Naltrexone for the Treatment of Amphetamine Dependence: A Randomized, Placebo-Controlled Trial
Abstract
Objective: Currently there is no approved pharmacotherapy for amphetamine dependence. Recent human laboratory studies have demonstrated that naltrexone modulates some of the reinforcing effects of amphetamine. The aim of this study was to investigate the efficacy of naltrexone in comparison with placebo in reducing relapse to amphetamine use in amphetamine-dependent patients. Method: Eighty patients who met DSM-IV criteria for amphetamine dependence were randomized to 12 weeks of double-blind naltrexone (50 mg) or placebo treatment. Patients visited the clinic twice weekly to receive medication and relapse prevention therapy and leave urine samples, which were analyzed for drug toxicology and for assessing adherence to medication via detection of naltrexone’s metabolite (6-β-naltrexol). The main outcome measure was abstinence from amphetamine use, as indicated by the total number of negative amphetamine urine samples during 12 weeks of treatment. All missing urine samples were defined for the analysis as positive for amphetamine. Results: Overall, 55 patients (68.8%) completed the trial. The intention-to-treat analysis showed that the naltrexone group had a significantly higher number of amphetamine-negative urine samples compared with the placebo group. Survival analyses showed that the treatment groups differed in rate of continuous abstinence, in both the intention-to-treat and completer samples, in favor of naltrexone treatment. There was a significant reduction in craving levels and self-reported consumption of amphetamine in the naltrexone group compared with the placebo group. Treatment with naltrexone was well tolerated in this sample. Conclusions: This trial demonstrated the efficacy of naltrexone in reducing amphetamine use in amphetamine-dependent individuals.
Amphetamine abuse and dependence represent a major public health problem with growing psychiatric, social, and economic consequences. The total number of amphetamine abusers worldwide is estimated at 34 million, which exceeds the combined number of cocaine and heroin abusers (1) . In Sweden, amphetamine is the most commonly abused substance after cannabis and alcohol (2) . Thus far, treatment of amphetamine users in emergency departments and psychiatric clinics has focused on management of the acute and short-term consequences of amphetamine dependence, such as withdrawal symptoms, amphetamine-induced psychosis, and associated depression. However, treatment targeting only acute or short-term problems has proved insufficient for alleviating recurrent craving and preventing relapse to compulsive drug use (3) . To date, an efficacious pharmacotherapy has remained elusive, despite extensive research on the neurobiological effects of amphetamine (4) .
The reinforcing effects of amphetamine are principally mediated via stimulation of the cortico-mesolimbic dopamine system (5) . However, other neurotransmitter systems modulate brain dopamine, such as the μ-opioid receptors, which are located on the mesolimbic dopamine neurons (6) . Furthermore, certain brain regions known to have a role in appetitive behaviors, such as the ventral tegmental area and the substantia nigra, contain neurons in which dopamine and opioids coexist (7 , 8) . The important functional interaction of the dopamine-opioid system specifically in stimulant abuse is evident from results of preclinical and clinical studies. For example, in rat studies, chronic administration of cocaine has been shown to increase brain m-opioid receptor binding in regions relevant to reward (such as the amygdala and the nucleus accumbens), and this effect seems to be mediated through dopamine receptors, which is evident in the fact that coadministration of dopamine receptor antagonists blocks this effect (9 , 10) . A recent positron emission tomography study demonstrated an increase in m-opioid receptor binding in limbic areas in chronic cocaine abusers, and this increased binding was correlated with self-reported craving (11) .
Although these changes were observed after chronic cocaine use, a similar effect could be expected in amphetamine use, based on the common pharmacological mechanisms of these drugs. Clinical data from our earlier studies using naltrexone, an opioid antagonist, with healthy volunteers (12) and with amphetamine-dependent patients (13) demonstrated that naltrexone blunted the subjective effects of amphetamine in both populations. In the patients, naltrexone also reduced craving for amphetamine. These results suggest that there could be a dopaminergic-opioid interaction in amphetamine use as well. The finding that an acute dose of naltrexone attenuated the subjective effects of amphetamine under experimental laboratory conditions demonstrates a potential pharmacotherapeutic efficacy of naltrexone for the treatment of amphetamine dependence.
