Selective Muscarinic Receptor Agonist Xanomeline as a Novel Treatment Approach for Schizophrenia
Abstract
Objective: There are significant unmet needs in the treatment of schizophrenia, especially for the treatment of cognitive impairment, negative syndrome, and cognitive function. Preclinical data suggest that agonists with selective affinity for acetylcholine muscarinic receptors provide a potentially new mechanism to treat schizophrenia. The authors studied xanomeline, a relatively selective muscarinic type 1 and type 4 (M 1 and M 4 ) receptor agonist, to determine if this agent is effective in the treatment of schizophrenia. Method: In this pilot study, the authors examined the efficacy of xanomeline on clinical outcomes in subjects with schizophrenia (N=20) utilizing a double-blind, placebo-controlled, 4-week treatment design. Outcome measures included the Positive and Negative Syndrome Scale (PANSS) for schizophrenia, the Brief Psychiatric Rating Scale (BPRS), the Clinical Global Impression (CGI) scale, and a test battery designed to measure cognitive function in patients with schizophrenia. Results: Subjects treated with xanomeline did significantly better than subjects in the placebo group on total BPRS scores and total PANSS scores. In the cognitive test battery, subjects in the xanomeline group showed improvements most robustly in measures of verbal learning and short-term memory function. Conclusions: These results support further investigation of xanomeline as a novel approach to treating schizophrenia.
Schizophrenia is a severe chronic illness characterized by symptoms that can be divided into three broad categories: positive symptoms, such as hallucinations and delusions; negative symptoms, such as emotional blunting and lack of motivation; and cognitive domain symptoms, such as working memory, attention, and information processing deficits (1) . All of the currently available antipsychotic drugs are effective in improving the positive symptoms of schizophrenia (2) and are presumed to have this effect predominantly by antagonizing various dopamine receptors (3) . Some studies of the newer, so-called atypical antipsychotics have demonstrated modest efficacy in ameliorating the negative and cognitive symptoms of schizophrenia (4) . However, these benefits have not been observed in larger trials, and the observed modest effects still leave the majority of patients significantly disabled (5) . Moreover, several methodological issues may confound the interpretation of these findings. For example, changes in negative symptoms may be pseudospecific to changes in overall symptomology. That is, negative symptoms may improve as an indirect consequence of overall improvement, not as a direct result of the treatment. In addition, differential effects on negative symptoms may result from differences in adverse effects and not efficacy, as the side effects of some drugs make negative symptoms worse (6) . Furthermore, a substantial number of patients do not respond, or are only partially responsive, to current medications. Therefore, the need exists for novel therapeutic approaches, especially therapies that are effective in multiple domains of schizophrenia, particularly if they can have such effects via mechanisms other than the dopamine receptor blockade (7) .
A number of neurotransmitter systems modulate the function of dopamine pathways in the CNS. Muscarinic acetylcholine receptors are well recognized as having a modulatory function on dopamine (8 , 9) . Muscarinic receptor antagonists have been utilized for decades to enhance function in dopamine-deficient states such as Parkinson’s disease. Although the antipsychotic-like actions of muscarinic cholinomimetics, such as arecoline, were first described in the literature many years ago (10) , such findings remain relatively unexamined in the current literature.
Central muscarinic receptors are involved in various functions, including movement regulation, nociception, and cognition (11) . Five muscarinic receptor subtypes (M 1 –M 5 ) that are widely distributed in the CNS have been cloned (12) . A postmortem study revealed abnormalities in these muscarinic receptors in several areas of the brain in subjects with schizophrenia (13 , 14) . Unfortunately, many of these muscarinic receptor subtypes have opposing actions in central neuronal systems, and the therapeutic potential of these receptors has been difficult to establish due to the lack of selective agents for the different receptors. Recently, selective muscarinic ligands have been developed that have shown promise in preclinical studies (15) .
