Electroconvulsive Therapy Augmentation in Clozapine-Resistant Schizophrenia: A Prospective, Randomized Study
Abstract
Objective:
Up to 70% of patients with treatment-resistant schizophrenia do not respond to clozapine. Pharmacological augmentation to clozapine has been studied with unimpressive results. The authors examined the use of ECT as an augmentation to clozapine for treatment-refractory schizophrenia.
Method:
In a randomized single-blind 8-week study, patients with clozapine-resistant schizophrenia were assigned to treatment as usual (clozapine group) or a course of bilateral ECT plus clozapine (ECT plus clozapine group). Nonresponders from the clozapine group received an 8-week open trial of ECT (crossover phase). ECT was performed three times per week for the first 4 weeks and twice weekly for the last 4 weeks. Clozapine dosages remained constant. Response was defined as ≥40% reduction in symptoms based on the psychotic symptom subscale of the Brief Psychiatric Rating Scale, a Clinical Global Impressions (CGI)-severity rating <3, and a CGI-improvement rating ≤2.
Results:
The intent-to-treat sample included 39 participants (ECT plus clozapine group, N=20; clozapine group, N=19). All 19 patients from the clozapine group received ECT in the crossover phase. Fifty percent of the ECT plus clozapine patients met the response criterion. None of the patients in the clozapine group met the criterion. In the crossover phase, response was 47%. There were no discernible differences between groups on global cognition. Two patients required the postponement of an ECT session because of mild confusion.
Conclusions:
The augmentation of clozapine with ECT is a safe and effective treatment option. Further research is required to determine the persistence of the improvement and the potential need for maintenance treatments.
As many as 30% of patients with schizophrenia respond poorly to standard treatment with antipsychotic medications (1). Clozapine is the only medication shown to be effective in antipsychotic-refractory patients (2, 3), but in as many as 45% to 70% of these patients, symptoms are also resistant to substantial improvement with clozapine (2, 4). Limited options are available for individuals with clozapine-resistant symptoms. Several augmentation strategies have been evaluated, including addition of another antipsychotic, mood stabilizers, anxiolytics, antidepressants, and glutamatergic agents; however, no agent has shown unequivocal efficacy in this population. Meta-analyses of randomized placebo-controlled studies have found little or no advantage of augmentation with antipsychotics (5–8). The treatment of this subgroup of patients remains an enormous challenge, with significant public health implications.
Preliminary data from nonrandomized open-label studies suggest that addition of ECT may be an effective alternative for patients with clozapine-resistant symptoms. ECT, while initially introduced for the treatment of schizophrenia in the 1930s, is currently most commonly used for the treatment of depression. ECT and antipsychotics are considered to exhibit synergistic effects (9). In 36 patients with symptoms resistant to typical antipsychotics, Kupchik et al. (10) found that 67% of these patients benefitted from the combination of clozapine and ECT, clozapine, or ECT alone. In an open-label study in 11 clozapine nonresponders, Kho et al. (11) found the combination of clozapine plus ECT to be efficacious. Havaki-Kontaxaki et al. (12) reviewed all studies of clozapine-ECT combination in schizophrenia or schizoaffective patients with strictly defined clozapine-resistant symptoms, concluding that preliminary evidence exists for the safety and short-term efficacy of this combination. The main limitation of this review was the small sample size (N=21) with data from one open-label trial and six case studies. Finally, Masoudzadeh and Khalilian (13) compared ECT, clozapine, and ECT-clozapine combination in schizophrenia patients with treatment resistant symptoms and noted a Positive and Negative Syndrome Scale score reduction of 46% in the clozapine group, 40% in the ECT group, and 71% in the clozapine-ECT group. They concluded that the combination was significantly better than either treatment alone.
Therefore, our group initiated a pilot study of the effects of adding ECT to clozapine. In an open-label prospective study, we used bilateral ECT to treat 15 patients with schizophrenia who had not responded to clozapine or partially responded to clozapine, despite adequate doses for more than 12 weeks. Nine of these patients (60%) met the a priori response criterion of a 40% decrease in symptom ratings as measured by the psychotic symptom subscale of the Brief Psychiatric Rating Scale (BPRS) (14). Based on these encouraging preliminary data, we have now conducted the first prospective randomized study of the efficacy and side-effect profile of ECT plus clozapine in schizophrenia patients with clozapine-resistant symptoms.
