Citalopram, Methylphenidate, or Their Combination in Geriatric Depression: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
Objective:
The authors evaluated the potential of methylphenidate to improve antidepressant response to citalopram, as assessed by clinical and cognitive outcomes, in elderly depressed patients.
Method:
The authors conducted a 16-week randomized double-blind placebo-controlled trial for geriatric depression in 143 older outpatients diagnosed with major depression comparing treatment response in three treatment groups: methylphenidate plus placebo (N=48), citalopram plus placebo (N=48), and citalopram plus methylphenidate (N=47). The primary outcome measure was change in depression severity. Remission was defined as a score of 6 or less on the Hamilton Depression Rating Scale. Secondary outcomes included measures of anxiety, apathy, quality of life, and cognition.
Results:
Daily doses ranged from 20 mg to 60 mg for citalopram (mean=32 mg) and from 5 mg to 40 mg for methylphenidate (mean=16 mg). All groups showed significant improvement in depression severity and in cognitive performance. However, the improvement in depression severity and the Clinical Global Impressions improvement score was more prominent in the citalopram plus methylphenidate group compared with the other two groups. Additionally, the rate of improvement in the citalopram plus methylphenidate group was significantly higher than that in the citalopram plus placebo group in the first 4 weeks of the trial. The groups did not differ in cognitive improvement or number of side effects.
Conclusions:
Combined treatment with citalopram and methylphenidate demonstrated an enhanced clinical response profile in mood and well-being, as well as a higher rate of remission, compared with either drug alone. All treatments led to an improvement in cognitive functioning, although augmentation with methylphenidate did not offer additional benefits.
Despite progress in antidepressant therapies, a considerable number of depressed elderly patients either develop a chronic course or relapse frequently after periods of improvement (1, 2). Elderly patients appear to have a less robust response than younger adults, with a higher rate of recurrence, lower effect sizes, and lower remission rates (around 30%) in response to first-line antidepressant treatment (1–6).
Cognitive impairment in late-life depression is common and is associated with frontostriatal systems dysfunction, inadequate treatment response (7, 8), functional impairment in instrumental activities of daily living (9), and increased risk of conversion to dementia (10). Cognitive deficits often persist despite successful treatment of depression (11–15).
Methylphenidate is a dopamine reuptake inhibitor, and as monotherapy it has been shown to be effective and safe in a few open and controlled studies in elderly patients (16–21). The use of dopaminergic agents like methylphenidate in geriatric depression could be important, given that dopamine neurotransmission diminishes with aging (16–18). Methylphenidate has demonstrated efficacy in executive dysfunction targets, including attention deficits, apathy, and withdrawal (16–18).
To our knowledge, this is the first randomized trial to assess the use of methylphenidate to improve antidepressant response in geriatric depression. We compared the clinical efficacy and safety of three treatments representing three different mechanisms of action with respect to dopaminergic function: methylphenidate and placebo (mostly dopaminergic), citalopram and placebo (mostly serotonergic), and methylphenidate and citalopram (mixed). Based on our preliminary observations, we hypothesized that the combined use of citalopram and methylphenidate would lead to faster and improved antidepressant response, with improvements in mood, function, and cognition.
Method
Participants
From August 2008 to September 2012, 510 individuals were screened by telephone, of whom 203 were invited for a diagnostic interview. The details of the study were described to potential participants, and written informed consent was obtained in accordance with the procedures set by the UCLA Institutional Review Board.
Inclusion criteria included a current episode of unipolar major depressive disorder according to DSM-IV-TR criteria; a score ≥16 on the 24-item Hamilton Depression Rating Scale (HAM-D) (22); and a score ≥26 on the Mini-Mental State Examination (MMSE) (23). Exclusion criteria were a history of any psychiatric disorder other than unipolar major depressive disorder with or without comorbid anxiety symptoms; severe or acute unstable medical illness, including the presence of either atrial or ventricular arrhythmia or acute ischemic features on baseline ECG; acute suicidal or violent behavior or history of suicide attempt within the past year; or any other CNS diseases. Patients had been free of psychotropic medications for at least 2 weeks before starting the trial.
Primary Outcome Measure
The primary outcome measure was improvement in residual depressive symptoms in the three treatment groups over time, using continuous HAM-D scores. We also analyzed the differences in the rate of response by week 4 of treatment to characterize the rate of response based on the methylphenidate titration schedule that ended at week 4.
Secondary Outcome Measures
We measured comorbid symptoms of anxiety, apathy, medical and vascular risk factors, health-related quality of life, and cognitive performance. Remission was defined as a score ≤6 on the HAM-D and was analyzed as a secondary outcome.
Randomization
Randomization was performed using a computer-generated schedule. Because there were three groups, we used block randomization to maintain balance over the course of the study with a random mix of block lengths of three and six to help preserve the blind. Allocation concealment was implemented using sealed, sequentially numbered boxes that were identical in appearance for the three treatment groups. In order to monitor the internal validity of the randomization and blinding in the trial, we created a guessing scale for the study staff in the first year of the trial (as we did in our pilot studies); the accuracy of our guessing for group assignment in two independent trials was 35%.