Xanomeline is one such novel muscarinic agonist with relative functional in vitro selectivity for the M 1 and M 4 receptor subtypes (16) , and it has exhibited functional dopamine antagonism and antipsychotic-like effects in rodent models despite its lack of affinity for any dopamine receptors. Xanomeline has been demonstrated to decrease dopamine cell firing in the ventral tegmental area and is efficacious in behavioral tests predictive of antipsychotic activity, namely reversal of dopamine agonist-induced rotation and disruption of conditioned avoidance responding (17) . In a model of sensorimotor gating deficits in schizophrenia (18) , xanomeline also reversed dopamine agonist-induced disruptions (19) . An atypical antipsychotic profile of xanomeline is evident by virtue of its low proclivity to induce catalepsy, which is indicative of a low likelihood of producing extrapyramidal side effects, and its ability to increase extracellular levels of dopamine in the prefrontal cortex (20) . In a clinical study of subjects with Alzheimer’s-type dementia, this compound demonstrated efficacy in improving the cognitive deficits associated with dementia and improved the behavioral symptoms of delusions and hallucinations (21) . A recent primate study also demonstrated that treatment with xanomeline is effective in blocking the behavioral effects of dopamine agonists (22) . Therefore, we examined the efficacy of xanomeline on clinical outcomes in subjects with schizophrenia in a pilot study utilizing a double-blind, placebo-controlled, 4-week treatment design. We hypothesized that xanomeline would improve psychotic symptoms as well as significantly ameliorate cognitive deficits in patients with schizophrenia.
Method
Subjects
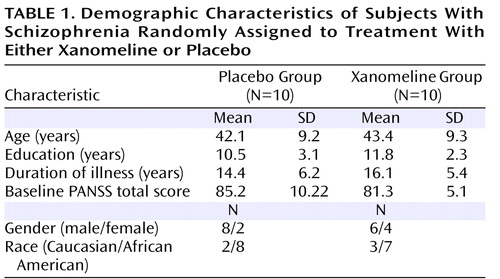
All clinical procedures were approved by the Institutional Review Board at Indiana University Medical Center. Study participants were inpatients admitted to the research unit at the Neuroscience Clinical Research Center at Indiana University Medical Center with a diagnosis of either schizophrenia or schizoaffective disorder. All subjects were hospitalized for acute exacerbation or poor treatment response. One subject had been free of antipsychotic medications for over 3 months, and another subject had been drug free for 1 month. Twelve subjects were taking atypical (second-generation) antipsychotics, and six subjects were taking typical (first-generation) antipsychotics prior to entering the study. All subjects exhibited either poor response or worsening symptoms with treatment and were medication free for 3 to 7 days before entering the initial placebo phase of the study.
The purpose of the study and its procedures were explained to all subjects, and informed consent was obtained. A total of 28 consecutively referred patients were screened; of the 24 subjects who qualified for the study, 20 entered the study and were randomly assigned to treatment after diagnostic and laboratory examinations. Diagnoses were made using the Structured Clinical Interview for DSM-IV (SCID-IV) and confirmed by a second clinical interview conducted by a psychiatrist.
Inclusion Criteria
Subjects meeting study criteria were between 18 and 60 years old, met DSM-IV criteria for schizophrenia or schizoaffective disorder as their only axis I disorder, and had a total Positive and Negative Syndrome Scale (PANSS) (23) score >60, scoring at least a 4 on one positive symptom item or a 3 on two positive symptom items. Female subjects of childbearing age were required to undergo pregnancy testing and take reasonable birth control precautions; all subjects were required to undergo a detailed psychiatric evaluation, blood tests (e.g., blood counts and routine chemistries), urine drug screening, and an ECG. In addition, subjects were required to have been off lithium therapy for at least 3 weeks prior to study entry and free of all antipsychotic medications; those subjects taking a depot antipsychotic could not have received a dose of medication for at least one injection cycle prior to study entry.