Taken together, the preclinical and human laboratory studies provide the rationale for a clinical trial evaluating the efficacy of naltrexone in reducing relapse to amphetamine use in amphetamine-dependent individuals.
Method
Participants
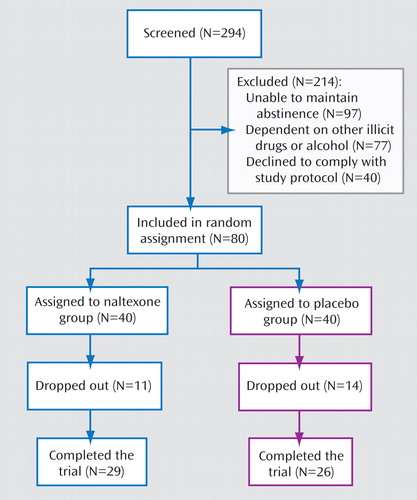
A total of 80 treatment-seeking amphetamine-dependent individuals 20–65 years of age were recruited from the Stockholm metropolitan area via advertisements in local newspapers and written information sent to social workers. The study was approved by the regional ethical review board in Stockholm and the Swedish Medical Products Agency, and the trial was conducted in accordance with the Good Clinical Practice Guideline of the International Conference on Harmonization and with the Declaration of Helsinki. Written informed consent was obtained from all patients.
To be eligible for the study, patients had to meet DSM-IV criteria for amphetamine dependence and to have used amphetamine on at least 12 days in the past 12 weeks. Exclusion criteria included a current DSM-IV diagnosis of any other substance dependence syndrome except nicotine; a history of a major psychiatric disorder (e.g., schizophrenia or psychosis) or a current psychiatric condition requiring medication; use of any opioid medication or illicit opiates in the past month; current use of benzodiazepines; traces of any illicit substance in the urine (amphetamine, benzodiazepines, cannabis, cocaine [benzoylecgonine], dextropropoxyphene, or opiates); a serious somatic disease (e.g., seizure disorder, glaucoma, arteriosclerosis, hyperthyroidism); serum aspartate or alanine aminotransferase activity greater than three times the upper reference range or a serum bilirubin concentration greater than twice the upper reference range; and pregnancy or lactation.
Study Design
This was a single-site randomized, double-blind, placebo-controlled trial of naltrexone for amphetamine dependence. Enrollment began in January 2003 and ended after 29 months. Initial eligibility was ascertained by telephone screening. All eligible patients had a 2-week lead-in period to assist with and confirm their current drug-free state. This process enabled the inclusion of patients who not only expressed a verbal motivation and commitment to seek treatment but also displayed some supporting behavioral evidence.
After the lead-in period, 80 patients were randomly assigned to receive either placebo or naltrexone treatment for 12 weeks. The randomization process was conducted by the Karolinska University Hospital pharmacy with software designed for the purpose. The randomization code was kept by the pharmacy and disclosed at the end of the trial. No interim analysis was performed. Patients were asked to attend the clinic twice weekly. On the first weekly visit, they met with a research nurse, filled out questionnaires, left supervised urine samples, and collected the weekly medication. On the second weekly visit, they received relapse prevention therapy from a licensed psychologist and left supervised urine samples. All urine samples were screened for amphetamine, benzodiazepines, cannabis, cocaine (benzoylecgonine), dextropropoxyphene, and opiates.
Assessments and Outcome Measures
At intake each patient underwent a physical examination and was assessed with the Structured Clinical Interview for DSM-IV. In addition, urine and blood analyses were conducted. Current symptoms of attention deficit hyperactivity disorder (ADHD) were assessed at baseline, first using a checklist of DSM-IV criteria and then a complete neuropsychiatric battery, including interviews with parents or siblings, to ascertain the diagnosis of ADHD. The Addiction Severity Index (14) was administered before (week 0) and after treatment (week 12). Weekly assessments included self-reports of drug consumption, which were obtained by the timeline followback interview (15) , and of craving for amphetamine, which was assessed on a 7-point visual analogue scale measuring craving at the present moment and over the past week (adapted from the Tiffany et al. cocaine craving scale [16] ). Monitoring of adverse effects was carried out by both the physician (through interview) and the patient (in self-ratings of intensity and duration) using a standardized form. At weeks 4, 8, and 12, liver enzyme and bilirubin concentrations were measured.
