatric illness and major malformations, such as substance use and illness severity, are represented in both the exposed and unexposed groups, limiting confounding. The most critical strength of the current study is the use of both medical records and participant interviews to provide in-depth information—often not included in other studies—to identify cases of malformations. The addition of medical record review after telephone interviews with subjects not only allows the research team to obtain more accurate information regarding the presence of the primary outcome (major malformations), but also allows for in-depth contextual information that is typically unavailable in medical records, such as lifestyle and demographic factors and detailed weekly medication use patterns. This method allows for an accurate picture of pharmacotherapy throughout pregnancy and information on factors that could confound the relationship between first-trimester exposure to second-generation antipsychotics and major malformations.
This study also has notable limitations. The extent to which results are generalizable to the larger population of women taking second-generation antipsychotics is unknown because the majority of women included in this analysis were white, married, college educated, and motivated to participate in a prospective, longitudinal study. The size of the Registry at present is an obvious limitation for the assessment of specific types of malformations. While the overall risk estimates of congenital malformations are generally reassuring and are less than the background risk noted by the Centers for Disease Control and Prevention of approximately 2%–2.5%, pregnancy registries typically evaluate the frequency of all malformations (31). The data available do not allow for estimates of specific malformations (e.g., cleft lip/palate, neural tube defects). To establish correlations, such as a threefold increase in the occurrence of a malformation with a frequency of 1 in 1,000 (like spina bifida or cleft lip), more than 2,000 exposed infants would need to be enrolled to establish this correlation with 80% power (16). In addition, with only four identified major malformations, the statistical power to identify risk factors is low, as illustrated by the wide confidence interval around the odds ratio. As a result, we were unable to adjust for potential factors that could be driving the relationship between first-trimester second-generation antipsychotic use and major malformations.
The sensitivity analysis examining confounding provides some information about the direction of bias, taking polytherapy into account. Based on this analysis, a number of factors may result in confounding and lead to a biased odds ratio estimate. According to our analysis and previous studies regarding risk factors related to both second-generation antipsychotic use and major malformations, potential confounders include diagnosis and severity of illness; whether the pregnancy was planned; maternal age; health and lifestyle indicators, such as BMI; and first-trimester use of other psychotherapeutic drugs, prenatal vitamins, alcohol, and cigarettes (32–36). These factors are related to both second-generation antipsychotic use and malformations in complex ways that make it difficult to fully understand the direction and magnitude of the bias without an adequate sample to examine these relationships. However, our sensitivity analyses indicate an upward bias in our results because most of the tested potential confounders attenuated the effect estimate, suggesting that the true relationship between second-generation antipsychotic use and major malformations is somewhere closer to the null. Having a serious mental illness (e.g., bipolar disorder, treatment-resistant depression) leads to the utilization of second-generation antipsychotics and other psychotropic medications and may also increase the likelihood of behaviors and exposures that increase the risk of poor pregnancy outcomes. This type of analysis is critical, as shown recently with respect to risk of selective serotonergic antidepressant exposure, where the observed risk estimate is attenuated after the contribution of underlying psychiatric morbidity is factored into the model (37).
In summary, our results suggest that the use of a second-generation antipsychotic during the first trimester does not substantively increase the risk of major malformations. Past findings have demonstrated that pregnancy does not protect against new or worsening illness in patients with psychiatric disorders and that the discontinuation of ongoing maintenance treatment in women with severe mood and psychotic disorders appears to carry a high risk of illness recurrence during pregnancy (38, 39). Therefore, the present findings challenge the frequently observed clinical practice of abruptly stopping maintenance treatment for psychiatric disorders during pregnancy. A major clinical implication of these findings is that for women with substantial psychiatric morbidity and good response to a second-generation antipsychotic, maintenance treatment with a second-generation antipsychotic during pregnancy may be the most prudent treatment option, similar to recommendations for continued treatment for pregnant women with other serious and chronic medical conditions, such as epilepsy (40).