Method
The study was conducted at The Zucker Hillside Hospital of the North Shore-LIJ Health System. The study was approved by the respective institutional review boards, and participants were recruited from the inpatient units of the Zucker Hillside Hospital at Glen Oaks, N.Y., and the Pilgrim State Psychiatric Center in Long Island, N.Y.
Study Design and Procedures
In an 8-week random-assignment study incorporating nonblinded treatment and blinded assessments, patients with antipsychotic- and clozapine-resistant schizophrenia were assigned to two treatment groups. One group received treatment as usual (clozapine group) for 8 weeks, and the other received a course of bilateral ECT in addition to their current pharmacotherapy regimen (ECT plus clozapine). Nonresponders from the clozapine group received an open 8-week trial of ECT (crossover trial) with the same schedule and procedures as the randomized ECT plus clozapine group.
Antipsychotic medication resistance was defined as a history of at least two failed trials of 400 mg of chlorpromazine equivalents for at least 4 weeks. Clozapine resistance was defined as a history of persistence of psychotic symptoms after a trial of clozapine of at least 12 weeks, at a blood level ≥350 ng/mL.
The inclusion criteria were diagnosis of schizophrenia according to DSM-IV criteria; age 18–60 years; duration of illness >2 years; resistance to at least two antipsychotics; clozapine resistance; a baseline BPRS score of at least moderate (score of 4) on one of the four psychotic items (hallucinatory behavior, suspiciousness, conceptual disorganization, and unusual thought content) of the psychotic symptom subscale or a score of 12 on these four items combined; a Clinical Global Impressions (CGI)-severity (15) rating of at least moderate (score of 4); capacity to give informed consent; and for women of childbearing capacity, a negative pregnancy test and patient agreement to use a medically accepted form of contraception.
Exclusion criteria were schizoaffective disorder; bipolar disorder; current affective episode; ECT within 6 months; history of epilepsy; severe neurological or systemic disorder that could significantly affect cognition, behavior, or mental status (other than tardive dyskinesia or neuroleptic-induced parkinsonism); psychoactive substance dependence (other than nicotine or caffeine) within 1 month prior to entering the study; a score >18 on the 24-item Hamilton Depression Rating Scale (HAM-D) (16); clinical determination that mood stabilizers that could not be discontinued were necessary; and pregnancy.
We also excluded patients with affective disorders and prominent depressive symptoms because ECT is well known to be effective in those situations, and we wanted to avoid contamination of our results by improvement solely driven by the treatment of the affective symptoms.
The primary outcome measure was response rate. The response criterion was defined a priori as improvement ≥40% based on the psychotic symptom subscale, a CGI-severity rating of mild or less (<3), and a CGI-improvement rating of much improved (≤2). For power calculations, we assumed a response rate of 40% in the ECT plus clozapine group and 10% in the clozapine group.
We chose to define response as a 40% reduction in symptoms based on the psychotic symptom subscale, compared with the traditional 20% in medication studies, recognizing that ECT adds additional levels of complexity and requires increased effort by both clinicians and patients.
Clozapine Group
Once randomly assigned, participants in both groups remained on the clozapine dose at which they entered the study for 8 weeks. Concurrent use of other antipsychotic medications and antidepressants was allowed as long as they were taken at a stable dose for at least 12 weeks before entering the study. Lorazepam, up to 6 mg per day, or diphenhydramine, up to 100 mg, were used as needed for anxiety, agitation, or insomnia.
ECT Plus Clozapine Group
ECT was performed with bilateral placement using the Thymatron-DGx device (Somatics, Lake Bluff, Ill.). Seizure threshold was determined at the first treatment. Dosing at subsequent treatments was given at 50% above threshold. The seizure threshold determination schedule and subsequent stimulus levels are shown in Table 1. Medications used during anesthesia were glycopyrrolate (0.1 mg–0.2 mg), methohexital (0.5 mg/kg–1 mg/kg), and succinylcholine (0.5 mg/kg–1 mg/kg). Participants signed a separate informed consent for ECT, and standard institutional procedures for ECT were followed.
| Seizure Threshold (% Energy) | Dose at Subsequent Treatment (% Energy) |
|---|---|
| 5 | 10 |
| 10 | 15 |
| 20 | 30 |
| 40 | 60 |
| 60 | 90 |
TABLE 1. ECT Dosing Algorithm
ECT was administered three times per week for the first 4 weeks, then twice weekly for the next 4 weeks. If patients met remission criteria before the completion of 8 weeks and showed a plateau in their improvement for two consecutive ratings, ECT was continued weekly through the end of 8 weeks.
