Methamphetamine Use and Schizophrenia: A Population-Based Cohort Study in California
Abstract
Objective:
Clinical investigators in Japan have long suggested that exposure to methamphetamine might cause a persistent schizophrenia-like psychosis. This possibility is discounted in the Western literature. To investigate the relationship between drug use and later schizophrenia, the authors conducted a large-scale cohort study of drug users initially free of persistent psychosis.
Method:
A population-based cohort study was conducted using data from California inpatient hospital discharge records from 1990 through 2000. Patients with methamphetamine-related conditions (N=42,412) and those with other drug use disorders (cannabis, cocaine, alcohol, and opioids) were propensity score-matched to individuals with primary appendicitis who served as a population proxy comparison group; the methamphetamine cohort was also matched to the other drug cohorts. Cox modeling was used to estimate differences between matched groups in the rates of subsequent admission with schizophrenia diagnoses.
Results:
The methamphetamine cohort had a significantly higher risk of schizophrenia than the appendicitis group (hazard ratio=9.37) and the cocaine, opioid, and alcohol groups (hazard ratios ranging from 1.46 to 2.81), but not significantly different from that of the cannabis group. The risk of schizophrenia was higher in all drug cohorts than in the appendicitis group.
Conclusions:
Study limitations include difficulty in confirming schizophrenia diagnoses independent of drug intoxication and the possibility of undetected schizophrenia predating drug exposure. The study's findings suggest that individuals with methamphetamine-related disorders have a higher risk of schizophrenia than those with other drug use disorders, with the exception of cannabis use disorders. The elevated risk in methamphetamine users may be explained by shared etiological mechanisms involved in the development of schizophrenia.
Epidemiological studies have suggested that use of cannabis may increase the risk of developing schizophrenia (1). However, an earlier literature, still controversial, suggests that abuse of methamphetamine could also trigger the development of persistent psychotic syndromes (2). Clinical investigations in Japan describing persistent psychosis in some methamphetamine users long after drug withdrawal support this possibility (3). In Western psychiatry, however, a prolonged psychosis in the drug-free state is not generally viewed as a feature of chronic methamphetamine exposure, and when such a psychosis is observed, it is thought to be explainable in toto by a preexisting (undiagnosed) psychotic disorder (4). Nevertheless, two Clinical Case Conference articles in this journal, spanning a 14-year period, have focused specifically on the still unresolved possibility that methamphetamine exposure may induce a persistent psychotic state (5, 6).
In this study, we used data from a large cohort sample to assess whether the incidence of schizophrenia is elevated in methamphetamine users who were free of psychosis before drug use. Because the stress-vulnerability theory of schizophrenia (7) suggests that heavy exposure to any drug of abuse, especially one that can cause acute psychosis, might interact with risk factors to increase vulnerability to development of persistent psychosis, we also included a second stimulant group (cocaine) as well as three nonstimulant drug use groups (cannabis, alcohol, and opioids) in our analyses.
Method
Data
The study was approved by the Research Ethics Board of the Centre for Addiction and Mental Health in Toronto. We used California inpatient hospital admissions data from 1990 through 2000. The California Office of Statewide Health Planning and Development (OSHPD) provided anonymized individual-level inpatient data collected from all California-licensed hospitals. The data set consisted of a record for each inpatient discharge from a licensed hospital (general acute care, acute psychiatric, chemical dependency recovery, and psychiatric health facilities) but excluded federal hospitals. Each medical record can contain up to 25 diagnoses per hospital admission episode. Inpatient data were screened by the OSHPD's automated data entry and reporting software program (MIRCal), and data fields with error rates of 0.1% or higher were returned to the hospitals for correction (8, 9). Reabstraction studies comparing OSHPD inpatient data files with original medical records found specificities for diagnoses ranging from 0.98 to 1.00 and sensitivities for diagnoses ranging from 0.88 to 1.00 (8–10).
Outcome Measure
The primary outcome variable was time to readmission with any of the four characteristic schizophrenia diagnoses specified in ICD-9 (code 295.1, disorganized type; 295.2, catatonic type; 295.3, paranoid type; 295.6, residual type). Other ICD-9 295 schizophrenia codes were excluded (e.g., schizophreniform, schizoaffective) to ensure use of the strictest definition of schizophrenia for comparison purposes. Reabstraction studies have found high agreement for schizophrenia-related diagnoses between medical chart information and ICD-9-based administrative data files, ranging from 90% to 100% (11–13).