Intervention Procedures
Participants were seen in-person weekly for the first 4 weeks, while the methylphenidate dosage was titrated, for evaluation of safety and detection of accelerated response, and every 2 weeks thereafter for the remainder of the 16-week study. Treatment with both drugs was initiated simultaneously after the baseline assessment in order to track accelerated response. Participants were given a weekly supply of their assigned study medications, which were prepared and dispensed by the UCLA Pharmacy in matching capsules: 20 mg/day of citalopram and 2.5 mg (or 1 capsule) of methylphenidate twice a day (with recommended dosing at 9 a.m. and 3 p.m.), or the matching number of capsules of placebo as a starting dose. We used flexible dosing of methylphenidate, ranging from 5 mg/day to 40 mg/day, based on the response and tolerability assessment at each weekly visit during the first 4 weeks of treatment. The dosage range was established in two of our pilot studies that were dedicated to the dose-finding and safety evaluation of the optimal methylphenidate dosage in older adults (18, 19). The methylphenidate dosage was increased at each visit if the patient had a Clinical Global Impressions Scale (CGI) (24) improvement score ≥3 and had experienced no serious adverse effects. The dosage was increased by 2.5 mg twice a day every 4 days between days 4 and 28 of treatment or until the patient had a CGI improvement score of 1 or 2. After day 28 of methylphenidate titration, patients remained on the same dosage through the end of the trial.
For patients in the citalopram groups who showed minimal improvement, with a CGI improvement score ≥3 by day 28 of treatment, the citalopram dosage was increased to 40 mg/day and continued to the end of the trial. That was the case for a majority of participants; for 13 patients, the citalopram dosage was increased to 60 mg/day at weeks 7–8 because of insufficient response.
Methylphenidate dosage could be decreased by two capsules, to a minimum of 5 mg/day, and citalopram dosage could be decreased to 20 mg/day. Participants who could not tolerate the minimum allowed dosage were discontinued from the trial. The use of concomitant rescue medications during the treatment trial was restricted to the use of lorazepam for anxiety (up to 1 mg/day).
Assessments
Mood, medical, and quality of life assessments.
Participants were evaluated with the HAM-D, the Montgomery-Åsberg Depression Rating Scale (25), and the CGI to measure depression severity and change over time. Measures of comorbid psychiatric symptoms that might be affected by the use of methylphenidate included the Hamilton Anxiety Rating Scale (26) and the Apathy Evaluation Scale (27). Medical comorbidity was assessed with the Cerebrovascular Risk Factor Prediction Chart (28) and the Cumulative Illness Rating Scale–Geriatrics (29). Health-related quality of life was assessed with the Medical Outcomes Study Short Form 36-Item Health Survey (SF-36) (30). The HAM-D was administered at all visits. The other clinical measures were administered at baseline and at the end of the study by two raters (H.L. and N.S.C.).
Cognitive assessments.
A comprehensive neuropsychological test battery (31) was administered at baseline and endpoint to assess five cognitive domains: memory (using the California Verbal Learning Test–II [long delayed free recall] and the Rey-Osterrieth Complex Figure Test [30-minute delayed recall]), language (using the Boston Naming Test, the FAS Verbal Fluency Task, and the animal naming test), attention/processing speed (using the WAIS-III digit span task, the Trail Making Test, part A, the Stroop Color Trial [Golden version]), executive functioning (using the Trail Making Test, part B, and the Stroop interference task [Golden version]), and visuospatial functioning (using the WAIS-III block design task and the Rey-Osterrieth Complex Figure Test [copy condition]).
We transformed raw scores to z-scores for each test score of interest for each participant. Z-scores were calculated from published normative data (31–33). For variables in which good performance was represented by lower values (e.g., the Trail Making Test), z-scores were reversed so that high z-scores represented good performance for all measures. These z-scores were averaged within each neuropsychological domain to produce composite scores and then averaged over all tests to calculate a global performance score. The Cronbach alpha coefficients for the five domains were as follows: memory, 0.87; language, 0.86; attention/processing speed, 0.82; executive functioning, 0.76; and visuospatial functioning, 0.89.
Safety and adherence assessments.
Vital signs and weight were measured at baseline and at each visit. A 12-lead ECG was performed at baseline and, if any cardiac complaints were present, at weeks 3 and 16. A physical examination was conducted at baseline and at week 16 or on early termination. Side effects were assessed at all visits by the UKU Side Effect Rating Scale (34). Treatment compliance was assessed by indirect measures of adherence, including questioning of the patients, returned pill count, and drug level measures at weeks 3, 8, and 16. Plasma levels of citalopram and its metabolites, as well as methylphenidate and ritalinic acid levels, were obtained. We explored the relationship between measures of adherence and outcomes.