Crossover ECT Trial
Participants from the clozapine group who did not respond to 8 weeks of continued pharmacotherapy received ECT augmentation with the same schedule of treatments and ratings as the ECT plus clozapine group for an additional 8 weeks.
Assessments and Raters
Eligible, consenting patients had a complete baseline medical and psychiatric evaluation. DSM-IV diagnosis was established using the Structured Clinical Interview for DSM-IV (17) by experienced raters. Patients were rated at baseline and weekly. Final rating assessments were performed at the end of week 8, except those from the cognitive battery, which was administered at week 9 (i.e., 1 week after the last ECT session).
The raters were masked to the clinical assignment. Psychopathology rating measures included the BPRS, CGI, HAM-D, the Schedule for Assessment of Negative Symptoms (18), and the Treatment Emergent Side Effects Scale (15), and rating assessments were performed weekly.
To enhance objectivity, we videotaped the initial (baseline) and final (week 8 or study exit) BPRS ratings. The tapes were edited to remove any revealing statements about treatment, and brief (2–5 seconds), nonessential portions from all tapes were erased to create 3–10 skips in a random fashion, thus balancing the presence of awkward skips that might be otherwise present mostly in the ECT tapes. An independent, masked rater rated the edited tapes. The average score between the two ratings at baseline and the last visit was used for data analyses.
A focused neuropsychological battery was performed at baseline and at week 9. The battery was designed to assess ECT-related effects, as well as measures of cognition relevant to schizophrenia, and included the standard and modified Mini-Mental State Examination (MMSE), the Rey Auditory Verbal Learning Test, the paired-word and story-recall measures subtests of the Randt Memory Battery, the letter-number span task, the Trail-Making Test, the Controlled Oral Word Association Test, the Competing Programs Test, and the Set Shifting Test.
Statistical Analysis
Histograms, q-q plots, and the Shapiro-Wilk test were used to assess distribution of continuous variables. Based on the distribution, an independent-samples t test or Wilcoxon rank-sum test was used to compare the baseline continuous variables between treatment groups. Chi-square test was used for categorical variables. Since missing values may be dependent on the observed outcomes, we assumed the missing data to be missing at random, and hence analysis of the longitudinal data was conducted utilizing a mixed-models approach. A random intercept in the mixed-models was used to account for correlation of measurements over time among the participants; the correlational type was assumed to be unstructured. The difference in slopes of the outcomes between the two treatment groups was assessed using group-by-time interaction term in the mixed models. Interrater reliability between the in-person and video ratings was assessed using both consistency and absolute agreement intraclass coefficients. The consistency intraclass coefficient between the ratings was 0.892 (95% confidence interval [CI]=0.783–0.948; F=17.5, df=28, 28, p<0.0001). The absolute agreement intraclass coefficient was 0.888 (95% CI=0.783–0.948; F=17.5, df=28, 28, p<0.0001). All analyses were adjusted for age.
Results
Participants
Participant screening and enrollment flow data are presented in Figure 1 (92 were screened; 54 met inclusion criteria; 13 refused participation; 41 signed consent). Thirty-nine individuals were randomly assigned (ECT plus clozapine group, N=20; clozapine group, N=19). The two groups were well matched on demographic variables, including sex, ethnicity, and psychopathology (Table 2). However, the ECT-treated group was significantly younger than the clozapine group; therefore, all analyses were adjusted for age. Of the 20 participants assigned to ECT plus clozapine, 17 completed the 8-week trial, two dropped out early and refused further ECT treatments, and one was removed from the study because of persistence of involuntary movements. In the clozapine group, 16 of the 19 patients completed the 8-week clozapine phase, and three patients dropped out because they refused to continue to participate in the ratings assessment while there was no change in their treatment. However, all three of the patients who dropped out agreed to participate in the crossover ECT phase, and all 19 participants were included in that phase.
