A Swedish National Twin Study of Lifetime Major Depression
Abstract
OBJECTIVE: Substantial evidence supports the heritability of lifetime major depression. Less clear is whether genetic influences in major depression are more important in women than in men and whether genetic risk factors are the same in the two sexes. It is not known whether genetic effects on major depression are constant across historical cohorts. METHOD: Lifetime major depression was assessed at personal interview by modified DSM-IV criteria in 42,161 twins, including 15,493 complete pairs, from the national Swedish Twin Registry. Twin models were evaluated by using the program Mx. RESULTS: Model fitting indicated that the heritability of liability to major depression was significantly higher in women (42%) than men (29%) and the genetic risk factors for major depression were moderately correlated in men and women. No significant differences were seen in the etiologic roles of genetic and environmental factors in major depression in three cohorts spanning birth years 1900–1958. CONCLUSIONS: In the largest sample to date, lifetime major depression was moderately heritable, with estimates similar to those in prior studies. In accord with some but not other previous investigations, this study suggests both that the heritability of major depression is higher in women than in men and that some genetic risk factors for major depression are sex-specific in their effect. No evidence was found for differences in the roles of genetic and environmental risk factors in major depression in birth cohorts spanning nearly six decades.
Major depression is a relatively common, multifactorial psychiatric disorder that is responsible for substantial disability worldwide (1). Major depression is familial, and most or all of the familial aggregation results from the impact of genetic factors (2, 3). A meta-analysis of the five methodologically rigorous twin studies published to date that included blind personal interviews produced statistically homogeneous results and an aggregate estimate of the heritability of major depression of 37% (3). To date, the largest of these individual twin studies have included 3,372 (4), 2,662 (5), and 2,974 (6) complete pairs. In this report, examining results of lifetime major depression as assessed at personal interview in over 15,000 complete pairs of twins from the national Swedish Twin Registry, we seek to address three questions.
First, in this large representative sample of twin pairs, can we replicate findings in previous studies suggesting that the heritability of major depression is moderate, in the range of 35%–40%?
Second, while some consensus has emerged in prior studies about the overall heritability of major depression, the impact of sex on the transmission of major depression has remained uncertain. Such sex effects, which can be quantitative or qualitative in nature, are difficult to study because their reliable detection requires large samples (C.A. Prescott and I.I. Gottesman, unpublished 1993 study). Studies have produced variable evidence for and against sex effects in genetic risk for major depression (6, 7–9). In the most powerful such study to date, performed in the Virginia Twin Registry—which utilized two waves of assessment and a “measurement model” to reduce the effect of diagnostic errors (6)—both quantitative and qualitative differences in the risk factors for DSM-III-R major depression in men and women were detected. Can we replicate these results in this large Swedish sample?
Third, heritability is not a characteristic of a disorder but rather of a disorder in a specific population at a specific time. We are unaware of prior efforts to determine the stability of estimates of the heritability of major depression across historical cohorts. In Sweden, prior studies found that the heritability estimates for alcohol abuse and regular tobacco consumption were constant in men born throughout the 20th century (10, 11). However, in women, the heritability of regular tobacco consumption increased dramatically during the same time period (11). Can we find any evidence that the etiologic roles of genetic and environmental factors in major depression differ across birth cohorts in Swedish twins?
Method
Sample
These data were collected as part of the Screening Across the Lifespan Twin study, based on the Swedish Twin Registry (12), which is formed from a nearly complete registration of all twin births in the country. Data collection was performed with a computer-assisted telephone interview, the purpose of which was to screen all twins regardless of their previous participation in activities of the Swedish Twin Registry or the vital status of their twin partner. Efforts were made to interview members of a pair within a month of each other. The most recent information on last name and address was linked to the telephone company’s files to obtain telephone numbers. Introductory letters describing the study were sent to a random sample of approximately 1,000 twin pairs each month. All screening data were collected over the telephone by trained interviewers (with adequate medical background) using a computer-based data collection system. If the twin was unable to be interviewed, portions of the interview were conducted with an informant (although no data on major depression were collected in these informant interviews).