Statistical Analysis
All data were entered into the database at the time of their collection and analyzed after completion of the trial. Safety analyses were performed using descriptive statistics and frequency distribution of dropouts. Patients in the three treatment groups were compared (using analyses of variance for continuous variables and chi-square tests for categorical variables) on all demographic and clinical measures at baseline to assess the success of the randomization procedures. All outcome results used intent-to-treat analyses. Mean longitudinal trajectories of HAM-D scores for patients who dropped out did not differ significantly from those of patients who completed the study. Hence, continuous HAM-D scores were analyzed using a mixed-effects general linear model, as implemented in PROC MIXED in SAS (SAS Institute, Cary, N.C.), under the missing-at-random assumption. Two mixed modeling approaches were used in modeling longitudinal HAM-D scores in the three groups. The first approach made less stringent assumptions on the shape of the HAM-D trajectories across time and included treatment group as the between-subject factor, time as the within-subject factor, and the interaction term between time and treatment group. Based on the shape of the HAM-D trajectories from the first analysis, the second mixed model included time as a continuous variable to target rates of change in HAM-D scores directly, employing a broken-line model, with different slopes in the three groups, from baseline to 4 weeks and from 4 weeks to end of study. Analysis included testing whether groups were significantly different in the slopes of these two linear segments (from 0–4 weeks and 4–16 weeks). In addition to the analyses of HAM-D scores, the proportion of participants who achieved remission was analyzed, using a chi-square test.
The secondary outcome measures were also analyzed using mixed-effects models, with group, time, and the group-by-time interaction as predictors. Post hoc analyses determined the significance of specific pairwise group differences and within-group changes. The significance threshold for the primary outcome measure was set at 0.05 (two-tailed).
Results
Figure 1 presents the CONSORT flow diagram for the study. After telephone screening and in-person diagnostic interviews, we enrolled 181 individuals in the study. Of these, 38 dropped out before randomization, so 143 were included in the randomization process and assigned to treatment groups (the intent-to-treat sample).
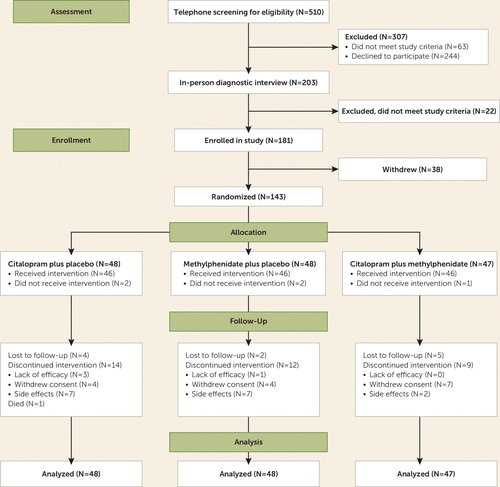
FIGURE 1. CONSORT Flow Diagram of Participants in a Randomized Placebo-Controlled Trial of Citalopram, Methylphenidate, or Their Combination in Geriatric Depression
Table 1 presents the baseline demographic and clinical characteristics of the three treatment groups. The average age of the study sample was 69.7 years (SD=7.3). The mean baseline score on the HAM-D was 18.9 (SD=2.9), and the mean MMSE score was 28.7 (SD=1.3). Fifty-nine participants (41.3%) met the criteria for treatment resistance, having had two adequate trials with antidepressants of two different classes; there were no group differences in proportion with treatment resistance. The proportion of women differed significantly between groups, as did baseline scores on the HAM-D and the Cumulative Illness Rating Scale–Geriatrics. We therefore controlled for these variables in the subsequent analyses.