Exclusion Criteria
Excluded from the study were female subjects who were pregnant or lactating, subjects who had received ECT within the last 3 months, subjects requiring psychotropic medications other than sedative/hypnotic medications (e.g., lorazepam, temazepam, or chloral hydrate) as needed, subjects with other significant neurological or medical conditions, subjects who met criteria for substance dependence within the last 3 months, subjects with a past history of serious violent or suicidal behavior, and subjects with significant electrocardiographic abnormalities.
Study Procedures
The following assessments were administered at baseline: PANSS; the Brief Psychiatric Rating Scale (BPRS) (24) ; the Clinical Global Impression scale (CGI) (25) ; the Simpson-Angus Rating Scale (26) , the Barnes Rating Scale for Drug-Induced Akathisia (27) , and the Abnormal Involuntary Movement Scale (AIMS) (28) for the assessment of extrapyramidal symptoms; and a neuropsychological test battery designed specifically to measure cognitive function in patients with schizophrenia. The battery included the following cognitive tests for each of the different domains: Continuous Performance Test, Identical Pairs Version; Stroop Color and Word Test; Wechsler Memory Scale; Wechsler Adult Intelligence Scale, third edition; Trail Making Test, parts A and B; word list memory and recall tests; Controlled Oral Word Association Test; Shipley Institute of Living Vocabulary Test; and Finger Tapping Test (total time of testing: approximately 2 hours). Safety assessments at baseline included an ECG, blood tests, orthostatic pulse and blood pressure measurements, weight, and temperature.
Subjects who met PANSS and CGI severity criteria began the study with one week of single-blind placebo treatment, during which subjects took placebo tablets three times a day. After 7 days, subjects who continued to meet PANSS and CGI severity criteria entered the double-blind phase of the study, during which half of the subjects were randomly assigned to xanomeline treatment (25 mg t.i.d.) and the other half randomly assigned to placebo treatment (three times a day). Two hours after the first dose of double-blind treatment (and after each dose escalation), orthostatic measurements of pulse rate and blood pressure were taken for each patient. Two days later (i.e., day 9 of the study), after obtaining an ECG and BPRS and CGI scores, the medication dose was increased in each group to either 50 mg t.i.d. of xanomeline or two placebo tablets three times a day. On day 11, after obtaining another ECG and BPRS and CGI scores, the dose of xanomeline and placebo were increased to 75 mg t.i.d and three tablets three times a day, respectively. If the subject was unable to tolerate the increase in dosage, it was attempted again after an additional 3 days. If the subject was still unable to tolerate the dose escalation, then the subject was maintained on the previous highest tolerated dose. After 3 weeks at the highest tolerated dose, subjects were tapered off the study drug over the next 5 days and discharged after stabilization with standard treatments.
Data Analysis
Twenty patients were randomly assigned to treatment in this pilot study. Since there was no existing data about the efficacy of xanomeline in treating the symptoms of schizophrenia before this study, there was no previous effect size available for performing a power analysis to determine the total number of patients needed.
The primary outcome measures of this study were total symptom scores and improvement in scores on the PANSS, BPRS, and CGI. Additional important outcome measures were scores on the PANSS subscales and changes in the cognitive test scores. Comparisons of these measures were conducted between placebo and xanomeline treatment groups utilizing ratings at last observation carried forward (last rating in the double-blind treatment phase for those subjects who completed at least 1 week of treatment) and after 3 weeks of treatment at the highest tolerated dose of the drug (completer analysis). Mean changes in the continuous outcomes were compared using analysis of covariance (ANCOVA), and t tests were used to assess the significance of the differences between group means. The study lacked power for meaningful comparisons of categorical outcomes. Therefore, completion/discontinuation rates and rates of adverse events are presented as descriptive summaries, not formal hypothesis tests.