Patient Groups
Primary population proxy: appendicitis group.
Patients with a primary appendicitis-related diagnosis were included in the appendicitis group if they had the following characteristics: 1) a primary diagnosis of an appendicitis-related condition (ICD-9 codes 540–542) at index admission; 2) no prior or concurrent indication (in relation to the index appendicitis admission) of any schizophrenia spectrum disorder (codes 295.x, schizophrenic disorders; 297.x, delusional disorders; 298.x, other nonorganic psychoses; or 301.2, schizoid personality disorder); 3) no concurrent diagnoses at index admission of drug-induced psychoses (code 292.1, drug-induced psychotic disorders); and 4) no prior, concurrent, or subsequent indication of any alcohol or drug use diagnosis. The codes used are listed in Table 1.
| Drug Category and ICD-9 Code | Description |
|---|---|
| Alcohol | |
| 303 | Alcohol dependence |
| 305.0 | Alcohol abuse |
| 980.0 | Alcohol (ethyl) poisoning |
| Methamphetamine | |
| 304.4 | Methamphetamine dependence |
| 305.7 | Methamphetamine abuse |
| 969.7 | Methamphetamine poisoning |
| E854.2 | Accidental methamphetamine poisoning |
| Cocaine | |
| 304.2 | Cocaine dependence |
| 305.6 | Cocaine abuse |
| 968.5 | Poisoning by cocaine |
| Opioids | |
| 304.0 | Opioid type dependence |
| 305.5 | Opioid abuse |
| 965.0 | Poisoning by opiates and related narcotics |
| Cannabis | |
| 304.3 | Cannabis dependence |
| 305.2 | Cannabis abuse |
| 969.6 | Poisoning by cannabis (derivatives) |
TABLE 1. ICD-9 Alcohol and Drug Codes Used to Identify Cohort Groups
Appendicitis was selected as the primary population proxy comparison condition because it is a relatively common reason for hospital admissions, is not associated with socioeconomic status (14), does not appear on theoretical grounds to be related to schizophrenia or substance use disorders, has a well-defined clinical course (15), and has been used successfully as a population proxy comparison condition in other epidemiological studies (16, 17). The incidence rate of schizophrenia in the unmatched appendicitis group in our study (13.9 per 100,000 years, 95% confidence interval=11.6–16.5, N=188,732; mean group follow-up time=4.88 years; 128 incident cases) was similar to that reported in a recent systematic review of literature (median=15.2 per 100,000 person-years; central 80% of the cumulative distribution, 7.7–43.0) (18).
Secondary population proxy: exclusion criteria group.
We created an additional population proxy comparison group based on appendicitis group assignment exclusion criteria 3 and 4 listed above. This exclusion criteria comparison group was created as follows: 1) we selected all individuals who were not assigned to any of the alcohol or drug cohorts as potentially eligible for the exclusion criteria comparison group; 2) we randomly selected one admission per individual in this initial group and designated the selected admission as the index admission; 3) to be eligible for the exclusion criteria comparison group, individuals could not have any prior or concurrent indication (in relation to their index admission) of drug use (see Table 1 for ICD-9 codes) or schizophrenia or schizophrenia-related conditions (as defined previously); and 4) eligible individuals could not have any drug episodes prior to or concurrent with their schizophrenia outcome or the end of the study period, whichever occurred first.
Drug cohorts.
Patients were assigned to only one of the following drug cohorts: methamphetamine, cocaine, opioids, cannabis, or alcohol. To be assigned to a drug cohort, an individual must have had 1) an ICD-9 diagnosis, in any diagnostic position in the medical record, indicating a condition in only one single drug category (Table 1) at index admission; 2) no prior or concurrent indication (in relation to the index drug admission) of schizophrenia spectrum disorders (ICD-9 codes 295.x, schizophrenic disorders; 297.x, delusional disorders; 298.x, other nonorganic psychoses; or 301.2, schizoid personality disorder); 3) no concurrent diagnoses at index admission of drug-induced psychoses (code 292.1, drug-induced psychotic disorders); and 4) no prior, concurrent, or subsequent indication (in relation to the index admission) of any alcohol or drug use diagnoses other than that of their assigned drug cohort as listed in Table 1. In other words, the algorithm excluded individuals from a drug group who had any ICD-9 diagnostic codes within a medical record or across records indicative of drug use other than that designated by their drug group membership. For example, individuals assigned to the methamphetamine group could have only methamphetamine-related ICD-9 diagnostic codes in any of their inpatient records (in any diagnostic position) from the time of their first discharge event to the time of the first schizophrenia admission (or the study end date).