Urine samples were screened for amphetamines by immunoassay with a cutoff level of 500 ng/ml. The confirmation analyses of the positive samples, which included amphetamine, methamphetamine, 3,4-methylenedioxyamphetamine, and 3,4-methylenedioxymethamphetamine (reporting limit=300 ng/ml), were performed using a liquid chromatography-tandem mass spectrometry method (17) .
Treatments
Naltrexone (50-mg capsules) and matching placebo capsules were obtained through the Karolinska University Hospital pharmacy and prepared for each patient in medication blister packages. Each week, participants received a blister package containing seven capsules, dispensed by the nurse. On the following visit, patients returned the empty blister package with any remaining capsules for pill count. Weekly urine samples were assayed for 6-β-naltrexol, the active metabolite of naltrexone, by mass spectrometry with a quantification limit of 1 μg/ml. Adherence to medication was assessed by detection of 6-β-naltrexol in urine and by pill counts.
All participants received standardized manual-based relapse prevention therapy (18) , delivered in 60-minute sessions once weekly by a licensed psychologist. All sessions were individualized and documented in the case record forms by the therapist.
Power Calculation
With the prediction of a between-group difference of 16% and a standard deviation of 20% (corresponding to a strong effect size according to Cohen’s definition), an effective sample size of 2×30 was calculated to give a corresponding power of 0.85. Given the high probability of dropouts, we increased the sample size to 2×40.
Data Analysis
The primary outcome measure of the study was the number of negative amphetamine urine samples during 12 weeks of treatment (of a total of 24 samples). All missing urine samples were defined as positive in the analysis. The primary analysis was carried out according to the intention-to-treat approach. Treatment efficacy was analyzed by repeated-measures analysis of variance (ANOVA) comparing naltrexone and placebo patients over 12 weeks. A secondary completer analysis on the primary outcome measure was conducted using the same statistical method; a completer was defined as per protocol as a patient who provided at least 16 of the total of 24 urine samples. Time-to-event (relapse) analysis was carried out for both the intention-to-treat and completer samples.
A Kaplan-Meier analysis was used to compute rates of continuous abstinence from amphetamine, in which the time-dependent survival (nonrelapse as measured by negative amphetamine urine samples) probabilities for both treatment groups were computed for both the intention-to-treat and completer samples.
The secondary outcome measures were self-reported use of amphetamine, alcohol, and other drugs; craving for amphetamine; and liver enzyme levels. All secondary measures were analyzed by repeated-measures ANOVA comparing naltrexone and placebo patients over 12 weeks of treatment.
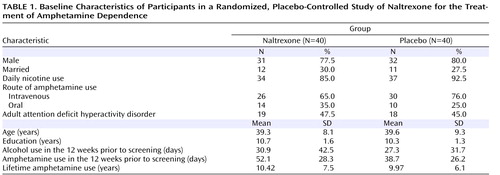
Patients were compared on baseline characteristics using chi-square tests for categorical variables and t tests for continuous variables in order to assess the efficiency of the randomization procedure in ensuring homogeneity between the two treatment groups.
Results
Of 294 screened individuals, 80 met the study criteria ( Figure 1 ). There were no significant differences between the two treatment groups in sociodemographic or clinical characteristics, except in amphetamine use prior to enrollment in the study. The naltrexone group reported 52.6 days of use, compared with 37.1 days for the placebo group. This difference could not be explained by any known demographic factor ( Table 1 ).
