Treatment-Resistant Bipolar Depression: A Randomized Controlled Trial of Electroconvulsive Therapy Versus Algorithm-Based Pharmacological Treatment
Abstract
Objective:
Electroconvulsive therapy (ECT) is regarded by many clinicians as the most effective treatment for treatment-resistant bipolar depression, but no randomized controlled trials have been conducted, to the authors’ knowledge. They compared efficacy measures of ECT and algorithm-based pharmacological treatment in treatment-resistant bipolar depression.
Method:
This multicenter, randomized controlled trial was carried out at seven acute-care psychiatric inpatient clinics throughout Norway and included 73 bipolar disorder patients with treatment-resistant depression. The patients were randomly assigned to receive either ECT or algorithm-based pharmacological treatment. ECT included three sessions per week for up to 6 weeks, right unilateral placement of stimulus electrodes, and brief pulse stimulation.
Results:
Linear mixed-effects modeling analysis revealed that ECT was significantly more effective than algorithm-based pharmacological treatment. The mean scores at the end of the 6-week treatment period were lower for the ECT group than for the pharmacological treatment group: by 6.6 points on the Montgomery-Åsberg Depression Rating Scale (SE=2.05, 95% CI=2.5–10.6), by 9.4 points on the 30-item version of the Inventory of Depressive Symptomatology–Clinician-Rated (SE=2.49, 95% CI=4.6–14.3), and by 0.7 points on the Clinical Global Impression for Bipolar Disorder (SE=0.31, 95% CI=0.13–1.36). The response rate was significantly higher in the ECT group than in the group that received algorithm-based pharmacological treatment (73.9% versus 35.0%), but the remission rate did not differ between the groups (34.8% versus 30.0%).
Conclusion:
Remission rates remained modest regardless of treatment choice for this challenging clinical condition.
Bipolar disorder is characterized by recurrent depressive, manic, or mixed episodes and a relapsing illness course. Bipolar disorder subtypes I and II are defined according to the severity of the manic episodes, although the long-term course is mainly dominated by depressive symptoms (1).
There are numerous pharmacological treatment options for the manic episodes but not the depressive episodes (2). Lithium, divalproex, carbamazepine, lamotrigine, quetiapine, olanzapine, and fluoxetine exert some beneficial effects (3–6), while the use of antidepressants is controversial (7, 8). Despite some differences in first-line choices, pharmacological treatment algorithms for bipolar depression include the same pharmacological agents (3, 9–12). It is difficult to document systematic evidence for the selection of any specific therapeutic choice for treatment-resistant bipolar depression (13).
Many clinicians regard electroconvulsive therapy (ECT) as the most effective acute treatment in severe treatment-resistant mood and psychotic disorders (14). The use of ECT in bipolar depression has not been extensively studied, but for severe refractory bipolar depression it is a second-line option in most guidelines (3, 9–12). These recommendations are based on clinical experience and the results from nonrandomized studies and a meta-analysis comparing the efficacy of ECT in unipolar versus bipolar depression (15–18). To our knowledge, no randomized controlled trials of ECT for the treatment of bipolar depression have been reported.
This study compared efficacy outcomes of ECT and algorithm-based pharmacological treatment in treatment-resistant bipolar depression, using repeated Montgomery-Åsberg Depression Rating Scale (MADRS) (19) assessments as the primary outcome after a 6-week intervention period and, as secondary outcomes, repeated assessments with the Inventory of Depressive Symptomatology–Clinician-Rated, 30-item version (20), and the Clinical Global Impressions Scale for Bipolar Disorder (CGI-BP) (21), the response and remission rates, and the times to response and remission.
Method
Overview
This multicenter study was not sponsored by industry and was carried out in Norway at the Division of Psychiatry, Haukeland University Hospital, Bergen; Østmarka Department of Psychiatry, St. Olav University Hospital, Trondheim; Division of Psychiatry, Stavanger University Hospital, Stavanger; Department of Emergency Mental Health Services and Gerontopsychiatric Unit, Oslo University Hospital, Ullevaal; Psychiatry Clinic, Oslo University Hospital, Aker; and Psychiatry Clinic, Østfold County Hospital, Fredrikstad. The randomized controlled trial compared measures of efficacy for ECT and algorithm-based pharmacological treatment in 73 acutely ill inpatients with bipolar disorder who were experiencing treatment-resistant depression and were recruited from April 2008 to May 2011. After being randomly assigned and before starting the new treatment, the patients entered a washout phase if they were taking concomitant medication not allowed by the study protocol (length of washout: five times drug half-life for patients assigned to ECT and a varying time for patients assigned to pharmacological treatment). The detailed study protocol was published previously (22). The results from the 6-week treatment period are presented here.
Subjects
The patients were 26 to 79 years old and currently depressed, with a MADRS score of 25 or higher. They fulfilled the DSM-IV-TR criteria (23, 24) for bipolar disorder subtype I or II. Additional criteria for inclusion were an indication for ECT as determined by the participating clinicians and treatment resistance as defined by a lack of response to two trials (lifetime) with antidepressants and/or mood stabilizers with documented efficacy in bipolar depression (lithium, lamotrigine, quetiapine, or olanzapine) in adequate doses for at least 6 weeks or until cessation of treatment due to side effects.