Treatment decisions regarding discontinuation or maintenance of treatment with second-generation antipsychotics during pregnancy are complex, and clinicians and patients must balance the potential risks of pharmacologic treatment with the risks of untreated psychiatric illness. Quantification of this risk-benefit decision is optimized by using the best available data gathered in the most rigorous fashion. Given recent FDA guidance regarding the importance of pregnancy registries, carefully collected data may help clinicians and patients make informed choices about treatment, taking into account patient wishes, clinical history, and carefully obtained safety data.
1 : Prevalence of atypical antipsychotic use in psychiatric outpatients: comparison of women of childbearing age with men. Arch Women Ment Health 2014; 17:583–586Crossref, Medline, Google Scholar
2 : Prevalence and trends in the use of antipsychotic medications during pregnancy in the U.S., 2001-2007: a population-based study of 585,615 deliveries. Arch Women Ment Health 2013; 16:149–157Crossref, Medline, Google Scholar
3 : Increasing off-label use of antipsychotic medications in the United States, 1995-2008. Pharmacoepidemiol Drug Saf 2011; 20:177–184Crossref, Medline, Google Scholar
4 : Fetal antipsychotic exposure in a changing landscape: seeing the future. Br J Psychiatry 2013; 202:321–323Crossref, Medline, Google Scholar
5 : Evaluating the postmarketing experience of risperidone use during pregnancy: pregnancy and neonatal outcomes. Drug Saf 2007; 30:247–264Crossref, Medline, Google Scholar
6 : Use and safety of antipsychotic drugs during pregnancy. J Psychiatr Pract 2009; 15:183–192Crossref, Medline, Google Scholar
7 : The reproductive safety profile of mood stabilizers, atypical antipsychotics, and broad-spectrum psychotropics. J Clin Psychiatry 2002; 63(Suppl 4):42–55Medline, Google Scholar
8 : Clinical utilization of atypical antipsychotics in pregnancy and lactation. Ann Pharmacother 2004; 38:1265–1271Crossref, Medline, Google Scholar
9 : Olanzapine-exposed pregnancies and lactation: early experience. J Clin Psychopharmacol 2000; 20:399–403Crossref, Medline, Google Scholar
10 : Atypical antipsychotic drugs and pregnancy outcome: a prospective, cohort study. J Clin Psychopharmacol 2013; 33:453–462Crossref, Medline, Google Scholar
11 : Pregnancy outcome of women using atypical antipsychotic drugs: a prospective comparative study. J Clin Psychiatry 2005; 66:444–449Crossref, Medline, Google Scholar
12 : Maternal use of antipsychotics in early pregnancy and delivery outcome. J Clin Psychopharmacol 2008; 28:279–288Crossref, Medline, Google Scholar
13 : Pregnancy outcomes following maternal exposure to second-generation antipsychotics given with other psychotropic drugs: a cohort study. BMJ Open 2013; 3:e003062Crossref, Medline, Google Scholar
14 : Atypical antipsychotics in the treatment of schizophrenia during pregnancy and the postpartum. Am J Psychiatry 2006; 163:2064–2070Link, Google Scholar
15 : Pregnancy exposure to olanzapine, quetiapine, risperidone, aripiprazole and risk of congenital malformations. A systematic review. Basic Clin Pharmacol Toxicol 2015; 116:315–320Crossref, Medline, Google Scholar
16 : The AED (antiepileptic drug) pregnancy registry: a 6-year experience. Arch Neurol 2004; 61:673–678Crossref, Medline, Google Scholar
17 : A prospective cohort study of antipsychotic medications in pregnancy: the first 147 pregnancies and 100 one year old babies. PLoS One 2014; 9:e94788Crossref, Medline, Google Scholar
18 : Pregnancy registries: differences, similarities, and possible harmonization. Epilepsia 2010; 51:909–915Crossref, Medline, Google Scholar