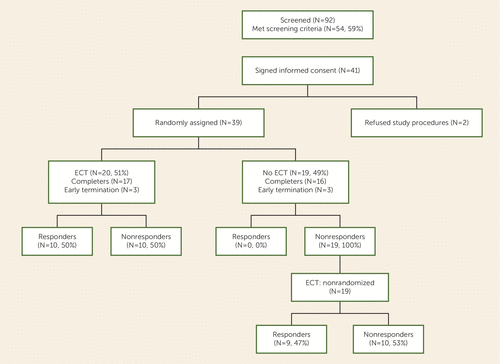
FIGURE 1. CONSORT Diagram for Patients With Clozapine-Resistant Schizophrenia
| Variable | ECT Plus Clozapine Group (N=20) | Clozapine Group (N=19) | ||
|---|---|---|---|---|
| N | % | N | % | |
| Sex | ||||
| Male | 15 | 75 | 13 | 68.4 |
| Female | 5 | 25 | 6 | 31.6 |
| Race/ethnicity | ||||
| Caucasian | 11 | 55 | 10 | 52.6 |
| African American | 5 | 25 | 6 | 31.6 |
| Hispanic | 2 | 10 | 3 | 15.8 |
| Other | 2 | 10 | 0 | 0 |
| Mean | SD | Mean | SD | |
| Age (years)a | 35.70 | 2.27 | 42.78 | 1.82 |
| Brief Psychiatric Rating Scale (BPRS) total score | 45.68 | 1.87 | 46.42 | 2.55 |
| BPRS psychotic symptom subscale score | 16.58 | 0.86 | 16.89 | 0.9 |
| Clinical Global Impressions-severity score | 5.35 | 0.02 | 5.53 | 0.22 |
| Hamilton Depression Rating Scale score | 14.90 | 1.64 | 16.79 | 1.58 |
| Mini-Mental State Examination (MMSE) score | 22.60 | 1.2 | 22.2 | 1.0 |
| Modified MMSE score | 44.78 | 1.89 | 39.76 | 39.76 |
| Clozapine level (ng/mL) | 854.8 | 278.1 | 828.9 | 267.5 |
TABLE 2. Demographic and Clinical Characteristics of Patients With Clozapine-Resistant Schizophrenia
Clinical Efficacy Results
Ten of the 20 patients (50%) in the ECT augmentation and none (0%) of the patients in the clozapine group met the a priori response criterion. If the less conservative but commonly used response criterion of a 20% reduction in symptoms were used, we would have 12 (60%) responders. Conversely, if an even stricter criterion were used, such as a 50%, 60%, or 70% reduction in symptoms based on the psychotic symptom subscale, we would have nine (45%), six (30%), or three (15%) responders, respectively. None of the clozapine patients would have responded with any of the above criteria. Patients randomly assigned to ECT augmentation were found to have significantly greater reduction in ratings on the BPRS-psychosis subscale and the CGI-severity scale compared with patients in the clozapine group over time (Figure 2, Figure 3). Post hoc analyses revealed that the ECT plus clozapine group had significantly lower psychotic symptom subscale ratings by week 3, and this significant difference persisted for the remainder of the 8-week trial.
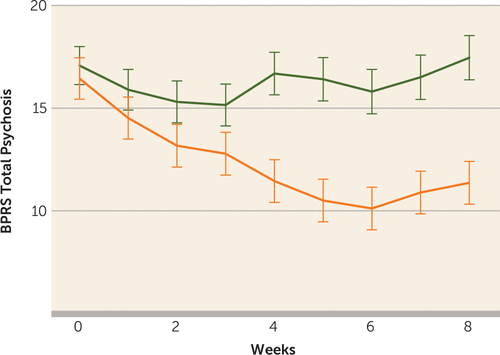
FIGURE 2. Changes in Brief Psychiatric Rating Scale (BPRS) Psychosis Subscale Results Over Timea
a The graph shows the changes in psychosis symptoms in the clozapine group (blue line; phase 1) and the ECT plus clozapine group (red line; phase 1). Treatment-by-time interaction: F=5.38, df=8, 238, p<0.0001. The degrees of freedom for mixed-models analysis were obtained using Satterthwaite’s method. Error bars represent standard deviations.