Patients were required to be sufficiently fluent in Norwegian to be able to provide informed consent and valid responses in psychometric testing. Exclusion criteria were having received ECT within the previous 6 months; a history of nonresponse to ECT; a rapid cycling course of illness, defined as at least four mood episodes within the previous 12 months; an unstable serious medical condition, including any clinically relevant laboratory abnormality; a condition assumed to affect neurocognitive function, such as Parkinson’s disease, multiple sclerosis, stroke, or substance dependence or abuse according to DSM-IV; pregnancy; inadequate contraception (in fertile women); elevated mood, defined as a score of 20 or higher on the Young Mania Rating Scale (25); or a high suicide risk according to the researcher’s clinical judgment.
Treatments
Electroconvulsive therapy.
The ECT procedures were standardized across all the study centers by using either the Thymatron System IV device (Somatics, Lake Bluff, Ill.) or (in one center only) the MECTA 5000 device (MECTA, Lake Oswego, Ore.). Stimulation electrodes were placed according to the d’Elia method (right unilateral electrode placement) (26). The pulse width was set to 0.5 ms. The initial stimulus dose was determined by an age-based, gender-adjusted method (22, 27). Treatment was administered three times a week for up to 6 weeks, with a maximum of 18 sessions. The procedures for anesthesia and determination of seizure adequacy (seizure duration, δ-waves, and clinical effect) followed a study protocol compatible with current standards of care, as described previously (22, 28).
Patients randomly assigned to ECT were switched to an algorithm-based pharmacological maintenance treatment (10, 22) if they reached remission, defined as a MADRS score of 12 or below, before the end of the 6-week treatment period.
Algorithm-based pharmacological treatment.
Patients in the pharmacological group were treated according to the treatment algorithm for bipolar depression of Goodwin and Jamison (10). The pharmacotherapy to be used was chosen before the randomization and took into account the patients’ medication histories. The algorithm was to be followed on a step-by-step basis. Patients who had experienced no effect or intolerable side effects while taking one of the medications listed in the algorithm could be switched to the next treatment option according to the algorithm. The following were allowed as adjuvant treatment: alimemazine (first-generation antihistamine, 10–30 mg/day), chlorpromazine (first-generation antipsychotic, 25–50 mg/day), chlorprothixene (first-generation antipsychotic, 20–40 mg/day), mianserin (tetracyclic antidepressant, 10 mg/day), oxazepam (anxiolytic, 15–45 mg/day), and zolpidem (hypnotic, 10 mg/day) or zopiclone (hypnotic, 7.5 mg/day). Judgment of treatment compliance was based on the patient’s self-report at each visit and serum level monitoring at week 3.
Randomization and Masking of Study Groups
The random assignment to ECT or pharmacological treatment was stratified independently in each study center by using the default random-number generator of SPSS 15 (SPSS, Chicago) with a random seed. The patient and treating psychiatrist were unblinded to treatment modality. To compensate for the unblinded rater, the MADRS and the Inventory of Depressive Symptomatology interviews at baseline and week 6 (or when the patient left the study) were audiotaped. Raters who were blinded to the treatment condition rated the audiotapes.
Assessments
The patients were diagnosed by experienced psychiatrists specifically trained in the use of the Structured Clinical Interview for DSM-IV Axis I Disorders (23) or the Mini International Neuropsychiatric Interview–Plus (29). The demographic and course of illness variables were collected at baseline by means of a Norwegian adaptation of the Network Entry Questionnaire used by the Bipolar Collaboration Network (30). The severity of symptoms was rated by administering the MADRS, the Inventory of Depressive Symptomatology, the CGI-BP, and the Young Mania Rating Scale at baseline and weekly thereafter. Additionally, patients in both treatment groups were seen and evaluated as clinically indicated by the treating clinicians, irrespective of the study protocol. Before the study, all participating raters were trained in the use of the MADRS and the 30-item Inventory of Depressive Symptomatology–Clinician Rated by one of the authors (U.F.M.) who has extensive experience in training raters in clinical trials. All clinicians rated at least 10 interviews and achieved an intraclass correlation (ICC) of at least 0.7 for both the MADRS and the Inventory of Depressive Symptomatology. During the study, 35 of 73 taped interviews were randomly selected for reliability testing by two separate raters blinded to the treatment status of the patients. The correlation between the blinded and regular raters was high (ICC>0.90).
The presence of substance abuse was determined by clinical interviews and urine tests.
Outcome Measures
The longitudinal profile of weekly MADRS scores during the 6-week treatment period was defined as the primary outcome measure. Secondary outcome measures were the longitudinal profiles of scores on the Inventory of Depressive Symptomatology and CGI-BP during the 6-week treatment period, the times to response and remission, a single end-of-treatment MADRS score, and the proportions of responders and remitters at the end of the 6-week treatment period. The end-of-treatment MADRS score had to be obtained within 8 days of the termination of the 6-week treatment period. Response was defined as a decrease in MADRS score of at least 50% from baseline. Remission was defined as a MADRS score of 12 or lower.