The ICD-9 coding framework does not distinguish between methamphetamine and other amphetamines. However, it is likely that the ICD-9 amphetamine-related codes can serve as reasonable proxies for methamphetamine-related conditions based on two lines of evidence. During the time frame of our study, almost all amphetamine-related admissions to substance abuse treatment in California were specifically for methamphetamine. From 1992 through 2000, there were 225,999 primary amphetamine-related inpatient and outpatient treatment admissions to publicly funded substance abuse treatment programs in California, and methamphetamine accounted for 96.4% of these episodes (19). Also, in California, Arizona, and Nevada, federal legislation to control methamphetamine precursors in order to reduce the manufacture and supply of methamphetamine produced statistically significant reductions in inpatient hospital admissions with the methamphetamine-related ICD-9 diagnostic codes we used in our study (20)—a pattern supporting the use of the ICD-9 amphetamine-related codes as sensitive indicators of methamphetamine use disorders.
Analysis
Propensity score matching and Cox regression.
We chose the propensity score matching method as our primary approach because research suggests that conventional regression methods with covariate adjustment can produce biased estimates of treatment or outcome effects if there is extreme imbalance in the background characteristics of the examined study groups (21). As can be seen in Table 2, our unmatched cohort groups varied considerably across age, race, and sex—all of which are significantly related to the incidence of schizophrenia—as well as patterns of readmission and the average amount of time from index admission to the end of the study period (which would lead to an ascertainment bias of schizophrenia across groups). Propensity score matching offered a technique to avoid possible confounding arising from such initial differences across cohorts.
| Race | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Black | White | Hispanic | Other | Age (Years) | Female | Visitsa | Follow-Up Time (Years)c | ||||||||||
| Group | N | % | N | % | N | % | N | % | Mean | SD | N | % | Mean | SD | Schizophrenia Eventsb | Mean | SD |
| Appendicitis (N=188,732) | 7,384 | 3.9 | 107,375 | 56.9 | 54,050 | 28.6 | 19,923 | 10.6 | 35.5 | 16.2 | 77,969 | 41.3 | 0.51 | 1.5 | 128 | 5.0 | 2.9 |
| Exclusion criteriad (N=10,056,583) | 749,206 | 7.4 | 6,098,589 | 60.6 | 2,193,678 | 21.8 | 1,015,110 | 10.1 | 48.2 | 21.9 | 6,650,615 | 66.1 | 0.63 | 1.5 | 4,545 | 5.1 | 2.9 |
| Methamphetamine (N=42,412) | 1,724 | 4.1 | 31,830 | 75.0 | 6,889 | 16.2 | 1,969 | 4.6 | 30.7 | 9.8 | 24,297 | 57.3 | 1.03 | 2.3 | 425 | 4.8 | 2.5 |
| Cocaine (N=39,390) | 19,777 | 50.2 | 12,664 | 32.2 | 5,524 | 14.0 | 1,425 | 3.6 | 34.3 | 9.6 | 19,321 | 49.1 | 1.92 | 3.5 | 425 | 5.6 | 2.8 |
| Alcohol (N=408,604) | 37,832 | 9.3 | 284,200 | 69.6 | 69,545 | 17.0 | 17,027 | 4.2 | 50.6 | 16.8 | 117,950 | 28.9 | 1.26 | 3.0 | 2,549 | 5.3 | 2.9 |
| Opioids (N=56,844) | 6,670 | 11.7 | 36,168 | 63.6 | 11,873 | 20.9 | 2,133 | 3.8 | 41.8 | 14.4 | 28,624 | 50.4 | 2.05 | 5.2 | 263 | 4.8 | 2.9 |
| Cannabis (N=23,335) | 4,692 | 20.1 | 14,480 | 62.1 | 3,145 | 13.5 | 1,018 | 4.4 | 27.0 | 10.6 | 13,828 | 59.3 | 0.82 | 2.0 | 182 | 3.9 | 2.6 |
TABLE 2. Characteristics of Patients Assigned to Initial Unmatched Cohort Groups in a Study of Use of Methamphetamine and Other Drugs and Schizophrenia
We used a 1:1 propensity score matching procedure (22) to create groups matched on age at index admission, race (white, black, Hispanic, other), sex, time from the index admission to the last date in the study file, California region of patient's residence, Charlson comorbidity index score (a clinical severity algorithm providing a weighted score based on the presence of major medical comorbidities diagnosed at the index admission in the study) (23), and number of hospital admissions following index admission until the outcome event or study end, whichever occurred first. Balance between variables in all of the propensity score-matched samples was assessed using standardized differences (d), where a value of d >10 represented a meaningful difference between groups (24).