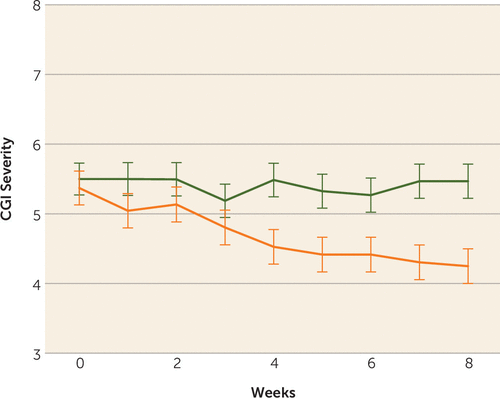
FIGURE 3. Changes in Clinical Global Impressions (CGI)-Severity Scale Ratings Over Timea
a The graph shows the changes in symptom severity in the clozapine group (blue line; phase 1) and the ECT plus clozapine group (red line; phase 1). Treatment-by-time interaction: F=5.00, df=8, 238, p<0.0001. The degrees of freedom for mixed-models analysis were obtained using Satterthwaite’s method. Error bars represent standard deviations.
In the crossover phase, nine (47.4%) of 19 patients met the response criterion. Overall, of the 39 patients who received ECT in the randomized and crossover arms, 19 (48.7%) had at least a 40% reduction in ratings on the psychotic symptom subscale after 8 weeks of treatment.
Regarding negative symptoms, there were no significant differences between the two groups and in group-by-time interactions in analyses with the Scale for the Assessment of Negative Symptoms on global measures for affective flattening (F=0.98, df=1, 39.3, p=0.33), alogia (F=0.03, df=1, 40.1, p=0.87), avolition-apathy, (F=0.76, df=1, 39.3, p=0.39), and anhedonia-asociality (F=1.87, df=1, 39.7, p=0.18). The above analysis was based on actual differences from baseline to final visit. A sensitivity analysis based on percent change scores in the above measures also showed no statistically significant differences between the groups (see table in the online data supplement).
Side Effects
One patient in the ECT plus clozapine group was removed from the study because of recurrence of preexisting involuntary “jerky” movements that raised clinical concerns of seizure activity. These concerns were not substantiated by electroencephalographic studies.
There were no side-effect differences between the two groups in any rated symptom, and there were no treatment-related side effects, except for two occasions when patients in the ECT plus clozapine group were rated as mildly confused and treatment was postponed until the next day.
We did not observe any peculiarities in EEG or motor seizure expression, or any prolonged seizures, during ECT. There were no spontaneous seizures among any of the study patients.
Cognitive Effects
Because of potential concerns about the cognitive side effects of ECT, we included an extensive neurocognitive battery. We assessed multiple cognitive domains, including global mental state, speed of processing, executive function, and episodic memory. The two treatment groups were well matched on each of the neurocognitive variables at baseline, including a global measure of cognitive function (using MMSE).
Neither group demonstrated a significant change in the global neurocognitive measures tested from baseline to endpoint. The mean MMSE scores for the ECT plus clozapine group were 22.6 (SD=1.2) at baseline and 23.1 (SD=1.2) at week 9, and mean scores for the clozapine group were 22.2 (SD=1.0) and 23.4 (SD=1.2), respectively.
Speed measures generally demonstrated significant group-by-time interactions such that the clozapine group improved slightly and the ECT plus clozapine group declined. For tests of visual and verbal memory, significant interaction effects were less consistently found. For executive function (Trail-Making Test, Part B, letter-number span task) interaction effects were not found. Results are presented in the second table of the online data supplement.
In general, our cognitive findings indicate that speed of processing was sensitive to the effects of ECT administration when measured at a subacute time point (i.e., within 1 week of the final 8-week ECT course). Results in the domains involving executive function and episodic memory were less consistently found. Clinically, the degree of cognitive burden shortly after the trial was felt to be consistent with the clinical experience with ECT.