Ethics
This was a substudy of the Bipolar Research and Innovation Network–Norway (BRAIN) study that commenced in 2004 (31). It was approved by the Regional Committee for Medical and Health Research Ethics (Mid-Norway), the Norwegian Data Inspectorate, and the Norwegian Medicines Agency. Patients provided written informed consent before study entry.
Statistics
All analyses were performed by using the SPSS 18.0 software package for Windows and R (32).
Descriptive analyses.
Means and standard deviations (SDs) were computed for continuous variables, while numbers and percentages were computed for categorical variables. Differences between groups in demographic and clinical variables were analyzed with two-sided independent-samples t tests. Categorical variables were analyzed with chi-square tests. The p value for significance was set at 0.05.
Missing data.
Data were registered as missing for the continuous outcome variables (MADRS, Inventory of Depressive Symptomatology, and CGI-BP) if the patient did not return to the final assessment within 8 days of the termination of the 6-week treatment period. This occurred with 14 patients, who were classified as dropouts because the final assessment was performed outside the predetermined time range, as shown in the flow diagram in Figure 1. However, analyses involving the full longitudinal profile of the scores on the MADRS, Inventory of Depressive Symptomatology, and CGI-BP do not require imputation of missing values, since the linear mixed-effects modeling accommodates missing data; the survival analyses handle this through censoring.
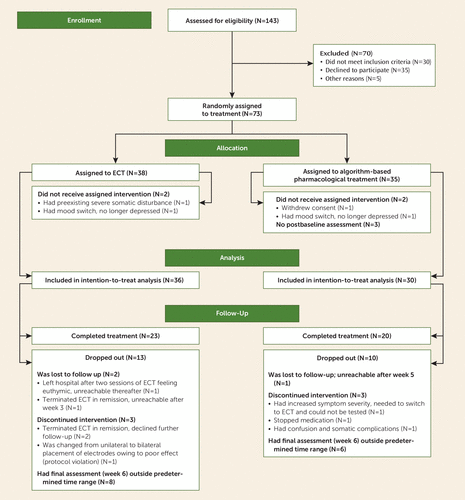
FIGURE 1. Patient Participation in a Randomized Controlled Trial of ECT Versus Algorithm-Based Pharmacological Treatment for Patients With Treatment-Resistant Bipolar Depression
Efficacy analyses.
The efficacy analyses used an intention-to-treat study group comprising all randomly assigned patients who had at least one postbaseline assessment. In analyses of the continuous efficacy outcomes, the longitudinal trajectories of scores on the MADRS and the Inventory of Depressive Symptomatology during the 6-week treatment period were compared for the ECT and pharmacological treatment groups by using linear mixed-effects modeling (33). Possible correlations due to the multicenter structure of the data were checked by means of a two-level model with subjects within centers. This analysis produced no changes in the results. The analysis involving the CGI-BP was based on bootstrapping owing to the nonnormality of the data.
Efficacy of treatment was also evaluated as times to response and remission with MADRS score as the outcome measure in Cox regression analyses. A frailty model was used to handle the multicenter structure. This analysis also produced no changes in the results.
In both the survival and linear mixed-effects modeling analyses, we used the exact number of days since the baseline assessment as the time variable. The number of weeks could also have been used, but since not all assessments were conducted exactly at days 7, 14, etc., using the week number would have decreased the sensitivity of the analyses.
Results
Patient Flow and Characteristics
An overview of the flow of patients through the study is shown in Figure 1. In total, 143 patients were assessed for eligibility, of whom 30 did not meet the inclusion criteria, 35 refused to participate, and five were not included for various other reasons. The remaining 73 patients were randomly assigned to the two treatments, four did not receive the assigned treatment, and another three had no postbaseline assessment, yielding an intention-to-treat efficacy group of 66, of whom 36 received ECT and 30 received algorithm-based pharmacological treatment.
Nine of the 66 patients (13.6%) in the modified intention-to-treat group dropped out of the study early. The final assessment was not performed within the predetermined time range for an additional 14 patients. Among the patients who dropped out were two patients in the ECT group who were lost to follow-up. The first left the hospital after only two ECT sessions, feeling euthymic and having a MADRS score of 14. That patient agreed to weekly follow-up but did not show up and was later found dead, apparently because of an accidental overdose of illicit substances. The second patient was in remission when ECT was stopped. Two other patients discontinued ECT when in remission and refused further follow-up, and another patient was removed from the study because of a protocol violation (switch to bilateral electrode placement after nine unilateral treatments).