The cohort of twins born between 1886 and 1958 was contacted between March 1998 and January 2003. Of the 61,005 living individuals age 42 or older at the time of screening, 44,919 (73.6%) responded. Of those who did not respond, 9,366 refused (15.4% of the cohort) while the others were not able to be traced or were not interviewable. The present analyses are based on 42,161 twins excluding 1) those interviewed by proxy, 2) those who terminated the interview prior to the depression section or for whom the interview improperly skipped out of the depression section, and 3) members of pairs with uncertain zygosity. According to standard Swedish practice, informed verbal consent was obtained prior to interview. This project was approved by the Swedish Data Inspection Authority, the Ethics Committee of the Karolinska Institute, and the Institutional Review Board of the University of Southern California. As detailed elsewhere (12), zygosity was assigned by using standard self-report items, which, when validated against biological markers, were 95%–99% accurate.
Assessment Methods
Interviews were conducted by using the computerized Composite International Diagnostic Interview Short Form (CIDI-SF) adapted from its original design for 12-month prevalence to assess lifetime prevalence (13). In the CIDI-SF, the evaluation of major depression was simplified by the elimination of criteria A5 (psychomotor agitation/retardation) and C (distress or impairment) and the simplification of criteria A3 (eliminating loss/gain of appetite) and A4 (eliminating hypersomnia). The version of the CIDI-SF adapted for this study contained a “skip-out” for individuals who, when asked about episodes of sad mood in the last year, volunteered they were taking antidepressants. The assumption was that such individuals were likely positive for a history of major depression but were unlikely to manifest symptoms in the last year because of treatment. Because of a procedural oversight, this skip-out was not eliminated when the CIDI-SF was adapted for lifetime prevalence.
In validating the CIDI-SF criteria for major depression against 1-year prevalence data with the full Composite International Diagnostic Interview, Kessler and Mroczek (unpublished written communication, Feb. 22, 1994) recommend using a cutoff of four or more of eight criteria contained in the CIDI-SF. We simulated the CIDI-SF assessment of major depression in our sample of 7,521 personally interviewed twins from the Virginia Twin Registry for whom we had a complete set of items for DSM-III-R adapted from the Structured Clinical Interview for DSM-III-R (14). Compared to the full criteria, we found the following estimates of sensitivity, specificity, and kappa: three or more criteria: 0.998, 0.865, 0.81 (SE=0.01); four or more criteria: 0.956, 0.954, 0.90 (SE=0.01); and five or more criteria: 0.759, 1.000, 0.81 (SE=0.01). Our data also suggested that four or more criteria for major depression from the CIDI-SF produced the optimal cutoff.
The sample contained 8,056 twins who completed the major depression section and met the criteria for major depression and 322 twins who skipped out of the section because they volunteered a history of antidepressant usage. We assessed whether a history of “taking antidepressants” was a valid substitute for a diagnosis of major depression by examining risk for major depression in co-twins of all twins with 1) neither a diagnosis of major depression nor a history of antidepressant usage, 2) a diagnosis of major depression, and 3) a history of antidepressant usage. When the analysis controlled for zygosity, age, and sex, the risk for major depression significantly differed across the three groups (χ2=162.55, df=2, p<0.0001). The three post hoc contrast analyses indicated that both group 2 (χ2=156.90, df=1, p<0.0001) and group 3 differed significantly from group 1 (χ2=9.01, df=1, p=0.003) but that groups 2 and 3 did not themselves differ significantly (χ2=0.16, df=1, p=0.69). With respect to risk for major depression in the co-twin, “taking antidepressants” was equivalent to having been assigned a diagnosis of major depression. Therefore, we considered as affected twins who either fulfilled the criteria for major depression or volunteered that they were taking or had taken antidepressants.