Clozapine Dosage and Plasma Levels
The mean clozapine dosage for the ECT treatment group at baseline was 525.0 mg/day (SD=224.3), and the mean dosage for the clozapine group was 511.1 mg/day (SD=171.0). There were no significant differences between groups. The clozapine daily doses remained unchanged for each patient throughout the course of the study. There were no significant differences between groups in mean plasma levels (clozapine plus norclozapine) at baseline and at endpoint.
ECT
The average number of ECT treatments in the randomized phase was 15.8 (SD=4.2), and the average number of treatments in the crossover phase was 14.3 (SD=5.3).
The stimulus dose expressed as “percent of energy” on the Thymatron device is presented in Table 1. The average stimulus levels at the beginning of the course and at the final ECT session are similar to those observed in clinical practice in patients not receiving clozapine.
Discussion
To our knowledge, this is the first prospective randomized controlled study with masked raters assessing the efficacy of ECT as an augmentation strategy for patients with schizophrenia resistant to or partially responsive to clozapine. The results of this study suggest that ECT is a safe and effective treatment option for these patients and confirm findings from smaller uncontrolled studies and from earlier case reports (11, 12). The response rates of 50% observed in the randomized arm and 47% in the crossover arm compare favorably to all other suggested augmentation strategies for clozapine in this population. The fact that none of the patients in the clozapine group met the response criteria was not unexpected because our selection criteria required a period of at least 8 weeks of clozapine treatment at a steady dose without clinical improvement. The lack of effects with regard to negative symptoms precludes the attribution of response to the improvement of negative symptoms that may often be misinterpreted as depressive symptoms and vice versa.
ECT is the oldest biological treatment used in modern psychiatry. It was introduced as a treatment for schizophrenia in the 1930s, at a time when no medications were available. With the introduction and widespread use of antipsychotic medications in the 1950s, its use in schizophrenia dramatically declined. Yet, there are certain conditions in the course of the illness when rapid results are required, such as cases with prominent catatonic symptoms, suicidality, and agitation or in cases of neuroleptic malignant syndrome (19). In these cases, ECT is used with very good results. In our study, we demonstrated that ECT also may be useful in patients with chronic schizophrenia with positive symptoms not adequately responsive to antipsychotic medications, including the last resort of pharmacotherapy, clozapine.
There are several aspects of our study and its design that are worth mentioning. We paid particular attention to patient selection in an effort to study the effects of ECT on purely schizophrenia patients. We excluded patients with prominent affective symptoms to avoid contamination of our data by improvements driven solely or partially by the treatment of these symptoms that respond to ECT. In order to maintain the blind in a study comparing treatments that are so obviously different, we implemented strict procedures, including videotaping of baseline and exit interviews and independent “off-line” ratings. We included a crossover arm to strengthen the results observed in the randomized phase. Perhaps the most significant element of the study design was our a priori response criterion. We defined response as a 40% reduction in symptoms based on the psychotic symptom subscale, a level twice as high as the traditional 20% used in most medication-resistant schizophrenia trials. This is in recognition of the fact that the administration of ECT adds additional levels of complexity and risk to the treatment of schizophrenia, requires increased effort by both clinicians and patients, and therefore should be met with increased expectations.
The finding that must be emphasized here is that in this group of patients with severe treatment-resistant symptoms, 50% responded with the demanding 40% reduction in symptoms criterion, and 60% responded with conventional response criteria. These are among the highest response rates ever recorded in this patient population.
There were several theoretical concerns regarding the concurrent use of ECT and clozapine, most notably the possibility of prolonged or spontaneous seizures. In our sample, we did not observe any such occurrences. On the contrary, there were instances that required us to administer stimuli at 100% of the device capacity in order to elicit an adequate seizure. This over-time increased resistance to seizure induction is in line with clinical experience and reflects the fact that ECT has known antiepileptic properties causing the seizure threshold to increase over the course of ECT treatment.
With regard to the cognitive effects of the combined treatment, we did not observe any unusual effects of ECT. Our patients were severely ill, and their cognitive performance was, as expected, relatively low, with an average baseline MMSE score of 22. The fact that there was no additional impairment after the course of ECT may reflect the fact that some aspects of cognitive function improved as a result of decreased disorganization, thus counteracting the expected transient memory impairment often seen with ECT.