Results
Demographic characteristics of subjects (N=20) randomly assigned to treatment in the study are presented in Table 1 . Subjects were tapered off any previous medications and randomly assigned to either placebo or xanomeline titrated up to 225 mg/day over 6 days. Subjects were assessed at baseline and during the trial using clinical and extrapyramidal symptom scales, a cognitive function test battery, and ECG, as well as laboratory safety measures. Eight out of 10 subjects and seven out of 10 subjects completed all study visits in the xanomeline and placebo groups, respectively. Two subjects in the xanomeline group and three subjects in the placebo group discontinued the study due to lack of efficacy. None of the subjects discontinued the study due to adverse events.
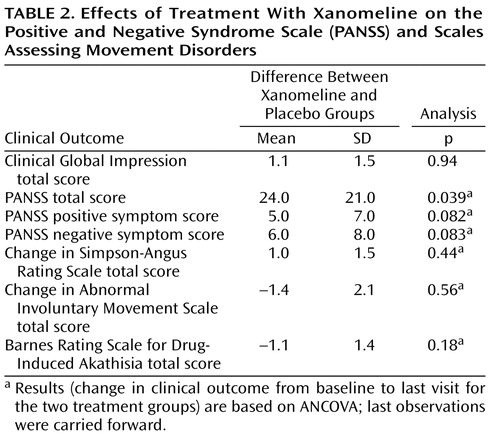
There was significant improvement in the xanomeline group in total BPRS scores by week 1 of treatment and continuing throughout the study period ( Figure 1 ). The xanomeline group also showed statistically significant improvements in total PANSS scores, as well as improvements in the PANSS positive and negative symptom subscales ( Table 2 ).
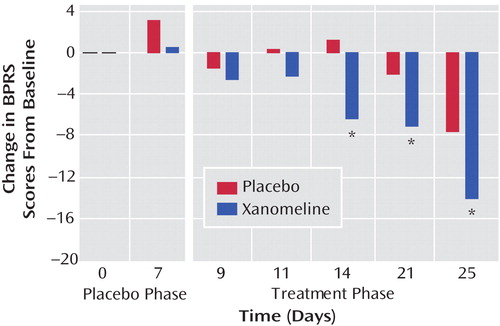
a Subjects in the xanomeline treatment group showed significantly greater improvement in clinical outcomes by week 1 of the treatment period.
* p<0.05.
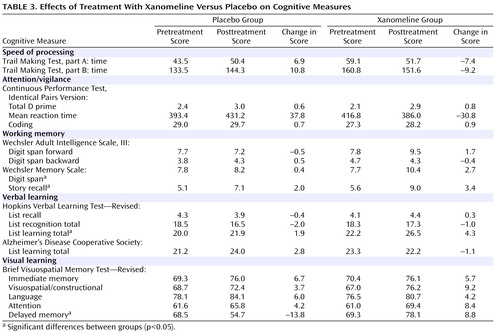
In the cognitive test battery, the xanomeline group showed the most robust improvements in measures of verbal learning and short-term memory function. Several tests reached the level of significance, including list learning, story recall, delayed memory, and digit span tests ( Table 3 and Figure 2 ). There were no significant improvements in tests on attention or speed of information processing.
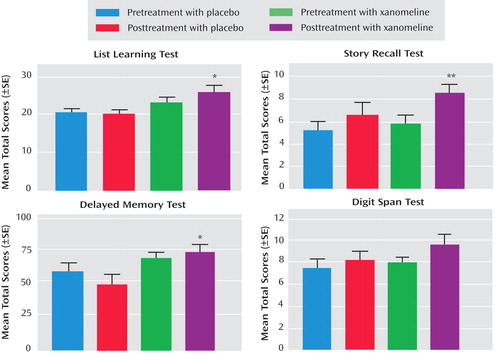
*p<0.05. **p<0.01.