Statistical Analysis
The goal of our genetic analysis was to decompose the variance of the liability to major depression into its genetic and environmental components. We assumed that twin resemblance arises from two latent factors: 1) additive genes (A), contributing twice as much to the monozygotic as to the dizygotic twin correlation (because monozygotic twins share all, while dizygotic twins share on average half, their segregating genes), and 2) shared or “common” environment (C), which contributes equally to the correlation in monozygotic and dizygotic twins. In addition to common environment (environmental experiences that make twins similar in their liability to major depression, such as parental rearing practices or social class), the model also contains individual-specific environment (E), which reflects measurement error and those environmental experiences (such as traumatic life events in adulthood) that make members of a twin pair different in their liability to major depression.
Using the software package Mx (15), we fit models by the method of maximum likelihood to data from all individual twins, including those without an interviewed co-twin. This method reduces the impact of cooperation bias and is a binary data maximum likelihood application of the “missing at random” principle expounded by Little and Rubin (16). Because prevalence rates changed substantially as a function of age in this sample, our calculation of tetrachoric correlations and model fitting included an age-dependent threshold that “subtracted out” the twin resemblance due to their perfect correlation for year of birth.
Analyzing all five twin-zygosity groups (i.e., female-female monozygotic and dizygotic, male-male monozygotic and dizygotic, and opposite-sex dizygotic pairs) enabled us to examine two distinct sex effects. The quantitative question asks whether the magnitude of genetic effects on major depression is the same in men and women. The qualitative question inquires whether the genetic risk factors for major depression in men and women are the same. The latter question involves the estimation of the parameter ra—the correlation in the additive genetic effects on the liability to major depression in men and women. If ra, or the genetic correlation, is zero or one, then the genetic factors that influence major depression in males and females are, respectively, entirely unrelated or identical.
Twice the difference in log -likelihood between the two models yields a statistic that is asymptotically distributed as chi-square with degrees of freedom equal to the difference in their numbers of parameters. We used the Akaike information criterion (17, 18) for model selection. The lower its value, the better is the balance between explanatory power and parsimony.
Our analyses assumed that the exposure to etiologically relevant environmental risk factors is equally correlated in monozygotic and dizygotic twin pairs. To test this equal environment assumption, we examined the available indices of the similarity of childhood environment (number of years living together in home of origin) and adult environment (current frequency of contact or personal meetings with the co-twin) to determine whether monozygotic twins may have had more similar environmental experiences than dizygotic twins (19). Using logistic regression while controlling for zygosity and sex, we tested, in same-sex pairs only, whether the mean score on these environmental measures predicted pair concordance for major depression.
Results
Descriptive Statistics
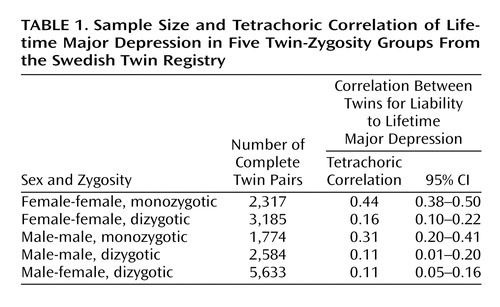
The 42,161 twins utilized in these analyses consisted of 15,493 complete pairs and 11,175 twins whose co-twin was not assessed. The number of complete pairs in each zygosity group is given in Table 1. The overall lifetime prevalence rate for major depression in the entire sample was 19.5% (SE=0.2%), and the rates in all women and men were 25.1% (SE=0.3%) and 13.2% (SE=0.2%), respectively.
Controlling for zygosity and sex, we found that twin pair resemblance for lifetime major depression was not predicted by the number of years the twins had lived together in the home of origin (χ2=0.98, df=1, n.s.) or the frequency of current contact (χ2=0.05, df=1, n.s.). It was, however, predicted, at nominally significant levels, by the frequency of current meetings (χ2=4.65, df=1, p=0.03). However, with a sample this large, significance alone is a poor guide to interpreting the importance of the association between variables. With zygosity and sex in the model, frequency of current meetings contributed a trivial additional proportion of variance to twin pair resemblance for lifetime major depression (0.2%).