19 : Bupropion in pregnancy and the prevalence of congenital malformations. Pharmacoepidemiol Drug Saf 2007; 16:474–484Crossref, Medline, Google Scholar
20 : Birth outcomes in pregnant women taking fluoxetine. N Engl J Med 1996; 335:1010–1015Crossref, Medline, Google Scholar
21 : Lithium and pregnancy. II. Hazards to women given lithium during pregnancy and delivery. BMJ 1973; 2:137–138Crossref, Medline, Google Scholar
22 : Preliminary findings from the National Register of Antipsychotic Medication in Pregnancy. Aust N Z J Psychiatry 2008; 42:38–44Crossref, Medline, Google Scholar
23 : North American antiepileptic drug pregnancy registry. Epilepsia 2004; 45:1465Crossref, Medline, Google Scholar
24 : Establishment of the National Pregnancy Registry for Atypical Antipsychotics. J Clin Psychiatry 2015; 76:986–989Crossref, Medline, Google Scholar
25 Cohen LS, Viguera AC, Masnik S, et al: The National Pregnancy Registry for Atypical Antipsychotics: Effects of Fetal Exposure on Risk for Major Malformations, in 53rd Annual Meeting of the Teratology Society. Tucson, Ariz, Jun 22–26, 2013Google Scholar
26 Cohen LS, Viguera AC, McInerney KA, et al: The National Pregnancy Registry for Atypical Antipsychotics: Effects of Fetal Exposure on Risk for Major Malformations and Extrapyramidal Symptoms, in American College of Neuropsychopharmacology 53rd Annual Meeting. Phoenix, Dec 7–11, 2014Google Scholar
27 : Need for inclusion and exclusion criteria for the structural abnormalities recorded in children born from exposed pregnancies. Teratology 1999; 59:1–2Crossref, Medline, Google Scholar
28 : Content and format of labeling for human prescription drug and biological products; requirements for pregnancy and lactation labeling. Final rule. Fed Regist 2014; 79:72063–72103Medline, Google Scholar
29 : Thalidomide and congenital abnormalities. Lancet 1961; 2:1358Crossref, Google Scholar
30 : Increased rate of major malformations in offspring exposed to valproate during pregnancy. Neurology 2005; 64:961–965Crossref, Medline, Google Scholar
31 : Annual summary of vital statistics: 2004. Pediatrics 2006; 117:168–183Crossref, Medline, Google Scholar
32 : Prepregnant weight in relation to risk of neural tube defects. JAMA 1996; 275:1089–1092Crossref, Medline, Google Scholar
33 : Periconceptional folic acid exposure and risk of occurrent neural tube defects. JAMA 1993; 269:1257–1261Crossref, Medline, Google Scholar
34 : Congenital malformations and maternal smoking during pregnancy. Teratology 1986; 34:65–71Crossref, Medline, Google Scholar
35 : Maternal age, obstetric complications, and the outcome of pregnancy. Obstet Gynecol 1983; 61:210–216Medline, Google Scholar
36 : The fetal alcohol syndrome. N Engl J Med 1978; 298:1063–1067Crossref, Medline, Google Scholar
37 : Antidepressant use in pregnancy and the risk of cardiac defects. N Engl J Med 2014; 370:2397–2407Crossref, Medline, Google Scholar
38 : Risk of recurrence in women with bipolar disorder during pregnancy: prospective study of mood stabilizer discontinuation. Am J Psychiatry 2007; 164:1817–1824Link, Google Scholar
39 : Relapse of major depression during pregnancy in women who maintain or discontinue antidepressant treatment. JAMA 2006; 295:499–507Crossref, Medline, Google Scholar
40 : Practice parameter update: management issues for women with epilepsy--focus on pregnancy (an evidence-based review): teratogenesis and perinatal outcomes: report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and American Epilepsy Society. Neurology 2009; 73:133–141Crossref, Medline, Google Scholar