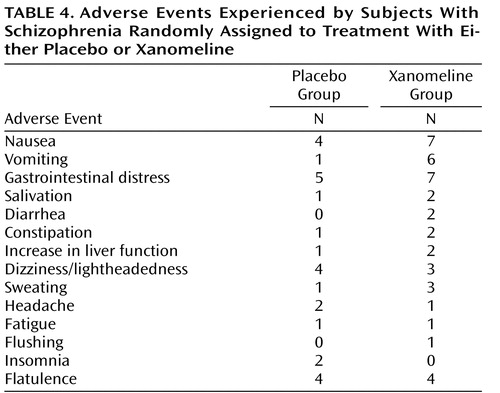
Adverse events, especially those associated with the gastrointestinal system, were typically reported more frequently by subjects in the xanomeline group than subjects in the placebo group ( Table 4 ). However, most of these adverse reactions were reported to be of mild or moderate severity, did not persist for long periods of time, and did not result in discontinuation of treatment for any patient. No significant changes in blood pressure, heart rate, and ECG measures were observed. Also, no significant changes in extrapyramidal symptoms, as measured by the Simpson-Angus Rating Scale, the Barnes Rating Scale for Drug-Induced Akathisia, or the AIMS, were observed ( Table 2 ).
To provide illustrative clinical information, case reports from one subject who responded well to treatment and one subject who responded poorly to treatment are presented in the Patient Perspectives.
Discussion
The central muscarinic system is a complex network with at least nine major projecting muscarinic cholinergic pathways with a wide distribution of terminals and postsynaptic receptors (11) . The different muscarinic receptor subtypes are widely distributed throughout the central and peripheral nervous systems, though regional segregation has been described (12) . The major functional roles of muscarinic receptor subtypes have been difficult to separate until recently due to a lack of selective pharmacological tools. However, the roles of muscarinic receptor subtypes and how they relate to potential treatment for schizophrenia are active areas of investigation (22 , 29γ8) . Most notably, M 4 and M 5 muscarinic receptor subtypes have demonstrated antipsychotic-like effects in preclinical models (22 , 36) . It has also been reported that mice lacking M 4 receptors were hyperresponsive to dopamine stimulation, suggesting a role for this receptor in the regulation of dopamine release and dopamine effects (8) .
Clinical research has suggested that the tegmental cholinergic system, which modulates both REM activity and tegmental dopamine projections, is involved in sleep abnormalities and psychosis (11) , and other studies have concluded that subjects with schizophrenia exhibit abnormalities in cholinergic muscarinic receptor activation (30 , 31) . Low muscarinic receptor binding in the prefrontal cortex of subjects with schizophrenia has been reported (14) , and studies of human hippocampal formation (13) and of the striatum (32) have also showed decreased muscarinic receptor binding in subjects with schizophrenia. This evidence suggests that although muscarinic receptors are broadly distributed and have overlapping distributions, there is selectivity in their functional role in the CNS and the potential exists to design novel drugs with specific actions, including for the treatment of schizophrenia.
The most consistent mechanisms associated with xanomeline are its agonist affinity for the muscarinic M 1 and M 4 receptors. Less consistently, xanomeline has been reported as having some affinity for serotonergic receptors (29) , although these results have not been found by other research groups (15) . Therefore, it is reasonable to hypothesize that xanomeline has benefit in treating schizophrenia.
The efficacy of xanomeline in Alzheimer’s-type dementia was previously evaluated in a multicenter (17 sites; N=343), double-blind, placebo-controlled study utilizing three doses of 25, 50, and 75 mg daily (21) . The treatment was administered for 6 months followed by a 1-month washout period. Compared to placebo, xanomeline treatment resulted in a significant dose-dependent improvement in cognitive function as assessed by the Alzheimer’s Disease Assessment Scale cognitive subscale. Maximal cognitive improvement occurred after 12 weeks of therapy. Dose-dependent improvements were also observed in coexisting psychotic symptoms of delusions, hallucinations, and suspiciousness. These antipsychotic effects became evident within a few days after starting xanomeline treatment. The present investigation showed statistically significant differences between xanomeline and placebo groups in PANSS total scores, as well as differences between groups in positive and negative symptom subscales and CGI scores. Consistent with the findings of the study of xanomeline in Alzheimer’s dementia (21) , the most frequent adverse events in the current investigation were gastrointestinal in nature.