One patient in the group assigned to pharmacological treatment was lost to follow-up after week 5. Three patients were removed from the study: one had an increase in symptom severity resulting in inability to comply with the testing procedure and a switch to ECT, one did not comply with the prescribed medication regimen, and one was removed from the study because of confusion and because an epileptic disorder was detected. The demographic and clinical baseline characteristics did not differ significantly between completers and noncompleters. The dropout rates were similar in the two groups. The demographic variables at baseline did not differ between the treatment groups (p>0.05 for all measures), as shown in Table 1. The only difference in baseline clinical characteristics was a higher rate of lifetime lithium use in the group assigned to pharmacological treatment.
| Measure | ECT (N=38) | Algorithm-Based Pharmacological Treatment (N=35) | Analysis | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | t | df | p | |
| Age at study inclusion (years) | 48.0 | 10.1 | 48.4 | 13.2 | 0.16 | 71 | 0.88 |
| Age at illness onset (years) | 15.9 | 6.7 | 19.0 | 11.3 | 1.34 | 47.3 | 0.19 |
| Duration of illness (years) | 31.8 | 12.2 | 27.7 | 10.6 | –1.45 | 65 | 0.16 |
| Number of episodes | |||||||
| Depressive | 22.3 | 24.2 | 17.4 | 14.1 | –0.94 | 57 | 0.36 |
| Hypomanic | 18.2 | 30.8 | 9.6 | 8.1 | –1.51 | 36.2 | 0.14 |
| Manic | 2.7 | 8.6 | 1.1 | 2.6 | –0.94 | 58 | 0.36 |
| Psychotic | 1.6 | 2.7 | 3.3 | 9.7 | 1.00 | 60 | 0.33 |
| Scores on rating scales | |||||||
| Montgomery-Åsberg Depression Rating Scalea | 39.1 | 7.5 | 38.0 | 7.4 | –0.60 | 71 | 0.55 |
| 30-item Inventory of Depressive Symptomatology–Clinician-Ratedb | 48.9 | 9.7 | 46.5 | 13.6 | –0.83 | 64 | 0.41 |
| Young Mania Rating Scalec | 3.5 | 2.8 | 3.2 | 2.3 | –0.55 | 71 | 0.59 |
| Clinical Global Impression for Bipolar Disorderd | 5.8 | 0.7 | 5.8 | 0.7 | –0.48 | 68 | 0.64 |
| N | % | N | % | χ2 | df | p | |
| Male gender | 21 | 55.3 | 16 | 45.7 | 0.67 | 1 | 0.42 |
| Bipolar disorder type I | 14 | 36.8 | 15 | 42.9 | 0.28 | 1 | 0.60 |
| Lifetime medication use | |||||||
| Antipsychotics | 30 | 78.9 | 30 | 87.5 | 0.57 | 1 | 0.55 |
| Antidepressants | 35 | 92.1 | 33 | 94.3 | 0.14 | 1 | 1.00 |
| Anticonvulsants | 33 | 86.8 | 25 | 71.4 | 2.65 | 1 | 0.15 |
| Lithium | 12 | 31.6 | 20 | 51.7 | 4.84 | 1 | 0.04 |
TABLE 1. Characteristics of Patients With Treatment-Resistant Bipolar Depression Randomly Assigned to ECT or Algorithm-Based Pharmacological Treatment
Treatment Variables
Treatment characteristics were recorded after each ECT session. Patients assigned to ECT received a mean of 10.6 treatments (SD=4.9) and a mean charge of 243.9 (SD=62.0) millicoulombs; their mean EEG seizure duration was 40.3 (SD=16.8) seconds.
The patients assigned to pharmacological treatment were prescribed antipsychotics, anticonvulsants, lithium, and antidepressants in various combinations. Only one patient received monotherapy (Table 2).
| Patient | Lithium: Mean Dosage (mg/day)a | Anticonvulsants, With Mean Dosage (mg/day)b | Antipsychotics, With Mean Dosage (mg/day) | Antidepressants (Classc), With Mean Daily Dosage (mg/day) | Time of Study Exit (week) | MADRSd Score at Study Exit |
|---|---|---|---|---|---|---|
| 1 | 124.5 | Lamotrigine, 300.0 | Venlafaxine (SNRI), 300.0 | 5 | 15 | |
| 2 | 166.0 | Lamotrigine, 37.5 | Quetiapine, 300.0 | Sertraline (SSRI), 75.0 | 6 | 5 |
| 3 | 210.0 | Valproate, 1200.0 | Olanzapine, 5.0 | Citalopram (SSRI), 20.0 | 5 | 21 |
| 4 | 193.0 | Lamotrigine, 58.3 | Quetiapine, 120.0 | 5 | 18 | |
| 5 | 207.5 | Quetiapine, 566.7 | Escitalopram (SSRI), 70.0 | 6 | 24 | |
| 6 | 45.5 | Lamotrigine, 41.7 | Perphenazine, 8.0 | Venlafaxine (SNRI), 225.0 | 6 | 24 |