| Variable | Citalopram Plus Placebo (N=48) | Methylphenidate Plus Placebo (N=48) | Citalopram Plus Methylphenidate (N=47) | p | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Female | 31 | 64.5 | 19 | 39.6 | 28 | 59.6 | 0.05 |
| Race/ethnicity | 0.9 | ||||||
| Caucasian | 36 | 75.0 | 35 | 72.9 | 37 | 78.7 | |
| African American | 5 | 10.4 | 5 | 10.4 | 5 | 10.6 | |
| Asian | 1 | 2.1 | 2 | 4.2 | 3 | 6.4 | |
| Hispanic | 6 | 12.5 | 5 | 10.4 | 3 | 6.4 | |
| Chronic course (>24 months) | 41 | 85.4 | 37 | 77.1 | 37 | 78.7 | 0.6 |
| Mean | SD | Mean | SD | Mean | SD | ||
| Age (years) | 70.1 | 7.1 | 70.0 | 7.1 | 68.9 | 7.6 | 0.7 |
| Education (years) | 15.8 | 2.7 | 16.0 | 2.4 | 15.2 | 3.0 | 0.3 |
| Age at depression onset (years) | 42.7 | 23.5 | 40.4 | 25.1 | 45.6 | 22.3 | 0.6 |
| Number of episodes | 3.8 | 3.8 | 4.0 | 5.4 | 3.3 | 2.9 | 0.7 |
| Duration of episode (months) | 51.6 | 45.6 | 50.2 | 47.2 | 38.6 | 31.8 | 0.3 |
| Hamilton Depression Rating Scale (24-item) | 18.3 | 2.3 | 19.8 | 3.6 | 18.7 | 2.9 | 0.04 |
| Montgomery-Åsberg Depression Rating Scale | 18.6 | 3.1 | 18.2 | 4.1 | 17.5 | 3.4 | 0.3 |
| Apathy Evaluation Scale | 31.3 | 8.6 | 31.0 | 10.6 | 30.0 | 10.1 | 0.8 |
| Hamilton Anxiety Scale | 8.9 | 2.4 | 9.9 | 3.2 | 8.7 | 2.9 | 0.07 |
| Short Form 36-Item Health Survey | |||||||
| Energy | 30.9 | 18.8 | 33.3 | 20.0 | 31.3 | 20.3 | 0.8 |
| Well-being | 44.2 | 17.6 | 47.3 | 15.6 | 45.6 | 17.2 | 0.7 |
| Role-emotional | 34.0 | 27.9 | 37.6 | 28.3 | 31.2 | 26.1 | 0.5 |
| Cumulative Illness Rating Scale–Geriatrics | 5.8 | 4.6 | 5.5 | 3.5 | 4.0 | 3.2 | 0.04 |
| Cerebrovascular Risk Factor Prediction Chart | 12.2 | 5.8 | 10.9 | 5.0 | 9.7 | 4.5 | 0.06 |
TABLE 1. Baseline Clinical and Demographic Characteristics of Older Patients Receiving Citalopram, Methylphenidate, or Their Combination for Depression
The use of lorazepam as a rescue drug was minimal, with doses of 0.5–1 mg given to 12 participants (four in the citalopram plus placebo group, six in the methylphenidate plus placebo group, and two in citalopram plus methylphenidate group). There were no significant differences between groups in use of lorazepam, and controlling for it did not affect the results.
The treatment groups did not differ significantly in mean dosages of citalopram or methylphenidate (citalopram plus placebo group: citalopram, 35.0 mg/day [SD=14.6]; methylphenidate plus placebo group: methylphenidate, 16.4 mg/day [SD=7.2]; citalopram plus methylphenidate group: citalopram, 32.3 mg/day [SD=13.5], methylphenidate, 16.2 mg/day [SD=8.1]).
Across groups, citalopram dosage was significantly associated with proportion of participants who achieved remission. Remission rates were 29.8% among participants receiving no citalopram (the methylphenidate plus placebo group), 41.9% among those receiving 20 mg/day of citalopram, 56.4% among those receiving 40 mg/day, and 69.2% among those receiving 60 mg/day (χ2=9.70, df=3, p=0.02). However, no clear relationship was observed between methylphenidate dosage and remission, with remission rates of 41.7% for those receiving no methylphenidate (the citalopram plus placebo group), 24.2% for those receiving 5–10 mg/day, 58.3% for those receiving 15–20 mg/day, and 53.9% for those receiving 20 mg/day (χ2=9.82, df=3, p=0.02).
Analyses of Change in HAM-D Score Over Time
Results of the mixed model, using time as a categorical effect and adjusting for gender and baseline medical burden, indicated a significant difference between groups in change in HAM-D score from baseline to study end (F=2.5, df=20, 137, p<0.001). Post hoc analyses revealed that the change in HAM-D score was significantly greater in the citalopram plus methylphenidate group compared with both the citalopram plus placebo group (t=2.4, df=137, p=0.02) and the methylphenidate plus placebo group (t=2.8, df=137, p=0.005). Adjustment for the measures of adherence, serum drug levels, and medical burden did not affect the results.
Rate of change in HAM-D response.
As shown in Figure 2, there were two distinct rates of change in the HAM-D trajectories over time, from baseline to week 4 and from week 4 to week 16. Hence, in order to target the rate of change in treatment response directly, we used a broken-line mixed-effects model with separate slopes for the three groups from baseline to week 4 and then from week 4 to week 16. From baseline to week 4, the citalopram plus methylphenidate group exhibited a significantly faster decrease in mean HAM-D score compared with the citalopram plus placebo group (slope difference=0.54, SE=0.24; t=2.21, df=125, p=0.03), but not compared with the methylphenidate plus placebo group (slope difference=0.07, SE=0.24; n.s.); the difference between the citalopram plus placebo and methylphenidate plus placebo groups fell short of significance (slope difference=0.46, SE=0.24; t=1.9, df=125, p=0.06). After week 4, the citalopram plus methylphenidate group showed a significantly faster decrease in mean HAM-D score compared with the methylphenidate plus placebo group (slope difference=0.19, SE=0.09; t=2.11, df=105, p=0.04), but not compared with the citalopram plus placebo group (slope difference=−0.01, SE=0.09; n.s.). The mean HAM-D score decreased significantly faster in the citalopram plus placebo group compared with the methylphenidate plus placebo group after week 4 as well (slope difference=0.20, SE=0.09; t=2.23, df=105, p=0.03). The predicted values of HAM-D scores obtained using this model are plotted in Figure S1 in the data supplement that accompanies the online edition of this article.