Amphetamine Abstinence
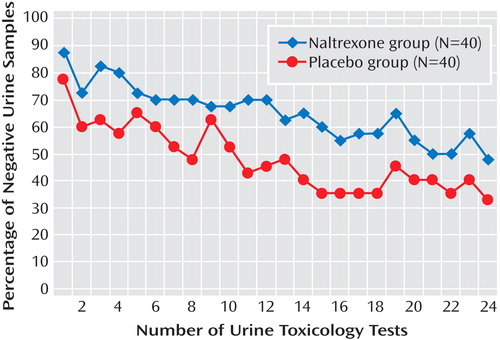
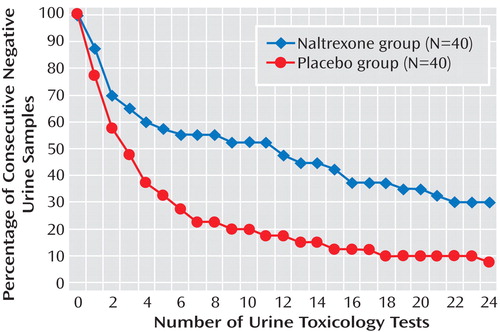
On the primary outcome measure, the intention-to-treat analysis showed that during treatment, the naltrexone group had a significantly higher mean number of amphetamine-negative urine samples than the placebo group (F=5.02, df=1, 78, p<0.05) ( Figure 2 ). There was also an effect of time in treatment (F=8.11, df=23, 56, p<0.05) showing that the mean number of negative urine samples became lower over time for both treatment groups. The mean percentage of negative urine samples during the 12-week trial was 65.2% (SD=36.1) for the naltrexone group and 47.7% (SD=33.7) for the placebo group. The time-to-event analysis for the intention-to-treat sample showed that the mean number of negative tests until a relapse occurred was 12.5 (SD=1.6) for the naltrexone group and 6.3 (SD=1.1) for the placebo group.
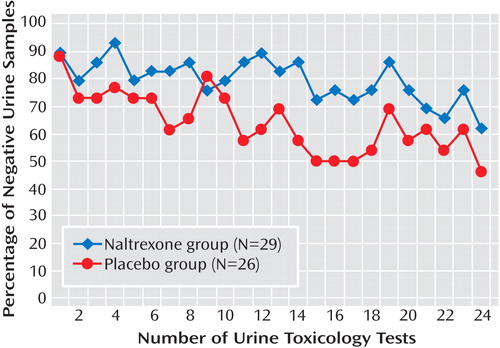
The same pattern was observed in the completer analysis, which showed that the naltrexone group had a significantly higher mean number of amphetamine-negative urine samples than the placebo group (F=4.15, df=1, 53, p<0.05) ( Figure 3 ). There was a decrease in the mean number of negative urine samples for both groups as an effect of time in treatment (F=3.36, df=23, 31, p<0.05). The mean percentage of negative urine samples during the 12-week trial was 79.7% (SD=28.8) for the naltrexone group and 64.1% (SD=27.9) for the placebo group. The time-to-event analysis (for completers) showed that the mean number of test occasions to a relapse was 15.5 (SD=1.8) for the naltrexone group and 8.2 (SD=1.6) for the placebo group.
Survival analyses were used to examine the rate of continuous abstinence from amphetamine. The results showed that the treatment groups differed in continuous abstinence rates, both in the intention-to-treat (Breslow test, t=6.36, p<0.05) and in the completer samples (Breslow test, t=5.34, p<0.05), in favor of naltrexone treatment ( Figure 4 displays results for the intention-to-treat sample). The effect size we obtained for the intention-to-treat sample was d=0.5, which is between “reasonably strong” and “strong” according to Cohen’s definition. There were no differences in effect of treatment on the variables of gender and ADHD diagnosis (data not shown).
Adherence to Treatment
Treatment retention
Of the 80 patients enrolled, 55 (68.8%) completed the study, with no difference in retention between the two treatment groups (naltrexone 72.5%, placebo 65.0%).
Adherence to medication
Analysis of urinary concentration of 6-β-naltrexol revealed that more than half the patients (25/40) in the naltrexone group adhered to medication (defined as the presence of metabolite in more than eight of 12 weekly tests). In addition, there was a positive correlation between adherence to medication and number of amphetamine-negative urine samples (r=0.69, p<0.05), that is, the higher the adherence to medication, the higher the abstinence rates for the naltrexone group.
Pill count
There was no difference between treatment groups with regard to the total number of blister packages and pills returned each week over the 12-week treatment.
Self-reported drug and alcohol consumption
The reduction in self-reported amphetamine use was greater in the naltrexone group than in the placebo group. Patients in the naltrexone group reported amphetamine use for 52.2% of the days during the 12 weeks preceding enrollment in the study compared with 5.5% of the days during the 12-week treatment period, whereas the patients in the placebo group reported amphetamine use for 38.7% of the days during the preenrollment period compared with 16.1% during the treatment period (F=78.38, df=1, 53, p<0.05).