| 7 | 156.8 | Lamotrigine, 800.0 | Olanzapine, 2.5 | Bupropion (NDRI), 150.0 | 5 | 20 |
| 8 | 166.0 | Lamotrigine, 100.0 | Olanzapine, 7.5 | Fluoxetine (SSRI), 20.0 | 6 | 3 |
| 9 | 332.0 | Lamotrigine, 45.0 | Quetiapine, 180.0 | Mirtazapine (NaSSa), 60.0 | 6 | 35 |
| 10 | 53.7 | Lamotrigine, 62.5 | Citalopram (SSRI), 12.0; venlafaxine (SNRI), 181.3 | 6 | 29 | |
| 11 | 166.0 | Olanzapine, 5.0 | Fluoxetine (SSRI), 32.0; paroxetine (SSRI), 10.0e | 6 | 30 | |
| 12 | Lamotrigine, 316.7 | Quetiapine, 283.3 | 6 | 22 | ||
| 13 | Lamotrigine, 200.0; valproate, 1500.0 | Olanzapine, 5.0 | Fluoxetine (SSRI), 40.0 | 6 | 15 | |
| 14 | Lamotrigine, 83.3 | Quetiapine, 204.2 | Mirtazapine (NaSSa), 27.5 | 6 | 10 | |
| 15 | Lamotrigine, 41.7 | Quetiapine, 75.0 | 6 | 11 | ||
| 16 | Lamotrigine, 25.0 | Quetiapine, 250.0 | 0 | 44 | ||
| 17 | Lamotrigine, 466.7 | Quetiapine, 208.3 | 5 | 4 | ||
| 18 | Lamotrigine, 350.0 | Olanzapine, 30.0 | Fluoxetine (SSRI), 40.0 | 0 | 57 | |
| 19 | Lamotrigine, 75.0; valproate, 900.0 | Olanzapine, 10.0 | Mirtazapine (NaSSa), 15.0 | 5 | 3 | |
| 20 | Lamotrigine, 16.7; valproate, 850.0 | Quetiapine, 383.3 | Mirtazapine (NaSSa), 30.0 | 6 | 21 | |
| 21 | Lamotrigine, 300.0 | Olanzapine, 5.0 | Escitalopram (SSRI), 20.0; mirtazapine (NaSSa), 15.0 | 6 | 43 | |
| 22 | Lamotrigine, 68.8 | Quetiapine, 300.0 | 5 | 25 | ||
| 23 | Lamotrigine, 100.0 | Quetiapine, 133.3 | 6 | 9 | ||
| 24 | Lamotrigine, 300.0 | Quetiapine, 100 | Venlafaxine (SNRI), 120.0; bupropion (NDRI), 150.0 | 6 | 23 | |
| 25 | Lamotrigine, 10.0; valproate, 1500.0 | Quetiapine, 50.0 | Bupropion (NDRI), 150.0 | 1 | 29 | |
| 26 | Lamotrigine, 10.0; valproate, 600.0 | Quetiapine, 110.0; olanzapine, 10.0 | 6 | 8 | ||
| 27 | Lamotrigine, 40.0 | Olanzapine, 20.0 | Fluoxetine (SSRI), 28.0 | 5 | 28 | |
| 28 | Lamotrigine, 41.7 | Quetiapine, 158.3 | Bupropion (NDRI), 205.0 | 3 | 51 | |
| 29 | Lamotrigine, 12.5; valproate, 600.0 | Quetiapine, 25.0 | 0 | 20 | ||
| 30 | Lamotrigine, 30.0; valproate, 450.0 | Olanzapine, 5.0; quetiapine, 450.0 | Venlafaxine (SNRI), 37.5f | 6 | 21 | |
| 31 | Lamotrigine, 115.0 | Olanzapine, 12.0 | 6 | 19 | ||
| 32 | Lamotrigine, 120.8; valproate, 1300.0 | Aripiprazole, 15.0g | 6 | 25 | ||
| 33 | Quetiapine, 545.8 | 6 | 21 |
TABLE 2. Individual Medications and Outcomes for Patients Receiving Algorithm-Based Pharmacological Treatment in a Comparison With ECT for Treatment-Resistant Bipolar Depression
Efficacy
Treatment with ECT was found to be significantly more effective than pharmacological treatment in the linear mixed-effects modeling analysis. The mean MADRS score at 6 weeks was 6.6 points lower in the ECT group (SE=2.05, 95% CI=2.5–10.6, p=0.002) (Figure 2). There was a significant interaction between the number of days since the baseline assessment and group (p=0.03); that is, the MADRS score changed at different rates in the two groups, resulting in a significant increase in the difference between groups. Similarly the mean score on the Inventory of Depressive Symptomatology at 6 weeks was 9.4 points lower in the ECT group (SE=2.49, 95% CI=4.6–14.3, p=0.0001). For the CGI-BP, the mean score was 0.7 points lower in the ECT group (SE=0.31, 95% CI=0.13–1.36, p=0.02) at the end of the 6-week treatment period. Including the bipolar subtype in the analyses did not significant affect these differences in rating scale scores.
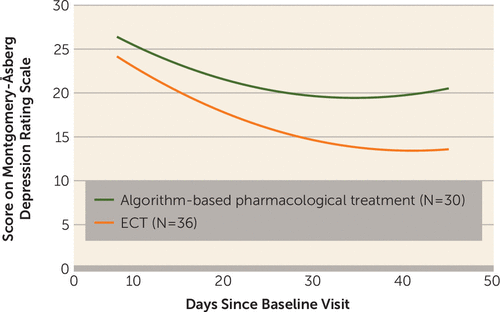
FIGURE 2. Change in Depression Severity in Patients With Treatment-Resistant Bipolar Depression Randomly Assigned to ECT or Algorithm-Based Pharmacological Therapya
a Linear mixed-effects analysis showed that the mean score at 6 weeks was 6.6 points lower in the ECT group (SE=2.05, 95% CI=2.5–10.6, p=0.002).