Cox regression.
The Cox proportional hazards method was used to compare differences in hazard of readmission with schizophrenia between the matched drug cohorts and population-proxy control groups and between the matched methamphetamine group and drug cohorts. All Cox models involving propensity score-matched groups used a robust variance estimator to account for the matched nature of the sample (25).
Results
Table 2 summarizes the characteristics of all eligible individuals assigned to the unmatched comparison and drug groups. Approximately 93% of individuals in the methamphetamine group (N=42,412) received a single ICD-9 diagnostic code for either methamphetamine abuse (304.4) or methamphetamine dependence (305.7).
Propensity Score Matching
None of the matched cohort groups listed in Tables 3 and 4 (or in Table S1 in the data supplement that accompanies the online edition of this article) had any meaningful standardized differences across any of the continuous variables or any level of the categorical variables used in the propensity score matching process. Hence, we followed the recommended approach and did not include any of the matching variables in the Cox modeling procedures (26).
| Appendicitis Cohort as Matcheda Reference Group | Exclusion Criteria Cohort as Matcheda Reference Group | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | Total Nb | Eventsc | Hazard Ratiod | 95% CI | p | Total Nb | Eventsc | Hazard Ratiod | 95% CI | p |
| Appendicitis | 352,024e | 102:83 | 1.23 | 0.93–1.63 | 0.15 | |||||
| Methamphetamine | 72,324 | 324:34 | 9.37 | 6.59–13.32 | <0.001 | 78,474 | 343:20 | 17.49 | 11.15–27.45 | <0.001 |
| Cocaine | 43,752 | 177:31 | 5.84 | 3.99–8.55 | <0.001 | 66,386 | 350:41 | 8.57 | 6.20–11.84 | <0.001 |
| Alcohol | 243,064 | 535:97 | 5.56 | 4.48–6.90 | <0.001 | 722,974 | 2028:313 | 6.47 | 5.75–7.29 | <0.001 |
| Opioids | 90,436 | 180:49 | 3.60 | 2.63–4.94 | <0.001 | 101,480 | 224:51 | 4.32 | 3.19–5.86 | <0.001 |
| Cannabis | 41,670 | 155:19 | 8.16 | 5.08–13.12 | <0.001 | 44,348 | 164:12 | 13.56 | 7.56–24.31 | <0.001 |
TABLE 3. Risk of Schizophrenia in Propensity Score-Matched Drug Cohorts and Population Proxy Comparison Groups
| Group (Reference Group)a | Hazard Ratiob | 95% CI | Total Nc | Eventsd | p |
|---|---|---|---|---|---|
| Methamphetamine (cocaine) | 1.46 | 1.15–1.85 | 30,040 | 170:140 | 0.002 |
| Methamphetamine (opioids) | 2.81 | 2.21–3.58 | 49,048 | 252:89 | <0.001 |
| Methamphetamine (alcohol) | 1.68 | 1.41–1.99 | 72,754 | 353:205 | <0.001 |
| Methamphetamine (cannabis) | 1.24 | 0.98–1.56 | 35,756 | 161:129 | 0.073 |
TABLE 4. Risk of Schizophrenia in the Methamphetamine Cohort Relative to Propensity Score-Matched Drug Comparison Groups
Cox Regression
Matched drug and population proxy cohorts.