Results of the present investigation should be considered in light of several limitations. First, this was a small pilot study. While signs of efficacy were detected, the sample size was too small to accurately quantify safety and tolerability risks. In addition, regarding cognitive assessments, the design of this study did not meet recently adopted standards for assessing efficacy in cognitive impairment associated with schizophrenia (39) . In addition, no adjustments for multiplicity were applied to the series of cognition outcomes. Therefore, it is likely that one or more of the significant differences observed in the cognitive battery were a false positive result. Moreover, improvements in cognition may have been pseudospecific (i.e., an indirect effect) and secondary to improvements in schizophrenia rather than a direct effect of xanomeline treatment. Similarly, no attempt was made to distinguish between primary and secondary negative symptoms, as the study design did not permit an interpretation of effect on primary affect restriction or amotivational pathology.
Nevertheless, this pilot study demonstrates that xanomeline has potential for positive therapeutic effects in multiple symptom domains for patients with schizophrenia. As xanomeline is an M 1 –M 4 receptor-preferring agonist and does not directly bind to dopamine receptors, these data suggest that xanomeline may have a unique mode of antipsychotic action and may provide a novel approach to treating schizophrenia. These results support further investigation of xanomeline as a novel approach to treating schizophrenia.

1. Mueser KT, McGurk SR: Schizophrenia. Lancet 2004; 363:2063–2072Google Scholar
2. Lehman AF, Lieberman JA, Dixon LB, McGlashan TH, Miller AL, Perkins DO, Kreyenbuhl J; American Psychiatric Association Steering Committee on Practice Guidelines: Practice Guideline for the Treatment of Patients With Schizophrenia, 2nd ed. Am J Psychiatry 2004; 161(suppl 2):1–56Google Scholar
3. Snyder SH: Pharmacology: the dopamine connection. Nature 1990; 347:121–122Google Scholar
4. Harvey PD, Green MF, McGurk SR, Meltzer HY: Changes in cognitive functioning with risperidone and olanzapine treatment: a large-scale, double-blind, randomized study. Psychopharmacology (Berl) 2003; 169:404–411Google Scholar
5. Volavka J, Czobor P, Sheitman B, Lindenmayer JP, Citrome L, McEvoy JP, Cooper TB, Chakos M, Lieberman JA: Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. Am J Psychiatry 2002; 159:255–262Google Scholar
6. Delisi LE, Nasrallah HA: The CATIE schizophrenia effectiveness trial. Schizophr Res 2005; 80:v–viGoogle Scholar
7. Miyamoto S, Duncan GE, Marx CE, Lieberman JA: Treatments for schizophrenia: a critical review of pharmacology and mechanisms of action of antipsychotic drugs. Mol Psychiatry 2005; 10:79–104Google Scholar
8. Gomeza J, Zhang L, Kostenis E, Felder C, Bymaster F, Brodkin J, Shannon H, Xia B, Deng C, Wess J: Enhancement of D 1 dopamine receptor-mediated locomotor stimulation in M 4 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A 1999; 96:10483–10488 Google Scholar
9. Tzavara ET, Bymaster FP, Davis RJ, Wade MR, Perry KW, Wess J, McKinzie DL, Felder C, Nomikos GG: M 4 muscarinic receptors regulate the dynamics of cholinergic and dopaminergic neurotransmission: relevance to the pathophysiology and treatment of related CNS pathologies. FASEB J 2004; 18:1410–1412 Google Scholar