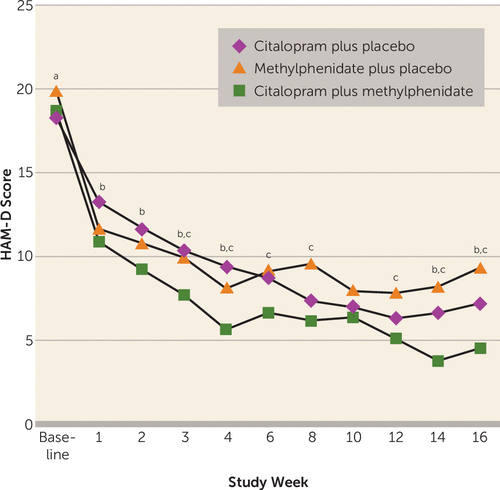
FIGURE 2. Change in 24-Item Hamilton Depression Rating Scale (HAM-D) Score Over Time, by Treatment Condition, Among Patients Receiving Citalopram, Methylphenidate, or Their Combination
a Statistically significant difference between citalopram plus placebo and methylphenidate plus placebo, p<0.05.
b Statistically significant difference between citalopram plus placebo and citalopram plus methylphenidate, p<0.05.
c Statistically significant difference between methylphenidate plus placebo and citalopram plus methylphenidate, p<0.05.
Secondary remitter analyses.
Twenty of 48 participants (41.7%) in the citalopram plus placebo group, 14 of 48 (29.2%) in the methylphenidate plus placebo group, and 29 of 47 (61.7%) in the citalopram plus methylphenidate group met the remission criterion (HAM-D score ≤6) at study end. These differences were significant (χ2=9.2, df=2, p=0.01) and were driven mostly by the differences in the remission rates between the citalopram plus methylphenidate and methylphenidate plus placebo groups (χ2=9.0, df=1, p=0.003), while the difference between the citalopram plus methylphenidate and citalopram plus placebo groups fell short of significance (χ2=3.4, df=1, p=0.07). Note that group differences in remission rates were not found significant at 12 weeks or 14 weeks. Because of high dropout rates, significant differences at 16 weeks must be interpreted with caution.
The analyses of group differences by remission status, partial response status, and nonresponse status also demonstrated significant differences favoring citalopram plus methylphenidate (χ2=9.9, df=4, p=0.04). In the citalopram plus placebo group, 20 participants remitted, 15 had a partial response, and 13 did not respond; in the methylphenidate plus placebo group, 14 remitted, 18 had a partial response, and 15 did not respond; and in the citalopram plus methylphenidate group, 29 remitted, eight had a partial response, and 11 did not respond. Relatively few participants achieved accelerated remission after 4 weeks of treatment, with no significant difference between groups (eight participants [17%] in the citalopram plus placebo group, seven [15%] in the methylphenidate plus placebo group, and 10 [21%] in the citalopram plus methylphenidate group).
Secondary Outcomes
Clinical global improvement.
When we combined participants with CGI improvement scores of 1 or 2 (very much and much improved, compared with those with minimal improvement or no change, or minimally worse), 27/32 (84.4%) in the citalopram plus methylphenidate group improved much or very much compared with either of the monotherapy groups (methylphenidate plus placebo, 13/33 [39.4%]; citalopram plus placebo, 17/30 [56.7%]; χ2=13.9, df=2, p=0.001); no significant difference was observed between the two monotherapy groups.
Analyses of change in secondary outcomes over time.
Changes in scores on the well-being subscale of the SF-36 also showed significant between-group differences favoring the citalopram plus methylphenidate group (F=4.8, df=2, 136, p=0.01) (Table 2). Changes in the anxiety, apathy, and psychological resilience measures did not differ between groups.
| Citalopram Plus Placebo (N=48) | Methylphenidate Plus Placebo (N=48) | Citalopram Plus Methylphenidate (N=47) | |||||
|---|---|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | Mean | SD | p |
| Hamilton Anxiety Rating Scale | –4.4 | 0.6 | –5.4 | 0.5 | –6.2 | 0.6 | 0.1 |
| Apathy Evaluation Scale | 7.1 | 1.5 | 7.1 | 1.4 | 9.1 | 1.4 | 0.5 |
| Clinical Global Impressions improvement scale | –1.4 | 0.2 | –0.8 | 0.2 | –1.4 | 0.2 | 0.001 |
| Short Form 36-Item Health Survey | |||||||
| Energy | 17.1 | 4.1 | 18.2 | 3.9 | 24.1 | 3.9 | 0.4 |
| Well-being | 25.0 | 3.6 | 13.9 | 3.5 | 28.5 | 3.5 | 0.01 |
| Role-emotional | 28.8 | 6.7 | 26.3 | 6.4 | 42.6 | 6.4 | 0.2 |
TABLE 2. Change Scores for Older Patients Receiving Citalopram, Methylphenidate, or Their Combination for Depressiona
Table S1 in the online data supplement presents estimated effect sizes for the selected measures over time.