The final Cox models demonstrated that individuals assigned to the methamphetamine group or to the other drug cohorts had a significantly greater risk of readmission with a schizophrenia diagnosis than individuals in the appendicitis or exclusion criteria cohorts (Table 3). The results generated from use of either of the population proxies demonstrated, in each drug group, similarly elevated risks of schizophrenia, similar patterns of statistical significance, and overlapping 95% confidence intervals for the corresponding hazard ratio estimates.
Matched methamphetamine and other drug cohort analyses.
In the final Cox models, individuals assigned to the methamphetamine group manifested a significantly greater risk of readmission with a schizophrenia diagnosis than individuals in the cocaine, alcohol, and opioid drug groups, but no difference in hazard in comparison with the matched cannabis group (Table 4).
Summary of Main Findings
Figure 1 presents a visual summary of the main findings. The panel on the left demonstrates a significantly elevated risk of schizophrenia in all of the drug groups relative to the appendicitis comparison group, with the methamphetamine group, followed by the cannabis cohort, having the highest hazard ratio point estimates. The panel on the right shows that the methamphetamine cohort has a significantly higher risk of schizophrenia than all of the drug comparison groups except the cannabis cohort.
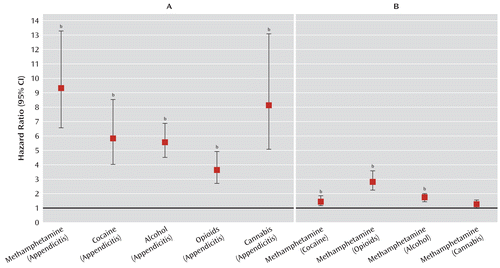
FIGURE 1. Risk of Schizophrenia in the Drug Cohorts Relative to the Population Proxy (Appendicitis) Comparison Group and in the Methamphetamine Cohort Relative to the Drug Cohort Comparison Groupsa
a Reference group in parentheses.
b Statistically significant difference, p<0.005.
Sensitivity Analyses
As shown in Table S1 in the online data supplement, we found that the Cox models with propensity score matching and traditional Cox modeling with covariate adjustment produced 1) the same pattern of statistical significance across each of the analyses listed in Tables 3 and 4; 2) the same pattern of elevated risk of schizophrenia in the methamphetamine group relative to the appendicitis comparison group and the other drug cohorts; 3) similar patterns of an elevated risk of schizophrenia in the other drug groups relative to the population proxy comparison groups; but 4) more conservative hazard ratio estimates in the propensity score matching approach in the drug cohorts relative to the population proxy comparison groups.
To reduce the possibility that our findings were affected by patients' having received psychosis-related diagnoses before the start date of the study (July 1, 1990), we repeated the analyses listed in Tables 3 and 4 but with a study start date of July 1, 1992, thus ensuring that the sample analyzed had at least a 2-year period without such diagnoses. The resulting Cox regression hazard ratio estimates and statistical significance patterns were similar (data not shown).
Discussion
We found that methamphetamine users who have been hospitalized have a much higher risk of receiving a subsequent diagnosis of schizophrenia than do matched population proxy groups. However, in comparison with the other drug use groups, the risk was similar to that of cannabis users and modestly higher than that of users of alcohol, cocaine, or opioids. Somewhat surprisingly, the risk of schizophrenia was also higher in users of all examined drugs compared with the proxy comparison groups.
Comparison With Existing Literature
Our finding of an elevated risk of developing schizophrenia in cannabis users not only supports similar findings from previous longitudinal studies (1) but also contributes to this literature by showing that an even greater risk of subsequent schizophrenia can be observed in a group of (likely) heavy cannabis users having a severity that warranted a hospital diagnosis. To our knowledge, no data are available from large-scale, sufficiently powered longitudinal studies specifically investigating the possible influence of the other drugs of abuse we examined here in relation to schizophrenia, and our findings therefore require independent replication (27). In this regard, one of the original Swedish cohort investigations (28), although focused on cannabis, reported a significant univariate association between risk of schizophrenia and use of stimulants. The authors noted, however, that this effect did not appear in the multivariate analyses, and the study design was not adequately powered to address this question.