Response and Remission Rates
At the end of the 6-week treatment period, the mean MADRS score was 14.7 (SD=7.4) in the ECT group and 19.9 (SD=10.0) in the pharmacological treatment group (t=1.91, df=41, p=0.07). These group means and also the p value of the comparison differ from the results of the linear mixed-effects modeling owing to the handling of missing values. Among patients who completed treatment, the response rate was higher in the ECT group, 73.9% (17 of 23), than in the pharmacological treatment group, 35.0% (seven of 20) (χ2=6.57, df=1, p=0.01), whereas the remission rate did not differ between the two groups: 34.8% (eight of 23) versus 30.0% (six of 20) (χ2=0.11, df=1, p=0.74). There was a nonsignificant tendency toward shorter times to response and remission in the ECT group, as shown in Figures 3 and 4.
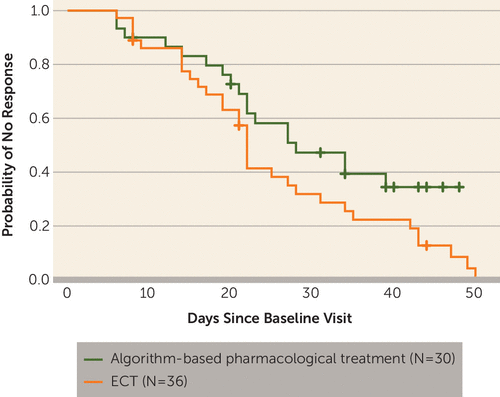
FIGURE 3. Time to Response for Patients With Treatment-Resistant Bipolar Depression Randomly Assigned to ECT or Algorithm-Based Pharmacological Therapya
a Time to response is depicted by a survival (Kaplan-Meier) plot. Response was defined as a reduction of at least 50% in the Montgomery-Åsberg Depression Rating Scale score from baseline. There was no significant difference between the groups (p=0.11, log-rank test).
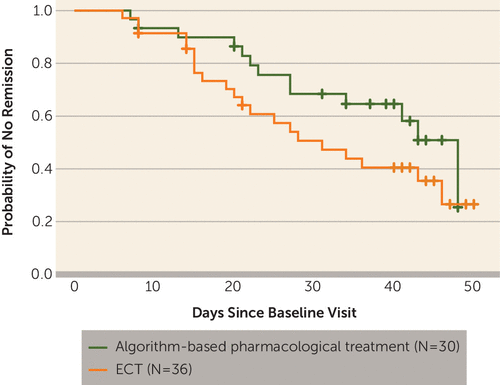
FIGURE 4. Time to Remission for Patients With Treatment-Resistant Bipolar Depression Randomly Assigned to ECT or Algorithm-Based Pharmacological Therapya
a Time to remission is depicted by a survival (Kaplan-Meier) plot. Remission was defined as reaching a Montgomery-Åsberg Depression Rating Scale score of 12 or less. There was no significant difference between the groups (p=0.14, log-rank test).
Adverse Events
The frequencies of psychic, neurologic, autonomic, and other adverse events are shown in Table 3. There was one death after discharge from the hospital, apparently due to overdose of illicit drugs.
| Symptom | ECT (N=36) | Algorithm-Based Pharmacological Treatment (N=30) | Relation to Treatment Procedure |
|---|---|---|---|
| Psychic adverse events | |||
| Concentration difficulties | 3 | Improbable | |
| Asthenia, lassitude, or increased fatigability | 1 | 2 | Improbable |
| Sleepiness or sedation | 3 | Possible | |
| Failing memory | 2 | 2 | Probable for the patients in the ECT group |
| Depression | 1 | Improbable | |
| Tension or inner unrest | 1 | 1 | Possible for the patient in the pharmacological group, probable for the patient in the ECT group |
| Increased duration of sleep | 1 | Possible | |
| Reduced duration of sleep | 2 | Possible | |
| Increased dream activity | 1 | Possible | |
| Emotional indifference | 1 | Possible | |
| Neurologic adverse event | |||
| Epileptic seizures | 1 | Improbable, patient had a complex partial seizure disorder from childhood that was unknown to the treating clinicians at inclusion in the study | |
| Autonomic adverse events | |||
| Reduced salivation | 1 | Possible | |
| Nausea or vomiting | 1 | Possible | |
| Constipation | 1 | Probable | |
| Orthostatic dizziness | 2 | Probable | |
| Increased tendency to sweat | 3 | 1 | Possible for the patient in the pharmacological group, improbable for the patients in the ECT group |
| Other adverse events | |||
| Rash | 1 | Probable | |
| Weight gain | 1 | Possible | |
| Weight loss | 1 | Improbable | |
| Diminished sexual desire | 2 | 4 | Possible |
| Orgasmic dysfunction | 1 | Possible | |
| Headache | 1 | Probable | |
| Death (due to an overdose of illicit drugs after discharge from hospital) | 1 | Improbable | |
| Medication overdose with possible suicidal ideation | 1 | 2 | Improbable |
| Self-strangulation attempt | 1 | Improbable | |
| Possible suicide attempt (patient jumped from a cliff) | 1 | Improbable | |
| Tooth damage | 1 | Probable | |
| Rib fracture | 1 | Improbable; the rib fracture occurred as the result of an accident unrelated to treatment |
TABLE 3. Significant Adverse Events and Relation to Treatment Procedure for Patients With Treatment-Resistant Bipolar Depression Randomly Assigned to ECT or Algorithm-Based Pharmacological Treatmenta
Discussion
To our knowledge, this is the first randomized controlled trial comparing the effects of ECT and pharmacological treatment in treatment-resistant bipolar depression. The main finding is that ECT is more effective than pharmacological treatment in the acute phase.