Cognitive outcomes.
There were no significant differences between groups in baseline neuropsychological performance. Table 3 presents baseline-to-endpoint change scores (using z-scores) for each composite domain and for the global performance score.
| Citalopram Plus Placebo (N=48) | Methylphenidate Plus Placebo (N=48) | Citalopram Plus Methylphenidate (N=47) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Raw Score Change by Test | Z-Score Change | SE | Raw Score Change by Test | Z-Score Change | SE | Raw Score Change by Test | Z-Score Change | SE | p |
| Attention/processing speed | 0.24 | 0.10 | –0.006 | 0.09 | 0.05 | 0.09 | 0.2 | |||
| WAIS-III digit span subtest | 2.07 | 0.25 | 0.37 | |||||||
| Trail Making Test, part A (seconds)b | –6.25 | –1.78 | –0.36 | |||||||
| Stroop Color Trial | 3.82 | 0.52 | –0.4 | |||||||
| Language | 0.26 | 0.09 | 0.23 | 0.09 | 0.27 | 0.08 | 0.9 | |||
| FAS test | 3.5 | 4.09 | 3.48 | |||||||
| Animal naming test | 1.29 | –0.47 | 0.73 | |||||||
| Boston naming test | 3.2 | 1.84 | 1.36 | |||||||
| Executive functioning | 0.13 | 0.12 | 0.28 | 0.12 | –0.02 | 0.11 | 0.2 | |||
| Trail Making Test, part B (seconds)b | –21.71 | –8.98 | 8.0 | |||||||
| Stroop Color-Word Trial | 2.55 | 0.82 | –0.19 | |||||||
| Visuospatial functioning | –0.10 | 0.11 | 0.01 | 0.11 | 0.16 | 0.10 | 0.2 | |||
| WAIS-III block design subtest | 1.56 | 2.55 | –0.1 | |||||||
| Rey-Osterrieth Complex Figure Test, copy condition | 0.05 | –0.41 | 0.71 | |||||||
| Memory | –0.03 | 0.10 | 0.12 | 0.09 | –0.05 | 0.09 | 0.4 | |||
| California Verbal Learning Test–II, long-delay free recall | 0.31 | 0.11 | –0.39 | |||||||
| Rey-Osterrieth Complex Figure Test, 30-minute delayed recall | 3.09 | 2.34 | 1.28 | |||||||
| Global performance score | 0.10 | 0.05 | 0.15 | 0.05 | 0.10 | 0.05 | 0.7 | |||
TABLE 3. Between-Treatment Comparison of Cognitive Z-Score Change by Composite Domain, and Raw Score Change by Neuropsychological Test, Among Older Patients Receiving Citalopram, Methylphenidate, or Their Combination for Depressiona
Between-group analyses revealed no significant differences in cognitive change (across all neuropsychological domains and the global performance score) from baseline to endpoint. In our within-group analyses, we observed variable improvement in cognitive functioning. First, both the methylphenidate plus placebo and citalopram plus methylphenidate groups demonstrated significant improvement on the global performance score (methylphenidate plus placebo group: t=−2.91, df=136, p=0.004; citalopram plus methylphenidate group: t=−2.04, df=136, p=0.04). All treatment groups significantly improved in language (citalopram plus placebo group: t=−2.81, df=136, p=0.01; methylphenidate plus placebo group: t=−2.61, df=136, p=0.01; citalopram plus methylphenidate group: t=−3.25, df=136, p=0.002). The methylphenidate plus placebo group additionally improved in executive functioning (t=−2.45, df=136, p=0.02). Finally, the citalopram plus placebo group demonstrated significant improvement in attention (t=−2.43, df=136, p=0.02). No within-group changes were noted in memory or visuospatial functioning. Across comparisons, change in HAM-D score from baseline to endpoint did not moderate improvement in cognitive functioning.
Dropout and Tolerability Analysis
The three groups did not differ significantly in time to dropout (citalopram plus placebo group: mean=33.2 days, SD=21.2, range=8–86; methylphenidate plus placebo group: mean=27.8 days, SD=15.1, range=7–48; citalopram plus methylphenidate group: mean=41.6 days, SD=31.3, range=7–98). The groups did not differ in number of side effects, dropout rate, or dropout reasons. After randomization, 16 participants dropped out because of side effects (seven each in the citalopram plus placebo and methylphenidate plus placebo groups and two in the citalopram plus methylphenidate group), three because of lack of efficacy, and 26 for other reasons (see Table S2 in the data supplement for details).