Our findings are consistent with and extend the perspective of our colleagues in Japan who propose that methamphetamine can produce prolonged psychotic syndromes in some individuals lacking preexisting psychosis (29). However, the Japanese position relies almost exclusively on case-series studies of methamphetamine users admitted to psychiatric hospitals with already occurring psychotic conditions (30), which cannot provide incidence estimates of persistent psychotic syndromes in this group. Our study extends this literature by providing comparative incidence data on the occurrence of a persistent psychotic condition among individuals diagnosed with methamphetamine use disorders. Approximately 1% of the hospital patients diagnosed with methamphetamine use disorders in our study were readmitted with a subsequent schizophrenia diagnosis, and this finding suggests that methamphetamine use severe enough to warrant a hospital diagnosis might be associated with the development of a schizophrenia-like persistent psychotic syndrome in a small subset of users.
Our primary results relied on Cox regression modeling with propensity score matching. As to the ongoing question of the relative merits of propensity score versus conventional regression approaches, systematic reviews (26, 31) have found that the two approaches generate similar parameter estimates and patterns of statistical significance in a large majority of instances (∼85%–90%), with propensity score matching methods tending to yield more conservative results—a pattern we found in our head-to-head comparisons of the two approaches (see Table S1 in the online data supplement). At this time, however, there do not appear to be any criterion standards to determine whether either of the approaches provides a truer result (26).
Strengths and Limitations
Our record linkage study has a number of strengths, including the ability to track a large population-based sample of methamphetamine users over a substantial period. Given the high cost and common obstacles (e.g., participant loss to follow-up) associated with long-term longitudinal studies of severe drug users, especially in regard to estimation of low-incidence conditions such as schizophrenia, our record linkage approach may be the only feasible design available to address this question. Nonetheless, there are inherent limitations associated with our study. While our group assignment algorithm excluded individuals with indications of multiple drug abuse or dependence, some undetected use of other drugs was likely. Such polydrug use, especially the possible influence of cannabis consumption across groups, may have contributed to the similar incidence patterns seen among the drug cohorts. Also, study subjects were limited to substance users having a severity sufficient to receive a hospital diagnosis, and thus a dose-response relationship could not be assessed.
Compared with patients in the appendicitis group, those in the methamphetamine cohort may have died at a higher rate during follow-up or had lower rates of health care insurance and, as a result, less access to medical care—two factors that could have led to an underestimation of the incidence rate of schizophrenia in our analyses of the methamphetamine and appendicitis groups. In addition, the mortality rates of the drug groups may have differed, and the lack of linked death records in our study may have introduced bias into our comparisons between the methamphetamine group and the other drug cohorts. Also, our identification of incident schizophrenia cases relied on inpatient admissions, an approach that would not have captured individuals with schizophrenia diagnosed in other settings. Nonetheless, a systematic review of the incidence of schizophrenia found that detection of schizophrenia outcomes across studies did not differ significantly by method of case ascertainment (e.g., use of hospital records, face-to-face interviews, and community-based surveys) (18).
Diagnostic validity is a significant concern, especially given the absence of a clear etiopathogenetic understanding of schizophrenia (32). In the United States, clinical diagnoses of schizophrenia during the 1990–2000 period would have been guided by the criteria established by APA (DSM-III-R from 1990 to 1994 and DSM-IV from 1994 to 2000), which in turn would be translated to an ICD-9-CM diagnosis for coding purposes. The use of “crosswalk” documents (33) helping coders determine the ICD-9-CM code corresponding to the DSM diagnosis makes it likely that DSM-III-R and DSM-IV clinical diagnoses of schizophrenia would be similarly coded in the ICD-9 classification. Both systems seek to distinguish primary psychotic disorders such as schizophrenia from drug-induced psychotic disorders. In the absence of independent chart reviews, however, there is uncertainty as to whether diagnoses of schizophrenia were justified or whether patients were correctly assessed as not having schizophrenia at study entry. However, our supplemental analyses, which ensured that individuals had at least a 2-year period without any psychosis-related inpatient events prior to their index admission, limits to some extent the possibility of reverse causality. Also, we cannot confirm that patients with a schizophrenia diagnosis were assessed over a prolonged drug-free interval to exclude a drug-induced psychotic state.