There are limitations of the study that need to be addressed. First, there was no placebo arm, since sham ECT studies are not considered ethical. Second, the number of patients was relatively low, and our sample included only inpatients. However, this is the largest study of this nature, and the comparative effects between the two groups are very strong. Third, there was an age difference between the two groups, with the clozapine group being older. One might assume that this may make symptoms in this group more resistant to treatment. Nevertheless, all group differences remained significant after correcting for age, and, more importantly, the older clozapine group had an equally strong response to ECT in the crossover phase. Finally, the duration of the study was relatively short and did not allow for conclusions regarding the long-term effects of ECT in this population. As in the case of the treatment of depression with ECT, it is likely that maintenance treatment is required for those patients who respond to ECT plus clozapine, but this should be the focus of further research.
Conclusions
The augmentation of clozapine with ECT for the treatment of clozapine-resistant schizophrenia is a safe and effective treatment option. In this severely ill group of patients with treatment-resistant symptoms, for whom there are few clinical alternatives, we demonstrated a 50% response rate using a conservative 40% reduction in symptoms criterion, and 60% response rate using conventional response criteria. To our knowledge, these are the highest response rates reported with any type of clozapine augmentation. Further research is required to determine the persistence of the results and the need for maintenance treatments.
1 : Defining treatment refractoriness in schizophrenia. Schizophr Bull 1990; 16:551–561Crossref, Medline, Google Scholar
2 : Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988; 45:789–796Crossref, Medline, Google Scholar
3 : Effects of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am J Psychiatry 1994; 151:20–26Link, Google Scholar
4 : A prospective study of clozapine in treatment-resistant schizophrenic patients, I: preliminary report. Psychopharmacology (Berl) 1989; 99(suppl):S68–S72Crossref, Medline, Google Scholar
5 : Does the addition of a second antipsychotic drug improve clozapine treatment? Schizophr Bull 2009; 35:458–468Crossref, Medline, Google Scholar
6 : Clozapine combined with different antipsychotic drugs for treatment resistant schizophrenia. Cochrane Database Syst Rev 2009; 3:CD006324Medline, Google Scholar
7 : Antipsychotic combinations vs monotherapy in schizophrenia: a meta-analysis of randomized controlled trials. Schizophr Bull 2009; 35:443–457Crossref, Medline, Google Scholar
8 : Augmentation of clozapine with a second antipsychotic: a meta-analysis of randomized, placebo-controlled studies. Acta Psychiatr Scand 2009; 119:419–425Crossref, Medline, Google Scholar
9 : The combined use of electroconvulsive therapy and antipsychotics in patients with schizophrenia. J ECT 2005; 21:75–83Crossref, Medline, Google Scholar
10 : Combined electroconvulsive-clozapine therapy. Clin Neuropharmacol 2000; 23:14–16Crossref, Medline, Google Scholar
11 : Electroconvulsive therapy for the treatment of clozapine nonresponders suffering from schizophrenia: an open label study. Eur Arch Psychiatry Clin Neurosci 2004; 254:372–379Crossref, Medline, Google Scholar
12 : Concurrent administration of clozapine and electroconvulsive therapy in clozapine-resistant schizophrenia. Clin Neuropharmacol 2006; 29:52–56Crossref, Medline, Google Scholar
13 : Comparative study of clozapine, electroshock and the combination of ECT with clozapine in treatment-resistant schizophrenic patients. Pak J Biol Sci 2007; 10:4287–4290Crossref, Medline, Google Scholar
14 : Anchoring the BPRS: an aid to improved reliability. Psychopharmacol Bull 1988; 24:112–117Medline, Google Scholar
15 : ECDEU Assessment Manual for Psychopharmacology, Revised. Bethesda, Md, US Department of Health, Education, and Welfare, 1976Google Scholar
16 : A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
17 : Structured Clinical Interview for DSM-IV (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1995Google Scholar
18 : Negative v positive schizophrenia: definition and validation. Arch Gen Psychiatry 1982; 39:789–794Crossref, Medline, Google Scholar
19 : The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging: A Task Force Report of the American Psychiatric Association, 2nd ed. Washington, DC, American Psychiatric Publishing, 2001Google Scholar