Results from self-reports and urine toxicology for other drugs (nicotine, alcohol, marijuana, cocaine, and benzodiazepines) showed no differences between the naltrexone and placebo groups at enrollment or during treatment.
Amphetamine craving scale
The two treatment groups were similar in mean craving scores (7-point scale) at week 0 (3.5 [SD=1.4]). There was also an interaction effect between treatment and time (F=5.0, df=3, p<0.05), indicating that the naltrexone group had a greater reduction in craving scores over 12 weeks as compared with the placebo group, and this was evident from week 4 onward.
Addiction Severity Index
There were no differences between the treatment groups in scores on the Addiction Severity Index.
Safety and tolerability
Safety results were based on data for all patients who received at least one dose of study medication. The most frequently reported adverse effects of naltrexone were nausea, headache, fatigue, and gastrointestinal discomfort. A total of 14 patients in the naltrexone group reported these side effects and rated them as mild. None of the participants discontinued the study as a result of any adverse clinical events; discontinuations were due rather to an inability to comply with the treatment protocol (i.e., weekly attendance at the clinic). There were no differences between groups with regard to liver enzyme levels (λ-glutamyltransferase, aspartate, alanine aminotransferase) or bilirubin, either at baseline or during treatment.
Discussion
To our knowledge, this is the first study to report the efficacy of naltrexone for the indication of amphetamine dependence. The study demonstrated a significant effect of medication on reducing amphetamine use, with an effect size of 0.5 for the intention-to-treat sample, which is comparable to the effect size of naltrexone for alcohol dependence. Treatment with naltrexone reduced the percentage of amphetamine-positive urine samples in patients with chronic amphetamine dependence. The mechanism by which naltrexone mediates this effect is not clear. It has been established (13 , 19 , 20) that the therapeutic effect of naltrexone does not result from any nonspecific effect, such as a pharmacokinetic interaction. A more probable mechanism is a reduction of the mood-altering or other subjective effects of the stimulant (e.g., euphoria or liking the drug’s effect), leading to a reduction in drug use. Our findings indicate that a 12-week treatment with naltrexone leads to a sustained effect on the behavioral and subjective correlates of amphetamine dependence, that is, a reduction in drug consumption and craving.
In our sample, in which daily doses of amphetamine use before enrollment ranged from 0.5 g to 2 g, adherence to treatment was generally good. An analysis of the participants who adhered to the study medication (as assessed by the presence of 6-β-naltrexol in urine) was performed to elucidate more clearly the treatment effects of naltrexone. In the naltrexone group, 62.5% of patients exhibited a continuous measure of adherence to medication. Adherence to medication was correlated with reduction in drug use, indicating that adequate exposure to the medication was an important factor in the change in amphetamine use patterns. The duration of effect of amphetamine is 6–10 hours, while the plasma half-life of a 50-mg dose of naltrexone is 10–12 hours, with an ability to block the μ receptor for more than 72 hours (21) . This, in combination with reductions in amphetamine use and craving, suggests that the duration of inhibition of the opioid receptors by a 50-mg dose of naltrexone was sufficient to counteract the reinforcing effect of amphetamine. Attendance at the relapse prevention sessions was similar for the two groups, and this may have contributed to the observed reduction in drug use in the placebo group as well. While the absence of a biomarker for adherence to placebo medication is a limitation of the study, the results indicate that the ability of naltrexone to promote a clinically significant reduction in drug consumption may be highly dependent on medication adherence.
At the start of treatment, the mean craving scores for the naltrexone and placebo groups were similar. Continued treatment with naltrexone, however, led to a reduction in craving scores as compared with placebo treatment over 12 weeks, and this was evident from week 4 onward. Previous treatment studies of alcohol and cocaine dependence have reported that naltrexone may affect the likelihood of future drug use by decreasing both craving for and liking of the drug (22 , 23) . The clinical significance of this finding is that despite exposure to amphetamine, the reduction in craving for the drug with naltrexone treatment in turn reduces the risk of relapse and helps patients maintain the motivation (enhanced by the relapse prevention therapy) to remain drug free. Thus, it may be that the decrease in drug consumption with naltrexone treatment results from its action on reducing the pleasure and/or high along with the craving. The reduction of these pleasurable effects, along with a greater control over the craving-driven impulses, could also be expected to reduce the likelihood that a patient will increase the daily dose of amphetamine to achieve a larger effect.