There is also concern about whether the primary population proxy appendicitis comparison group was appropriately chosen. However, as mentioned previously, the unmatched appendicitis group in our study manifested an incidence rate of schizophrenia similar to that in a recent review of literature. In addition, the appendicitis group and our secondary population proxy group had similar incidence rates of schizophrenia, and analyses using either comparison group produced similar results. To address the potential ascertainment bias that might have occurred if comparison subjects had a different propensity to visit a hospital that would provide a psychiatric diagnosis, we matched cohorts on number of hospital admissions.
Conclusions
Our findings add to the growing literature on cannabis as a risk factor for schizophrenia and, in addition, suggest that methamphetamine use sufficient to warrant a hospital diagnosis may also be a risk factor. We do have some skepticism about the suggestion in our data that the risk of subsequent development of schizophrenia is elevated in all of the major drug-of-abuse groups. This finding, which requires replication, was unanticipated, in large part because of the apparent absence in the literature of large-scale longitudinal studies (except those examining cannabis users) sufficiently powered to have addressed this issue (27). In the context of the stress-vulnerability (7) and dopamine sensitization (34) hypotheses of schizophrenia, it could in fact be argued that the propensity for development of a persistent psychosis could be elevated after significant use of any drug of abuse, given that all such drugs are “stressors” and that most act on the dopamine system. It has also been proposed, for example, that drugs of abuse (e.g., alcohol) might cause brain structural abnormalities that could increase the risk of schizophrenia in genetically susceptible individuals (35). The relationship between methamphetamine use and schizophrenia could involve a common etiology, shared genetic and environmental (e.g., low socioeconomic status) vulnerability factors (36), or a premorbid state (e.g., depression, anxiety, and poor cognitive functioning) prompting substance use (as an attempt at self-medication) and pathological drug-induced sensitization (34), in which repeated exposure to a dopaminergic stimulant induces a hyperdopaminergic state sufficient to induce psychosis in vulnerable individuals.
Schizophrenia can be a difficult diagnosis to establish, especially in chronic methamphetamine users, and clinicians need to be vigilant in monitoring their substance-abusing patients for signs of a developing persistent psychotic condition. There are important prognostic and treatment implications accompanying a diagnosis of schizophrenia. Current evidence links schizophrenia with impairment in both neurocognition and social cognition, as well as with significant functional disability over time. Antipsychotic medications, which often have significant side effects, are considered a cornerstone of treatment programs for schizophrenia, and current recommendations suggest that these be continued throughout life (37). Thus, an incorrect diagnosis can have profound negative implications. Clinicians should therefore be tentative in their coding until they are fairly certain that psychosis associated with methamphetamine use can reasonably be resolved into a chronic, drug-independent condition. Finally, our findings bring again into focus the ongoing debate—not addressed in this study—of whether persistent substance-related psychosis is in any way distinct (in pathogenesis, symptom features, course, and treatment response) from that classified as schizophrenia and hence requires a separate diagnostic classification (38).
1. : An overview of systematic reviews on cannabis and psychosis: discussing apparently conflicting results. Drug Alcohol Rev 2010; 29:304–317Crossref, Medline, Google Scholar
2. : An overview of methamphetamine-induced psychotic syndromes. Addict Disord Their Treat 2008; 7:143–156Crossref, Google Scholar
3. : Relapse of paranoid psychotic state in methamphetamine model of schizophrenia. Schizophr Bull 1992; 18:115–122Crossref, Medline, Google Scholar
4. : Amphetamine psychosis: clinical variations of the syndrome, in Amphetamine and Its Analogs: Pharmacology, Toxicology, and Abuse. Edited by Cho AKSegal DS. New York, Academic Press, 1994, pp 387–411Google Scholar
5. : Remission of persistent methamphetamine-induced psychosis after electroconvulsive therapy: presentation of a case and review of the literature. Am J Psychiatry 2010; 167:17–23Link, Google Scholar
6. : When does amphetamine-induced psychosis become schizophrenia? Am J Psychiatry 1996; 153:812–815Link, Google Scholar
7. : Psychosocial stress and psychosis: a review of the neurobiological mechanisms and the evidence for gene-stress interaction. Schizophr Bull 2008; 34:1095–1105Crossref, Medline, Google Scholar
8. : New Way to Edit: Discharge Data Review. Sacramento, California Office of Statewide Health Planning and Development, 1990Google Scholar
9.
10.