10. Fulcher JH Jr, Gallagher WJ, Pfeiffer CC: Comparative lucid intervals after amobarbital, CO 2 , and arecoline in the chronic schizophrenic. AMA Arch Neurol Psychiatry 1957; 78:392–395 Google Scholar
11. Yeomans JS: Role of tegmental cholinergic neurons in dopaminergic activation, antimuscarinic psychosis and schizophrenia. Neuropsychopharmacology 1995; 12:3–16Google Scholar
12. Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR: Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci 1991; 11:3218–3226Google Scholar
13. Crook JM, Tomaskovic-Crook E, Copolov DL, Dean B: Decreased muscarinic receptor binding in subjects with schizophrenia: a study of the human hippocampal formation. Biol Psychiatry 2000; 48:381–388Google Scholar
14. Crook JM, Tomaskovic-Crook E, Copolov DL, Dean B: Low muscarinic receptor binding in prefrontal cortex from subjects with schizophrenia: a study of Brodmann’s areas 8, 9, 10, and 46 and the effects of neuroleptic drug treatment. Am J Psychiatry 2001; 158:918–925Google Scholar
15. Felder CC, Bymaster FP, Ward J, DeLapp N: Therapeutic opportunities for muscarinic receptors in the central nervous system. J Med Chem 2000; 43:4333–4353Google Scholar
16. Shannon HE, Bymaster FP, Calligaro DO, Greenwood B, Mitch CH, Sawyer BD, Ward JS, Wong DT, Olesen PH, Sheardown MJ: Xanomeline: a novel muscarinic receptor agonist with functional selectivity for M 1 receptors. J Pharmacol Exp Ther 1994; 269:271–281 Google Scholar
17. Shannon HE, Hart JC, Bymaster FP, Calligaro DO, DeLapp NW, Mitch CH, Ward JS, Fink-Jensen A, Sauerberg P, Jeppesen L, Sheardown MJ, Swedberg MD: Muscarinic receptor agonists, like dopamine receptor antagonist antipsychotics, inhibit conditioned avoidance response in rats. J Pharmacol Exp Ther 1999; 290:901–907Google Scholar
18. Braff DL, Geyer MA: Sensorimotor gating and schizophrenia: human and animal model studies. Arch Gen Psychiatry 1990; 47:181–188Google Scholar
19. Stanhope KJ, Mirza NR, Bickerdike MJ, Bright JL, Harrington NR, Hesselink MB, Kennett GA, Lightowler S, Sheardown MJ, Syed R, Upton RL, Wadsworth G, Weiss SM, Wyatt A: The muscarinic receptor agonist xanomeline has an antipsychotic-like profile in the rat. J Pharmacol Exp Ther 2001; 299:782–792Google Scholar
20. Perry KW, Nisenbaum LK, George CA, Shannon HE, Felder CC, Bymaster FP: The muscarinic agonist xanomeline increases monoamine release and immediate early gene expression in the rat prefrontal cortex. Biol Psychiatry 2001; 49:716–725Google Scholar
21. Bodick NC, Offen WW, Levey AI, Cutler NR, Gauthier SG, Satlin A, Shannon HE, Tollefson GD, Rasmussen K, Bymaster FP, Hurley DJ, Potter WZ, Paul SM: Effects of xanomeline, a selective muscarinic receptor agonist, on cognitive function and behavioral symptoms in Alzheimer disease. Arch Neurol 1997; 54:465–473Google Scholar
22. Andersen MB, Fink-Jensen A, Peacock L, Gerlach J, Bymaster F, Lundbaek JA, Werge T: The muscarinic M 1 /M 4 receptor agonist xanomeline exhibits antipsychotic-like activity in Cebus apella monkeys. Neuropsychopharmacology 2003; 28:1168–1175 Google Scholar
23. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261–276Google Scholar
24. Andersen J, Larsen JK, Schultz V, Nielsen BM, Kørner A, Behnke K, Munk-Andersen E, Butler B, Allerup P, Bech P: The Brief Psychiatric Rating Scale: dimension of schizophrenia—reliability and construct validity. Psychopathology 1989; 22:168–176Google Scholar