Discussion
To our knowledge, this is the first randomized placebo-controlled trial designed to test the clinical efficacy and tolerability of combination treatment with methylphenidate and citalopram to improve antidepressant response in geriatric depression compared with either drug as monotherapy (that is, with either drug plus placebo). We detected an improved response in the combination treatment group, as evidenced by greater improvement in the two continuous measures of depression and in global clinical improvement. We also observed a faster rate of response in depressive symptoms in the combination treatment group in the first 4 weeks of treatment compared with the citalopram plus placebo group, and compared with the methylphenidate plus placebo group for the remainder of the trial, while the rates of response for the two monotherapy groups differed significantly in the last 12 weeks of the trial. We observed a significantly higher remission rate in the citalopram plus methylphenidate group compared with the methylphenidate monotherapy group, and a nonsignificantly (p=0.07) higher rate in comparison with the citalopram monotherapy group. These findings potentially offer clinicians guidance on the use of these drugs to achieve faster remission in depressed older adults, although they may not translate into higher remission rates over longer treatment periods.
Citalopram treatment appeared to be beneficial for cognition, although augmentation with methylphenidate did not offer additional benefits. However, participants treated with methylphenidate demonstrated improvement in the global cognitive performance score. We also noted improvements in the well-being subscale of the SF-36 in the methylphenidate plus citalopram group compared with the two monotherapy groups. We did not observe group differences in other clinical measures or in side effects. Overall, the outcomes are encouraging for mental health providers, given the limited number of successful treatment strategies available to enhance antidepressant response with additional benefits in function in geriatric depression.
To date, a limited number of studies have suggested that stimulant medications may be especially useful in older adults (18–21, 35–38). Two large series of medically ill mixed-age patients (36, 38) suggested the value of adjunctive dextroamphetamine (range, 2.5–30 mg/day) and methylphenidate (range, 5–30 mg/day) in relieving depression, with an onset of response within 48 hours. Our findings on cognitive outcomes are generally consistent with the literature, as they suggest that cognition improves after acute antidepressant treatment (39), although some studies do not support this finding (12, 15).
Our study has several limitations. We used a convenience sample of outpatients with major depression of moderate severity. Therefore, the results may not be applicable to patients with acute medical illnesses or with more severe depression. Because of the use of methylphenidate, we excluded individuals with a history of substance abuse and severe anxiety disorders, which also may limit the generalizability of the results. Although we were interested in acceleration of response with the use of methylphenidate, the titration was relatively slow because of concerns for safety in elderly patients, but the rate of remission within the first 4 weeks of methylphenidate treatment was still more rapid than would be expected after 16 weeks of treatment with citalopram. Finally, our results with regard to citalopram dosing should be interpreted cautiously and with consideration of the 2011 U.S. Food and Drug Administration recommendation (40) that citalopram dosing be limited to 40 mg/day in younger adults and 20 mg/day in the elderly because of potential cardiac side effects, as well as recently published data confirming an increase in the QTc interval with citalopram use in older patients with dementia (41). In addition, ideally, the comparison of different drug dosing on remission rates would require a fixed-dose comparison trial. Finally, a high dropout rate is a limitation of the study. Our analysis did not suggest that any missing data were not missing at random, and hence mixed-effects modeling of our primary outcome measure (HAM-D score) was carried out under the missing-at-random assumption. However, group differences in remission rates at 16 weeks should be interpreted with caution because of high dropout rates, and further studies are needed to ascertain the reasons for group differences in remission rates.
Despite these limitations, our study is the first comprehensive and well-controlled trial to address the potential of methylphenidate to enhance clinical and cognitive outcomes in geriatric depression. Combination treatment with citalopram and methylphenidate resulted in higher remission rates and shorter time to remission than citalopram monotherapy (62% compared with 42%), with no significant differences in frequency of adverse events. The combination treatment may offer a means of improving the efficacy and rate of response to treatment in late-life depression.