Tetrachoric correlations and 95% confidence intervals (CIs) are seen for the five twin-zygosity groups in Table 1. Three patterns are noteworthy. First, the correlations are substantially higher in monozygotic than in dizygotic pairs. Second, the correlations in the same-sex female-female pairs exceed those seen in the same-sex male-male pairs. Third, the resemblance in the opposite-sex dizygotic pairs is lower than that which would be predicted from the female-female and male-male dizygotic pairs.
Model Fitting—Entire Sample
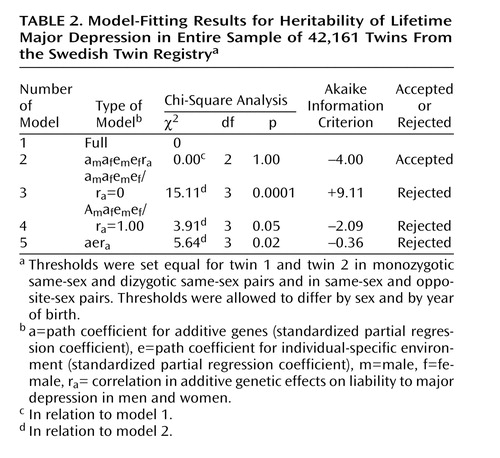
Working from a saturated model, we were able to constrain the thresholds for lifetime major depression to be equal in twin 1 and twin 2 in pairs, in monozygotic and dizygotic same-sex pairs and in same-sex and opposite-sex pairs. We then began our formal model fitting with a full model (model 1 in Table 2) with only two thresholds (one for men and one for women), which, as already described, were modified as a function of age (–2 log likelihood=39,457.0, df=42,136).
Model 2 constrained the shared environmental parameters for both females and males to zero, and this produced no change in fit and improved the Akaike information criterion to –4.00. From this model, we tested three additional submodels. Model 3 constrained the genetic correlation (ra) between men and women to zero and could be strongly rejected both by chi-square and Akaike information criteria (Table 2). Model 4 constrained ra to unity and could be rejected by both criteria. Model 5 constrained the estimates of the genetic and unique environmental components to be equal in men and women and also could be rejected by both criteria.
Parameter Estimates
The parameter estimates were identical for our full and best-fit submodel—model 2—and were as follows (with 95% CIs from model 2): am2=0.29 (95% CI=0.19–0.38), af2=0.42 (95% CI=0.36–0.47), em2=0.71 (95% CI=0.62–0.81), ef2=0.58 (95% CI=0.53–0.64), and ra=+0.63 (95% CI=+0.31–+0.99). Three results are notable. First, we found no evidence that shared environmental risk factors were of etiologic importance for major depression in either males or females. Second, the heritability of liability to major depression was substantially greater in women than in men. Third, the genetic correlation of the liability to major depression in men and women, estimated at 0.63, suggests that a substantial proportion of the genetic risk factors for this disorder are sex-specific in their action. When parameter estimates were constrained to be equal across the two sexes (model 5), the estimated heritability of major depression was 0.38.
Analysis of Age Cohorts
We divided the sample into three age groups, with approximately equal numbers of complete twin pairs in each, representing the birth years 1900–1938, 1939–1948, and 1949–1958. We then fit the full model to each age cohort, allowing both thresholds and parameter estimates to vary across the cohort. This model achieved a fit of –2 log likelihood of 39,623.5 (df=42,140). We then simplified the model by setting to zero all the shared environmental parameters, with an improvement in fit against the full model (χ2=0.00, df=6, p=0.99; Akaike information criterion=–12.00). We then constrained all the remaining genetic and environmental parameters to equality across the three cohorts, permitting thresholds to differ by both sex and cohort. This produced a further substantial improvement in the Akaike information criterion against the full model (χ2=2.58, df=12, p=0.99; Akaike information criterion=–21.42), suggesting that the etiologic roles of genetic and environmental factors in lifetime major depression did not differ across the broad age range of this sample. The parameter estimates of this model (am2=0.29, af2=0.42, em2=0.71, ef2=0.58, and ra=+0.64) were nearly identical to those obtained with our best-fit standard model. Because of the social and historical importance of World War II, we also divided our sample into those born before and after the conclusion of that war (i.e., in 1945 or earlier and in 1946 or later). The best-fit model constrained all genetic and environmental parameters to equality across these two cohorts.