25. Haro JM, Kamath SA, Ochoa S, Novick D, Rele K, Fargas A, Rodríguez MJ, Rele R, Orta J, Kharbeng A, Araya S, Gervin M, Alonso J, Mavreas V, Lavrentzou E, Liontos N, Gregor K, Jones PB; SOHO Study Group: The Clinical Global Impression-Schizophrenia scale: a simple instrument to measure the diversity of symptoms present in schizophrenia. Acta Psychiatr Scand Suppl 2003; 416:16–23Google Scholar
26. Simpson GM, Angus JW: A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl 1970; 212:11–19Google Scholar
27. Barnes TR: A rating scale for drug-induced akathisia. Br J Psychiatry 1989; 154:672–676Google Scholar
28. Guy W (ed): ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington, DC, US Department of Health, Education, and Welfare, 1976, pp 534–537Google Scholar
29. Watson J, Brough S, Coldwell MC, Gager T, Ho M, Hunter AJ, Jerman J, Middlemiss DN, Riley GJ, Brown AM: Functional effects of the muscarinic receptor agonist, xanomeline, at 5-HT 1 and 5-HT 2 receptors. Br J Pharmacol 1998; 125:1413–1420 Google Scholar
30. Tandon R, Shipley JE, Greden JF, Mann NA, Eisner WH, Goodson JA: Muscarinic cholinergic hyperactivity in schizophrenia: relationship to positive and negative symptoms. Schizophr Res 1991; 4:23–30Google Scholar
31. Riemann D, Hohagen F, Krieger S, Gann H, Muller WE, Olbrich R, Wark HJ, Bohus M, Low H, Berger M: Cholinergic REM induction test: muscarinic supersensitivity underlies polysomnographic findings in both depression and schizophrenia. J Psychiatr Res 1994; 28:195–210Google Scholar
32. Dean B, Crook JM, Pavey G, Opeskin K, Copolov DL: Muscarinic1 and 2 receptor mRNA in the human caudate-putamen: no change in m1 mRNA in schizophrenia. Mol Psychiatry 2000; 5:203–207Google Scholar
33. Friedman JI: Cholinergic targets for cognitive enhancement in schizophrenia: focus on cholinesterase inhibitors and muscarinic agonists. Psychopharmacology (Berl) 2004; 174:45–53Google Scholar
34. Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ: Selective cognitive dysfunction in acetylcholine M 1 muscarinic receptor mutant mice. Nat Neurosci 2003; 6:51–58 Google Scholar
35. Bymaster FP, Felder CC: Role of the cholinergic muscarinic system in bipolar disorder and related mechanism of action of antipsychotic agents. Mol Psychiatry 2002; 7(suppl 1):S57–S63Google Scholar
36. Hamilton SE, Loose MD, Qi M, Levey AI, Hille B, McKnight GS, Idzerda RL, Nathanson NM: Disruption of the m1 receptor gene ablates muscarinic receptor-dependent M current regulation and seizure activity in mice. Proc Natl Acad Sci U S A 1997; 94:13311–13316Google Scholar
37. Gomeza J, Shannon H, Kostenis E, Felder C, Zhang L, Brodkin J, Grinberg A, Sheng H, Wess J: Pronounced pharmacologic deficits in M 2 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci U S A 1999; 96:1692–1697 Google Scholar
38. Matsui M, Motomura D, Fujikawa T, Jiang J, Takahashi S, Manabe T, Taketo MM: Mice lacking M 2 and M 3 muscarinic acetylcholine receptors are devoid of cholinergic smooth muscle contractions but still viable. J Neurosci 2002; 22:10627–10632 Google Scholar
39. Marder SR, Fenton W: Measurement and Treatment Research to Improve Cognition in Schizophrenia: NIMH MATRICS initiative to support the development of agents for improving cognition in schizophrenia. Schizophr Res 2004; 72:5–9Google Scholar