1 : Chronicity and relapse in geriatric depression. Biol Psychiatry 1989; 26:551–564Crossref, Medline, Google Scholar
2 : Maintenance treatment of major depression in old age. N Engl J Med 2006; 354:1130–1138Crossref, Medline, Google Scholar
3 : Efficacy of second generation antidepressants in late-life depression: a meta-analysis of the evidence. Am J Geriatr Psychiatry 2008; 16:558–567Crossref, Medline, Google Scholar
4 : Moderators of outcome in late-life depression: a patient-level meta-analysis. Am J Psychiatry 2013; 170:651–659Link, Google Scholar
5 : A model of placebo response in antidepressant clinical trials. Am J Psychiatry 2013; 170:723–733Link, Google Scholar
6 : Optimal length of antidepressant trials in late-life depression. J Clin Psychopharmacol 2005; 25(suppl 1):S34–S37Crossref, Medline, Google Scholar
7 : Executive dysfunction and the course of geriatric depression. Biol Psychiatry 2005; 58:204–210Crossref, Medline, Google Scholar
8 : Antidepressant medication and executive dysfunction: a deleterious interaction in late-life depression. Am J Geriatr Psychiatry 2010; 18:128–135Crossref, Medline, Google Scholar
9 : IADL functions, cognitive deficits, and severity of depression: a preliminary study. Am J Geriatr Psychiatry 2005; 13:244–249Crossref, Medline, Google Scholar
10 : Mild cognitive impairment: clinical characterization and outcome. Arch Neurol 1999; 56:303–308Crossref, Medline, Google Scholar
11 : Patterns of mild cognitive impairment after treatment of depression in the elderly. Am J Geriatr Psychiatry 2009; 17:308–316Crossref, Medline, Google Scholar
12 : Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry 2000; 157:1949–1954Link, Google Scholar
13 : Change in cognitive functioning following acute antidepressant treatment in late-life depression. Am J Geriatr Psychiatry 2009; 17:881–888Crossref, Medline, Google Scholar
14 : Persistent mild cognitive impairment in geriatric depression. Int Psychogeriatr 2007; 19:125–135Crossref, Medline, Google Scholar
15 : Persistence of cognitive impairment in geriatric patients following antidepressant treatment: a randomized, double-blind clinical trial with nortriptyline and paroxetine. J Psychiatr Res 2003; 37:99–108Crossref, Medline, Google Scholar
16 : Methylphenidate-enhanced antidepressant response to citalopram in the elderly: a double-blind, placebo-controlled pilot trial. Am J Geriatr Psychiatry 2006; 14:181–185Crossref, Medline, Google Scholar
17 : Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry 1998; 155:344–349Link, Google Scholar
18 : Combined treatment with methylphenidate and citalopram for accelerated response in the elderly: an open trial. J Clin Psychiatry 2003; 64:1410–1414Crossref, Medline, Google Scholar
19 : Methylphenidate augmentation of citalopram in elderly depressed patients. Am J Geriatr Psychiatry 2001; 9:298–303Crossref, Medline, Google Scholar
20 : Stimulants in the treatment of depression: a critical overview. J Clin Psychiatry 1989; 50:241–249Medline, Google Scholar
21 : Use of stimulants in depressed patients with medical illness, in Geriatric Psychopharmacology. Edited by Nelson JG. New York, Marcel Decker, 1998, pp 245–258Google Scholar
22 : A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
23 : “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198Crossref, Medline, Google Scholar
24 Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 218–222Google Scholar
25 : A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Crossref, Medline, Google Scholar
26 : The assessment of anxiety states by rating. Br J Med Psychol 1959; 32:50–55Crossref, Medline, Google Scholar
27 : Reliability and validity of the Apathy Evaluation Scale. Psychiatry Res 1991; 38:143–162Crossref, Medline, Google Scholar
28 : Comparison of probability of stroke between the Copenhagen City Heart Study and the Framingham Study. Stroke 1994; 25:802–807Crossref, Medline, Google Scholar
29 : Rating chronic medical illness burden in geropsychiatric practice and research: application of the Cumulative Illness Rating Scale. Psychiatry Res 1992; 41:237–248Crossref, Medline, Google Scholar
30 JE, R, NM, : Validating the SF-36 health survey questionnaire: new outcome measure for primary care. Br Med J 1992; 305:160–164Crossref, Medline, Google Scholar
31 : Neuropsychological Assessment, 4th ed. New York, Oxford University Press, 2004Google Scholar
32 : Normative data for a brief neuropsychological screening battery: Multicenter AIDS Cohort Study. Percept Mot Skills 1991; 73:539–550Crossref, Medline, Google Scholar
33 : Neuropsychological processes associated with normal aging. Dev Neuropsychol 1990; 6:279–290Crossref, Google Scholar
34 : The UKU Side Effect Rating Scale: a new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl 1987; 334:1–100Crossref, Medline, Google Scholar
35 : Double blind evaluation of methylphenidate (Ritalin) hydrochloride: its use in the management of institutionalized geriatric patients. J Am Med Assoc 1959; 169:1739–1741Crossref, Medline, Google Scholar
36 : Psychostimulants for secondary depression in medical illness. Psychosomatics 1991; 32:203–208Crossref, Medline, Google Scholar
37 : Augmentation strategies for treatment of unipolar major depression. Mod Probl Pharmacopsychiatry 1997; 25:34–55Crossref, Medline, Google Scholar
38 : Psychostimulant treatment of depressive disorders secondary to medical illness. J Clin Psychiatry 1986; 47:12–15Medline, Google Scholar
39 : Does antidepressant therapy improve cognition in elderly depressed patients? J Gerontol A Biol Sci Med Sci 2003; 58:M1137–M1144Crossref, Medline, Google Scholar
40 US Food and Drug Administration: FDA Drug Safety Communication: Revised recommendations for Celexa (citalopram hydrobromide) related to a potential risk of abnormal heart rhythms with high doses. Aug 24, 2011 (http://www.fda.gov/drugs/drugsafety/ucm297391.htm)Google Scholar
41 : Changes in QTc interval in the Citalopram for Agitation in Alzheimer’s Disease (CitAD) randomized trial. PLoS ONE 2014; 9:e98426Crossref, Medline, Google Scholar