Discussion
We sought in this article to examine three questions, which we review in turn.
Heritability of Lifetime Major Depression
In this large, nationally representative twin sample, when the parameters were constrained to equality in men and women, the heritability of major depression was estimated to be 38%. These results are in close agreement to those obtained previously from a meta-analysis of the methodologically rigorous twin studies of major depression, which estimated, across studies, that the heritability of major depression was 37%, with 95% CIs of 31%–42% (3). Since the methodology of this study closely resembled that of the previous population-based twin studies, such close agreement in results is reassuring and suggests that there are no major cross-population differences in the heritability of major depression in the largely European or European-derived populations represented in these studies. Furthermore, our results are in line with statistical theory, which would predict—in the absence of interpopulation differences—that estimates from our large sample should approximate the mean results from the previous smaller studies.
Sex Differences in Genetic Risk Factors for Major Depression
Prior evidence of quantitative or qualitative sex differences in genetic risk factors for major depression has been inconsistent, perhaps because of the low statistical power (unpublished 1993 study by Prescott and Gottesman). We attempted to replicate, in this large Swedish sample, our previous results utilizing two waves of interviews in our smaller population-based sample from the Virginia Twin Registry (6). In our Virginia sample, the heritability of major depression, including measurement error, was estimated at 40% in women and 31% in men, very similar to the figures of 42% and 29% found in the present analyses. In the Virginia sample, we estimated the genetic correlation in risk factors for major depression in men and women to be +0.55 (6), compared to the estimate of +0.63 in the Swedish sample.
Our results are also broadly similar to findings from the Australian volunteer twin registry, where the best-fit model estimated the heritability of lifetime major depression by DSM-III-R criteria to be 44% in women and 24% in men (5). We are also aware of two studies that provided separate estimates in men and women and examined self-reported depressive symptoms in twins who, as in this sample, were largely in late adult life. Both found higher heritability in women (20, 21). One of these studies, also from Sweden (21), approximated current “caseness” for major depression from the self-report questionnaire and found heritability estimates of 42% in women and 21% in men.
These results provide strong support for the hypothesis that both quantitative and qualitative sex differences exist in genetic risk factors for major depression. The proportion of population risk in major depression attributable to genetic factors appears to be moderately greater in women than in men. This result could be due to several factors that cannot be discriminated by standard twin analyses of a dichotomous trait like major depression, including, for example, greater genetic variance in liability to major depression in women or greater environmental variance in men. Such a finding could also arise if men are substantially less reliable in their reporting of lifetime major depression than are women. Such differences have been found in some (22, 23) but not other (24, 25) samples.
Our results also replicate our prior finding (and older evidence from family studies [26]) that the genetic correlation in liability to major depression between the sexes is substantially less than unity. These results imply the existence of genes that have different impacts on the risk for major depression in men and women. For example, genes might exist that would alter the risk for depression in women in response to the variable hormonal environment of the menstrual cycle and pregnancy (27, 28). Such genes would affect genetic risk for major depression in women but not in men. These twin findings are of particular interest in light of results from three genome scans for major depression, all of which detected genomic regions that appear to have different effects on the risk of illness in men and women (29–31). Furthermore, a linkage analysis of the personality trait of neuroticism (which has a substantial genetic correlation with liability to major depression [32, 33]) revealed distinct linked regions of the genome that were female-specific and male-specific in their effect (34).
Possible Birth Cohort Differences in Heritability of Major Depression
Because the sample size in this study was large and it contained a broad age range, it was possible to test whether the etiologic importance of genetic and environmental risk factors in major depression varies as a function of birth cohort. We took the simplest approach to this problem, dividing our sample into thirds and then examining similarities or differences in parameter estimates. We found no evidence to suggest differences in the role of genetic and environmental risk factors in the etiology of lifetime major depression in these three cohorts, which together spanned the years from 1900 to 1958. When we divided our sample into those born before and after the conclusion of World War II, we obtained the same result. Although this time period saw large changes in the social and economic conditions of Sweden, as we have previously found for alcohol abuse and regular tobacco use in Swedish men (10, 11), the heritability of lifetime major depression appears to be relatively insensitive to such historical trends.