Using a linear mixed-effects modeling approach, we found that the mean MADRS score, the primary outcome measure, differed by 6.6 points between the ECT and pharmacological treatment groups. Similarly significant differences of 9.4 and 0.7 points between the two treatment groups were found for the secondary outcome measures: the 30-item Inventory of Depressive Symptomatology–Clinician Rated and CGI-BP scores, respectively. In a meta-analysis (35) of a mixed group of patients with unipolar or bipolar depression, ECT was found to be significantly more effective than pharmacological treatment, with a mean difference of 5.2 points (95% CI=1.4–8.9) on the Hamilton Depression Rating Scale (36).
There was no difference between the (low) remission rates of the ECT group (34.8%) and the pharmacological treatment group (30.0%). The response rate for ECT was significantly higher at 73.9%, which was considered a relatively successful outcome in this cohort of ill patients, in comparison to the response rate of 35.0% for the medication group. It should be noted that measures based on response or remission are a dichotomization of the MADRS score and thus generally produce less powerful results than linear mixed-effects analyses. The response and remission rates for ECT in the present study are consistent with those found by Medda and coauthors (17) in an open trial of the effects of ECT on medication-resistant depression or mixed states in patients with bipolar disorder subtype I, at about 70% and 30%, respectively. Comparable results have also found been in patients suffering from treatment-resistant unipolar depression (37). In contrast, patient groups not defined as having medication resistance often have somewhat higher response rates and substantially higher remission rates (15, 16). This underscores the importance of describing the degree of treatment resistance in patients when comparing the effects of interventions in depression.
In the survival analyses, the times to response and remission did not differ significantly between the ECT and pharmacological treatment groups, but there was a tendency for both times to be shorter in the ECT group. These survival analyses involved time to the first occurrence of response or remission, and patients who dropped out were censored. Measurements made before such individuals drop out may contain valuable information, and we included them in the survival analysis. The results are not directly comparable to those of other studies, because of the analysis method used and the lack of previous randomized controlled trials comparing the effects of ECT and pharmacological treatment in bipolar depression. However, a rapid effect of ECT is often claimed, and a small study of patients with unipolar or bipolar depression found quicker responses among patients randomly assigned to ECT than in those assigned to paroxetine (38).
Unilateral placement of stimulation electrodes has been found to be slightly less effective than bilateral placement (35). However, studies using unilateral ECT at a low dose or with a short interelectrode distance, techniques known to be less effective, were included. Although a minority of patients do not respond to right unilateral ECT and need to be crossed over to bilateral ECT, a large scale study (39) found that high-dose unilateral ECT with d’Elia electrode placement (26) was as effective as bilateral ECT. Therefore, the modest remission rate found for ECT in our study was probably due not to the use of unilateral electrode placement but, rather, to the selection of patients with a low potential for remission.
The present study was subject to some limitations. Neither the patients nor the researchers were blinded, which may have biased the treatment outcomes. However, this is unlikely since the video-based ratings by the blinded raters were strongly correlated with the results of the initial evaluation. A group receiving sham ECT, to control for a possible placebo response in patients and bias in evaluators, was not included because of ethical considerations. The relatively small study group and high dropout rates limit the statistical power of the analysis and may be a source of type 2 errors (22). Despite there being few differences in the characteristics of depressive episodes between bipolar disorder subtypes I and II (40), and particularly in treatment-resistant depression, the inclusion of both subtypes may introduce heterogeneity. However, there are no indications that this should bias the findings, with this instead leading to type 2 errors. The most severely depressed patients were not included because of their inability to give informed consent or their psychiatrists’ opinion that they were in urgent need of ECT. We suspect that their exclusion reduced the observed effect of ECT, since there are some indications that ECT is particularly beneficial in cases of severe depression. Finally, the indications for and attitudes toward ECT in Norway may differ from those in other countries, with implications for the generalizability of the results of this study.
The requirement that ECT be indicated for patients according to the responsible psychiatrist may have caused bias across the centers. The number of patients recruited from each center was too low to correct for any such response differences. However, the severity of depression at inclusion did not differ significantly among the centers. Finally, although we did apply a recognized treatment algorithm, we cannot rule out that the algorithm used was not optimal. Thus, our study clearly requires replication with alternative algorithms and medication dosages.
The main strength of the current study is its randomized controlled design. Furthermore, the study was initiated by researchers and financed by public research funds and the participating hospitals. The psychiatric health care system in Norway is publicly funded and based on catchment area, ensuring a representative sample of patients with severe treatment-resistant bipolar depression. The use of an algorithm-based pharmacological treatment as a comparison condition for ECT made it possible for the researchers to include patients with resistance to several medications. This design ensures that the results may also be generalized to patients exposed to a high lifetime number of pharmacological treatments, which is common in bipolar disorder (41).