11. : Reliability of the recording of schizophrenia and depressive disorder in the Saskatchewan health care datafiles. Soc Psychiatry Psychiatr Epidemiol 1997; 32:191–199Crossref, Medline, Google Scholar
12. : Psychiatric diagnoses as reported to Medicaid and as recorded in patient charts. Am J Public Health 1980; 70:406–408Crossref, Medline, Google Scholar
13. : Agreement between administrative files and written medical records: a case of the Department of Veterans Affairs. Med Care 1998; 36:1324–1336Crossref, Medline, Google Scholar
14. : Appendectomy: a contemporary appraisal. Ann Surg 1997; 225:252–261Crossref, Medline, Google Scholar
15. : Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev 2004; 4:CD001546Medline, Google Scholar
16. : Incidence of Parkinson's disease among hospital patients with methamphetamine-use disorders. Mov Disord 2010; 25:2333–2339Crossref, Medline, Google Scholar
17. : An increased risk of stroke among young schizophrenia patients. Schizophr Res 2008; 101:234–241Crossref, Medline, Google Scholar
18. : A systematic review of the incidence of schizophrenia: the distribution of rates and the influence of sex, urbanicity, migrant status, and methodology. BMC Med 2004; 2:13Crossref, Medline, Google Scholar
19.
20. : Impacts of federal ephedrine and pseudoephedrine regulations on methamphetamine-related hospital admissions. Addiction 2003; 98:1229–1237Crossref, Medline, Google Scholar
21. : Estimating treatment effects using observational data. JAMA 2007; 297:314–316Crossref, Medline, Google Scholar
22. : Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998; 17:2265–2281Crossref, Medline, Google Scholar
23. : A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–383Crossref, Medline, Google Scholar
24. : A comparison of propensity score methods: a case-study estimating the effectiveness of post-AMI statin use. Stat Med 2006; 25:2084–2106Crossref, Medline, Google Scholar
25. : Propensity-score matching in the cardiovascular surgery literature from 2004 to 2006: a systematic review and suggestions for improvement. J Thorac Cardiovasc Surg 2007; 134:1128–1135Crossref, Medline, Google Scholar
26. : Propensity score methods gave similar results to traditional regression modeling in observational studies: a systematic review. J Clin Epidemiol 2005; 58:550–559Crossref, Medline, Google Scholar
27. : How strong is the evidence that illicit drug use by young people is an important cause of psychological or social harm? methodological and policy implications of a systematic review of longitudinal, general population studies. Drugs Educ Prevent Policy 2004; 11:281–297Crossref, Google Scholar
28. : Self reported cannabis use as a risk factor for schizophrenia in Swedish conscripts of 1969: historical cohort study. BMJ 2002; 325:1199Crossref, Medline, Google Scholar
29. : Clinical features of sensitization to methamphetamine observed in patients with methamphetamine dependence and psychosis. Ann NY Acad Sci 2004; 1025:279–287Crossref, Medline, Google Scholar
30. : Use and abuse of amphetamines in Japan, in Amphetamine and Its Analogs: Pharmacology, Toxicology, and Abuse. Edited by Cho AKSegal DS. New York, Academic Press, 1994, pp 415–435Google Scholar
31. : A review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methods. J Clin Epidemiol 2006; 59:437–447Crossref, Medline, Google Scholar
32. : Competing definitions of schizophrenia: what can be learned from polydiagnostic studies? Schizophr Bull 2007; 33:1178–1200Crossref, Medline, Google Scholar
33. : A crosswalk from DSM-III-R to ICD-9-CM. Am J Psychiatry 1989; 146:1315–1319Link, Google Scholar
34. : Neurochemical sensitization in the pathophysiology of schizophrenia: deficits and dysfunction in neuronal regulation and plasticity. Neuropsychopharmacology 1997; 17:205–229Crossref, Medline, Google Scholar
35. : The impact of substance use on brain structure in people at high risk of developing schizophrenia. Schizophr Bull 2011; 37:1066–1076Crossref, Medline, Google Scholar
36. : Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr Bull 2008; 34:1066–1082Crossref, Medline, Google Scholar
37.
38. : DSM-V research agenda: substance abuse/psychosis comorbidity. Schizophr Bull 2007; 33:947–952Crossref, Medline, Google Scholar