Limitations
These results should be interpreted in the context of two potentially significant methodologic limitations. First, the lifetime history of major depression was assessed at one point in time. If uncorrelated in twin pairs, unreliability of measurement is indistinguishable from the effects of true individual-specific environment (E) in our twin models. Since the test-retest reliability of psychiatric disorders in community samples is typically moderate (35–38), a significant proportion of what is called E in our models may reflect errors of measurement rather than real environmental effects (38).
Second, since our analyses included individuals with a history of mania, our parameter estimates reflect a lifetime history of depression occurring both in unipolar depressive illness and bipolar disorder. Since mania was assessed in this study (39), we repeated our analyses while censoring individuals with a lifetime history of mania by DSM-IV criteria (2.7% of the sample). The best-fit model produced parameter estimates similar to those obtained in the entire sample: am2=0.30, af2=0.43, em2=0.70, ef2=0.57, and ra=+0.53. Our original estimates do not appear to have been unduly influenced by treating as affected both individuals with a history of major depression and those with mania.
 |
 |
Received Dec. 9, 2004; revision received April 4, 2005; accepted April 25, 2005. From the Departments of Psychiatry and Human Genetics, Medical College of Virginia of Virginia Commonwealth University; the Department of Psychology, University of Southern California, Los Angeles; and the Department of Medical Epidemiology and Biostatistics, Karolinska Institute, Stockholm. Address correspondence and reprint requests to Dr. Kendler, Department of Psychiatry, Medical College of Virginia, P.O. Box 980126, Richmond, VA 23298-0126; [email protected] (e-mail). Supported in part by grant MH-49492 from NIMH, grant AG-08724 from the National Institute on Aging, the Swedish Scientific Council, and the Swedish Department of Higher Education.
1. Murray CJL, Lopez AD: Evidence-based health policy: lessons from the Global Burden of Disease study. Science 1996; 274:740–743Crossref, Medline, Google Scholar
2. Tsuang MT, Faraone SV: The Genetics of Mood Disorders. Baltimore, Johns Hopkins University Press, 1990Google Scholar
3. Sullivan PF, Neale MC, Kendler KS: Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry 2000; 157:1552–1562Link, Google Scholar
4. Lyons MJ, Eisen SA, Goldberg J, True W, Lin N, Meyer JM, Toomey R, Faraone SV, Merren J, Merla-Ramos M, Tsuang MT: A registry-based twin study of depression in men. Arch Gen Psychiatry 1998; 55:468–472Crossref, Medline, Google Scholar
5. Bierut LJ, Heath AC, Phil D, Bucholz KK, Dinwiddie SH, Madden PAF, Statham DJ, Dunne MP, Martin NG: Major depressive disorder in a community-based twin sample. Arch Gen Psychiatry 1999; 56:557–563Crossref, Medline, Google Scholar
6. Kendler KS, Gardner CO, Neale MC, Prescott CA: Genetic risk factors for major depression in men and women: similar or different heritabilities and same or partly distinct genes? Psychol Med 2001; 31:605–616Crossref, Medline, Google Scholar
7. McGuffin P, Katz R, Watkins S, Rutherford J: A hospital-based twin register of the heritability of DSM-IV unipolar depression. Arch Gen Psychiatry 1996; 53:129–136Crossref, Medline, Google Scholar
8. Kendler KS, Pedersen NL, Johnson L, Neale MC, Mathe AA: A pilot Swedish twin study of affective illness, including hospital- and population-ascertained subsamples. Arch Gen Psychiatry 1993; 50:699–706Crossref, Medline, Google Scholar