There was no evidence (by urine toxicology or timeline followback) of any increase in intake of alcohol or other drugs during treatment to compensate for the reduction in amphetamine consumption. The data provide evidence that the reduction in amphetamine use is mediated by a combination of relapse prevention therapy and medication treatment rather than a consequence of an increase in use of other drugs.
Treatment with naltrexone did not produce any serious adverse effects, and no dropouts were attributed to adverse events. This is consistent with earlier studies of naltrexone for alcohol- and amphetamine-dependent patients (24 , 25) , and it suggests that the reasonable adherence and retention rates observed in the naltrexone group are partly related to the lack of significant medication side effects.
There was no differential response to treatment with regard to gender or ADHD diagnosis. The presentation of an ADHD diagnosis among amphetamine-dependent patients was fairly high in our sample, approaching 50%, a proportion similar to that reported in another study (26) , which suggests that there could be a high prevalence of ADHD in the general amphetamine-dependent population.
In Sweden, the vast majority of amphetamine abused is the racemic mixture ( d/l -amphetamine), whereas in other countries, methamphetamine is more common. A quantitative analysis of different amphetamine derivatives in the urine revealed that 16% percent of positive samples displayed a high concentration of methamphetamine. Preclinical data have shown that pretreatment with naltrexone attenuated behavioral sensitization (27) and cue-induced methamphetamine-seeking behavior in rats (28) . Our results in this study, together with these preclinical findings, provide some preliminary evidence of naltrexone’s applicability in methamphetamine dependence as well.
Some limitations of this study must be acknowledged. The sample selected for the study did not achieve an equal representation of the genders. Treatment outcome was assessed for 3 months, and long-term effects in this population are unknown. In addition, future studies should also include patients with major depression to understand the impact of treatment with naltrexone on drug use and the symptoms of depression.
The results of this study extend the findings from previous human experimental laboratory studies (12 , 13) by providing evidence of the efficacy of naltrexone in reducing relapse to amphetamine use. Medication adherence and retention to treatment was high in the group treated with naltrexone, which may be regarded as further proof of concept for naltrexone as a treatment for amphetamine dependence.

1. United Nations Office on Drugs and Crime: World Drug Report 2005. Vienna, 2005Google Scholar
2. Swedish Council for Information on Alcohol and Other Drugs: Drug Trends in Sweden 2006. Report No 98. Stockholm, 2006Google Scholar
3. Dackis C, O’Brien C: Neurobiology of addiction: treatment and public policy ramifications. Nat Neurosci 2005; 8:1431–1436Google Scholar
4. Srisurapanont M, Jarusuraisin N, Kittirattanapaiboon P: Treatment for amphetamine dependence and abuse. Cochrane Database Syst Rev 2001(4):CD003022Google Scholar
5. Wise RA, Bozarth MA: A psychomotor stimulant theory of addiction. Psychol Rev 1987; 94:469–492Google Scholar
6. Llorens-Cortes C, Pollard H, Schwartz JC: Localization of opiate receptors in substantia nigra: evidence by lesion studies. Neurosci Lett 1979; 12:165–170Google Scholar
7. Moore RY, Bloom FE: Central catecholamine neuron systems: anatomy and physiology of the dopamine systems. Annu Rev Neurosci 1978; 1:129–169Google Scholar
8. Johnson RP, Sar M, Stumpf WE: A topographic localization of enkephalin on the dopamine neurons of the rat substantia nigra and ventral tegmental area demonstrated by combined histofluorescence-immunocytochemistry. Brain Res 1980; 194:566–571Google Scholar