To conclude, the current results show that ECT is more effective than pharmacological treatment in the acute phase of treatment-resistant bipolar depression, which supports ECT as a treatment option. The low remission rates found in this study highlight the need for research focusing on the detection of new and more effective treatment options for treatment-resistant bipolar depression.
1 : Long-term symptomatic status of bipolar I vs bipolar II disorders. Int J Neuropsychopharmacol 2003; 6:127–137Crossref, Medline, Google Scholar
2 : Challenges in the management of bipolar depression. J Clin Psychiatry 2005; 66(suppl 5):11–16Crossref, Medline, Google Scholar
3 : Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord 2013; 15:1–44Crossref, Medline, Google Scholar
4 ;
5 : Long-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trials. Am J Psychiatry 2004; 161:217–222Link, Google Scholar
6 : Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 2003; 60:1079–1088Crossref, Medline, Google Scholar
7 : Risk factors related to lifetime suicide attempts in acutely admitted bipolar disorder inpatients. Bipolar Disord 2012; 14:727–734Crossref, Medline, Google Scholar
8 : The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry 2013; 170:1249–1262Link, Google Scholar
9 ;
10 : Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression, 2nd ed. New York, Oxford University Press, 2007, pp 752–753Google Scholar
11 ;
12 : New treatment guidelines for acute bipolar depression: a systematic review. J Affect Disord 2011; 129:14–26Crossref, Medline, Google Scholar
13 : Refractoriness in bipolar disorder: definitions and evidence-based treatment. CNS Neurosci Ther 2012; 18:227–237Crossref, Medline, Google Scholar
14 : ECT in treatment-resistant depression. Am J Psychiatry 2012; 169:1238–1244Link, Google Scholar
15 : Ultra-brief pulse ECT in bipolar and unipolar depressive disorder: differences in speed of response. Bipolar Disord 2009; 11:418–424Crossref, Medline, Google Scholar
16 : Electroconvulsive therapy is equally effective in unipolar and bipolar depression. Acta Psychiatr Scand 2010; 121:431–436Crossref, Medline, Google Scholar
17 : Comparative response to electroconvulsive therapy in medication-resistant bipolar I patients with depression and mixed state. J ECT 2010; 26:82–86Crossref, Medline, Google Scholar
18 : Efficacy of electroconvulsive therapy in bipolar versus unipolar major depression: a meta-analysis. Bipolar Disord 2012; 14:146–150Crossref, Medline, Google Scholar
19 : A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Crossref, Medline, Google Scholar
20 : The Inventory for Depressive Symptomatology (IDS): preliminary findings. Psychiatry Res 1986; 18:65–87Crossref, Medline, Google Scholar
21 : Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Res 1997; 73:159–171Crossref, Medline, Google Scholar
22 : The study protocol of the Norwegian randomized controlled trial of electroconvulsive therapy in treatment resistant depression in bipolar disorder. BMC Psychiatry 2010; 10:16Crossref, Medline, Google Scholar
23 : Structured Interview for the Diagnostic and Statistical Manual of Mental Disorders, Patient Version, 4th ed. Washington, DC, American Psychiatric Press, 1997Google Scholar
24
25 : A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry 1978; 133:429–435Crossref, Medline, Google Scholar
26 : Unilateral electroconvulsive therapy. Acta Psychiatr Scand Suppl 1970; 215:1–98Medline, Google Scholar
27 : Electroconvulsive Therapy. New York, Oxford University Press, 2002, p 4Google Scholar
28
29 : The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 1998; 59(suppl 20):22–33Medline, Google Scholar
30 : The Stanley Foundation Bipolar Network, I: rationale and methods. Br J Psychiatry Suppl 2001; 41:s169–s176Crossref, Medline, Google Scholar
31 : Age at onset of first episode and time to treatment in in-patients with bipolar disorder. Br J Psychiatry 2009; 194:559–560Crossref, Medline, Google Scholar
32
33 : Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry 2004; 61:310–317Crossref, Medline, Google Scholar
34 : The UKU side effect rating scale: a new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl 1987; 334:1–100Crossref, Medline, Google Scholar
35
36 : A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23:56–62Crossref, Medline, Google Scholar
37 : Antidepressant pharmacotherapy failure and response to subsequent electroconvulsive therapy: a meta-analysis. J Clin Psychopharmacol 2010; 30:616–619Crossref, Medline, Google Scholar
38 : Electroconvulsive therapy vs paroxetine in treatment-resistant depression—a randomized study. Acta Psychiatr Scand 1997; 96:334–342Crossref, Medline, Google Scholar
39 : Bifrontal, bitemporal and right unilateral electrode placement in ECT: randomised trial. Br J Psychiatry 2010; 196:226–234Crossref, Medline, Google Scholar
40 : The comparative clinical phenotype and long term longitudinal episode course of bipolar I and II: a clinical spectrum or distinct disorders? J Affect Disord 2003; 73:19–32Crossref, Medline, Google Scholar
41 : Psychotropic medications for patients with bipolar disorder in the United States: polytherapy and adherence. Psychiatr Serv 2008; 59:1175–1183Link, Google Scholar