9. Kendler KS, Pedersen NL, Neale MC, Mathe AA: A pilot Swedish twin study of affective illness including hospital- and population-ascertained subsamples: results of model fitting. Behav Genet 1995; 25:217–232Crossref, Medline, Google Scholar
10. Kendler KS, Prescott CA, Neale MC, Pedersen NL: Temperance board registration for alcohol abuse in a national sample of Swedish male twins born 1902-1949. Arch Gen Psychiatry 1997; 54:178–184Crossref, Medline, Google Scholar
11. Kendler KS, Karkowski LM, Pedersen NL: Tobacco consumption in Swedish twins reared apart and reared together. Arch Gen Psychiatry 2000; 57:886–892Crossref, Medline, Google Scholar
12. Lichtenstein P, De Faire U, Floderus B, Svartengren M, Svedberg P, Pedersen NL: The Swedish Twin Registry: a unique resource for clinical, epidemiological and genetic studies. J Intern Med 2002; 252:184–205Crossref, Medline, Google Scholar
13. Kessler RC, Andrews G, Mroczek D, Ustun B, Wittchen H-U: The World Health Organization Composite International Diagnostic Interview Short Form (CIDI-SF). Int J Methods Psychiatr Res 1998; 7:171–185Crossref, Google Scholar
14. Spitzer RL, Williams JBW: Structured Clinical Interview for DSM-III-R (SCID). New York, New York State Psychiatric Institute, Biometrics Research, 1985Google Scholar
15. Neale MC, Boker SM, Xie G, Maes HH: Mx: Statistical Modeling, 5th ed. Richmond, Virginia Commonwealth University, Medical College of Virginia, 1999Google Scholar
16. Little RJA, Rubin DB: Statistical Analysis With Missing Data. New York, John Wiley & Sons, 1987Google Scholar
17. Akaike H: Factor analysis and AIC. Psychometrika 1987; 52:317–332Crossref, Google Scholar
18. Williams LJ, Holahan PJ: Parsimony-based fit indices for multiple-indicator models: do they work? Structural Equation Modeling 1994; 1:161–189Crossref, Google Scholar
19. Kendler KS: Overview: a current perspective on twin studies of schizophrenia. Am J Psychiatry 1983; 140:1413–1425Link, Google Scholar
20. McGue M, Christensen K: Genetic and environmental contributions to depression symptomatology: evidence from Danish twins 75 years of age and older. J Abnorm Psychol 1997; 106:439–448Crossref, Medline, Google Scholar
21. Jansson M, Gatz M, Berg S, Johansson B, Malmberg B, McClearn GE, Schalling M, Pedersen NL: Gender differences in heritability of depressive symptoms in the elderly. Psychol Med 2004; 34:471–479Crossref, Medline, Google Scholar
22. Aneshensel CS, Estrada AL, Hansell MJ, Clark VA: Social psychological aspects of reporting behavior: lifetime depressive episode reports. J Health Soc Behav 1987; 28:232–246Crossref, Medline, Google Scholar
23. Wells JE, Horwood LJ: How accurate is recall of key symptoms of depression? a comparison of recall and longitudinal reports. Psychol Med 2004; 34:1001–1011Crossref, Medline, Google Scholar
24. Fennig S, Schwartz JE, Bromet EJ: Are diagnostic criteria, time of episode and occupational impairment important determinants of the female:male ratio for major depression? J Affect Disord 1994; 30:147–154Crossref, Medline, Google Scholar
25. Kendler KS, Gardner CO, Prescott CA: Are there sex differences in the reliability of a lifetime history of major depression and its predictors? Psychol Med 2001; 31:617–625Crossref, Medline, Google Scholar
26. Faraone SV, Lyons MJ, Tsuang MT: Sex differences in affective disorder: genetic transmission. Genet Epidemiol 1987; 4:331–343Crossref, Medline, Google Scholar