9. Unterwald EM: Regulation of opioid receptors by cocaine. Ann N Y Acad Sci 2001; 937:74–92Google Scholar
10. Azaryan AV, Coughlin LJ, Buzas B, Clock BJ, Cox BM: Effect of chronic cocaine treatment on mu- and delta-opioid receptor mRNA levels in dopaminergically innervated brain regions. J Neurochem 1996; 66:443–448Google Scholar
11. Gorelick DA, Kim YK, Bencherif B, Boyd SJ, Nelson R, Copersino M, Endres CJ, Dannals RF, Frost JJ: Imaging brain mu-opioid receptors in abstinent cocaine users: time course and relation to cocaine craving. Biol Psychiatry 2005; 57:1573–1582Google Scholar
12. Jayaram-Lindstrom N, Wennberg P, Hurd YL, Franck J: Effects of naltrexone on the subjective response to amphetamine in healthy volunteers. J Clin Psychopharmacol 2004; 24:665–669Google Scholar
13. Jayaram-Lindström N, Konstenius M, Eksborg S, Beck O, Hammarberg A, Franck J: Naltrexone attenuates the subjective effects of amphetamine in patients with amphetamine dependence. Neuropsychopharmacology 2007; 33:1856–1863Google Scholar
14. McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M: The fifth edition of the Addiction Severity Index. J Subst Abuse Treat 1992; 9:199–213Google Scholar
15. Sobell LC, Sobell MB: A technique for assessing self-reported alcohol consumption, in Measuring Alcohol Consumption. Edited by Litten RZ, Allen J. Totowa, NJ, Humana Press, 1992, pp 44–72Google Scholar
16. Tiffany ST, Singleton E, Haertzen CA, Henningfield JE: The development of a cocaine craving questionnaire. Drug Alcohol Depend 1993; 34:19–28Google Scholar
17. Andersson M, Gustavsson E, Stephanson N, Beck O: Direct injection LC-MS/MS method for identification and quantification of amphetamine, methamphetamine, 3,4-methylenedioxyamphetamine and 3,4-methylenedioxymethamphetamine in urine drug testing. J Chromatogr B Analyt Technol Biomed Life Sci 2008; 861:22–28Google Scholar
18. Caroll KM: A Cognitive-Behavioral Approach: Treating Cocaine Addiction. NIH Publication Number 98-4308. Rockville, Md, National Institute on Drug Abuse, 1998Google Scholar
19. Swift RM, Whelihan W, Kuznetsov O, Buongiorno G, Hsuing H: Naltrexone-induced alterations in human ethanol intoxication. Am J Psychiatry 1994; 151:1463–1467Google Scholar
20. King AC, Volpicelli JR, Frazer A, O’Brien CP: Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology (Berl) 1997; 129:15–22Google Scholar
21. Lee MC, Wagner HN Jr, Tanada S, Frost JJ, Bice AN, Dannals RF: Duration of occupancy of opiate receptors by naltrexone. J Nucl Med 1988; 29:1207–1211Google Scholar
22. Schmitz JM, Stotts AL, Rhoades HM, Grabowski J: Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav 2001; 26:167–180Google Scholar
23. McCaul ME, Wand GS, Eissenberg T, Rohde CA, Cheskin LJ: Naltrexone alters subjective and psychomotor responses to alcohol in heavy drinking subjects. Neuropsychopharmacology 2000; 22:480–492Google Scholar
24. Croop RS, Faulkner EB, Labriola DF: The safety profile of naltrexone in the treatment of alcoholism: results from a multicenter usage study. Arch Gen Psychiatry 1997; 54:1130–1135Google Scholar
25. Jayaram-Lindstrom N, Wennberg P, Beck O, Franck J: An open clinical trial of naltrexone for amphetamine dependence: compliance and tolerability. Nord J Psychiatry 2005; 59:167–171Google Scholar
26. Jaffe C, Bush KR, Straits-Troster K, Meredith C, Romwall L, Rosenbaum G, Cherrier M, Saxon AJ: A comparison of methamphetamine-dependent inpatients childhood attention deficit hyperactivity disorder symptomatology. J Addict Dis 2005; 24:133–152Google Scholar
27. Chiu CT, Ma T, Ho IK: Attenuation of methamphetamine-induced behavioral sensitization in mice by systemic administration of naltrexone. Brain Res Bull 2005; 67:100–109Google Scholar
28. Anggadiredja K, Sakimura K, Hiranita T, Yamamoto T: Naltrexone attenuates cue- but not drug-induced methamphetamine seeking: a possible mechanism for dissociation of primary and secondary reward. Brain Res 2004; 1021:272–276Google Scholar