27. Kendler KS, Silberg JL, Neale MC, Kessler RC, Heath AC, Eaves LJ: Genetic and environmental factors in the aetiology of menstrual, premenstrual and neurotic symptoms: a population-based twin study. Psychol Med 1992; 22:85–100Crossref, Medline, Google Scholar
28. Seeman MV: Psychopathology in women and men: focus on female hormones. Am J Psychiatry 1997; 154:1641–1647Link, Google Scholar
29. Nash MW, Huezo-Diaz P, Williamson RJ, Sterne A, Purcell S, Hoda F, Cherny SS, Abecasis GR, Prince M, Gray JA, Ball D, Asherson P, Mann A, Goldberg D, McGuffin P, Farmer A, Plomin R, Craig IW, Sham PC: Genome-wide linkage analysis of a composite index of neuroticism and mood-related scales in extreme selected sibships. Hum Mol Genet 2004; 13:2173–2182Crossref, Medline, Google Scholar
30. Holmans P, Zubenko GS, Crowe RR, DePaulo JR Jr, Scheftner WA, Weissman MM, Zubenko WN, Boutelle S, Murphy-Eberenz K, MacKinnon D, McInnis MG, Marta DH, Adams P, Knowles JA, Gladis M, Thomas J, Chellis J, Miller E, Levinson DF: Genomewide significant linkage to recurrent, early-onset major depressive disorder on chromosome 15q. Am J Hum Genet 2004; 74:1154–1167Crossref, Medline, Google Scholar
31. Abkevich V, Camp NJ, Hensel CH, Neff CD, Russell DL, Hughes DC, Plenk AM, Lowry MR, Richards RL, Carter C, Frech GC, Stone S, Rowe K, Chau CA, Cortado K, Hunt A, Luce K, O’Neil G, Poarch J, Potter J, Poulsen GH, Saxton H, Bernat-Sestak M, Thompson V, Gutin A, Skolnick MH, Shattuck D, Cannon-Albright L: Predisposition locus for major depression at chromosome 12q22-12q23.2. Am J Hum Genet 2003; 73:1271–1281Crossref, Medline, Google Scholar
32. Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ: A longitudinal twin study of personality and major depression in women. Arch Gen Psychiatry 1993; 50:853–862Crossref, Medline, Google Scholar
33. Fanous A, Gardner CO, Prescott CA, Cancro R, Kendler KS: Neuroticism, major depression and gender: a population-based twin study. Psychol Med 2002; 32:719–728Crossref, Medline, Google Scholar
34. Fullerton J, Cubin M, Tiwari H, Wang C, Bomhra A, Davidson S, Miller S, Fairburn C, Goodwin G, Neale MC, Fiddy S, Mott R, Allison DB, Flint J: Linkage analysis of extremely discordant and concordant sibling pairs identifies quantitative-trait loci that influence variation in the human personality trait neuroticism. Am J Hum Genet 2003; 72:879–890Crossref, Medline, Google Scholar
35. Prusoff BA, Merikangas KR, Weissman MM: Lifetime prevalence and age of onset of psychiatric disorders: recall 4 years later. J Psychiatr Res 1988; 22:107–117Crossref, Medline, Google Scholar
36. Rice JP, Rochberg M, Endicott J, Lavori PW, Miller C: Stability of psychiatric diagnoses: an application to the affective disorders. Arch Gen Psychiatry 1992; 49:824–830Crossref, Medline, Google Scholar
37. Williams JBW, Gibbon M, First MB, Spitzer RL, Davies M, Borus J, Howes MJ, Kane J, Pope HG, Rounsaville B, Wittchen H-U: The Structured Clinical Interview for DSM-III-R (SCID), II: multisite test-retest reliability. Arch Gen Psychiatry 1992; 49:630–636Crossref, Medline, Google Scholar
38. Foley DL, Neale MC, Kendler KS: Reliability of a lifetime history of major depression: implications for heritability and co-morbidity. Psychol Med 1998; 28:857–870Crossref, Medline, Google Scholar
39. Soldani F, Sullivan PF, Pedersen NL: Mania in the Swedish Twin Registry: criterion validity and prevalence. Aust NZ J Psychiatry 2005; 39:235–243Crossref, Medline, Google Scholar



