Specificity of Prefrontal Dysfunction and Context Processing Deficits to Schizophrenia in Never-Medicated Patients With First-Episode Psychosis
Abstract
OBJECTIVE: Context processing is a cognitive construct associated with activity in the middle frontal gyrus. Schizophrenia-related deficits in context processing tasks have been associated with prefrontal cortical dysfunction. This study evaluated whether prefrontal cortical dysfunction related to context processing occurred in first-episode, never-medicated schizophrenia patients, whether this dysfunction also occurred in patients with nonschizophrenia psychosis, and whether this dysfunction was related to psychotic symptom expression. METHOD: A modified version of the AX continuous performance task was conducted during event-related functional magnetic resonance imaging in 18 never-medicated, first-episode schizophrenia patients, 12 never-medicated patients with first-episode nonschizophrenia psychosis, and 28 comparison participants without psychiatric disorder. RESULTS: In-scanner measures of errors and interference reaction time showed that the schizophrenia patients had a specific deficit in context processing. Trials with greater context processing demands corresponded to activity in the middle frontal gyrus (Brodmann’s area 9) in the comparison subjects and in the patients with nonschizophrenia psychosis, but not in the schizophrenia patients. Individual differences in prefrontal cortical dysfunction were associated with context processing measures and disorganization symptoms. The schizophrenia patients also showed increased activity in the anterior (Brodmann’s area 10) and inferior prefrontal cortices (Brodmann’s area 45/46) when they were maintaining context over a delay. CONCLUSIONS: Prefrontal dysfunctions related to context processing were found only in schizophrenia patients early in the course of the illness, and these dysfunctions were related to disorganization symptoms. Instead of using context processing during a continuous performance task, schizophrenia patients may use an inefficient encoding and retrieval strategy.
Schizophrenia patients’ deficits in executive functions have been consistently associated with a dysfunction of the prefrontal cortex (1, 2). However, important questions remain regarding such disturbances, including the nature of the relationship between frontal deficits and cognitive impairments, their specificity to schizophrenia, their association with clinical symptoms, and the influence of potentially confounding factors such as performance confounds, medication effects, and chronicity of illness. The current study was designed to address these questions in a group of never-medicated, first-episode psychosis patients.
Although many studies have shown prefrontal dysfunctions in schizophrenia patients, few have attempted to show that this pathology is specific to the diagnosis of schizophrenia. Among studies that have compared medicated patients, three have reported hypofrontality in schizophrenia patients, compared to depressed or bipolar disorder patients (3–5), whereas one study found no prefrontal differences between schizophrenia and bipolar disorder patients (6). To our knowledge, only one study has examined this pathology in unmedicated patients, and that study found lower levels of activity in the inferior prefrontal cortex in depressed, bipolar disorder, and schizophrenia patients, relative to healthy comparison subjects (7). Thus, the question of diagnostic specificity remains important and controversial.
In addition to demonstrating diagnostic specificity, another way to strengthen the association between schizophrenia and prefrontal cortical dysfunctions is to demonstrate a relationship between the extent of dysfunction and psychotic symptoms. Various symptom factors of schizophrenia, such as reality distortion, disorganization, and negative (poverty) symptoms, have each been associated with prefrontal dysfunctions (1, 8–10). Such varying findings might be explained by a moderate association of all of these symptom types with prefrontal dysfunction, such that some correlations occasionally reach significance. To date, differences between correlations have not generally been considered. Thus, we also sought to test whether changes in regional activity are more correlated with some symptom factors, relative to others.
Despite the frequency of reports of prefrontal dysfunctioning and various associations with symptom manifestations, the evidence to date does not yet make a compelling case that prefrontal dysfunction is more than an artifact of other illness-related factors. Among the confounding factors that may spuriously lead to observations of prefrontal dysfunction are poor task performance, medication effects, and chronicity. Task performance can be a confound if the experimental procedures do not address whether a lower level of prefrontal activity is the cause of cognitive impairment or whether there are fewer demands on the prefrontal cortex because psychiatric patients’ cognitive processes are impaired for some other reason (11). Furthermore, poor task performance and lower levels of prefrontal activity may both correlate with or be caused by lower levels of motivation. The current study adopted statistical procedures to allow examination of data from trials with accurate performance only, to help control for on-task performance. Medication and chronicity effects also frustrate our ability to infer a causal relationship between prefrontal dysfunction and the illness, because one cannot rule out the possibility that an observed dysfunction is associated with pharmacological differences between psychiatric patients and comparison subjects or rule out the diverse secondary effects of adapting to a mental illness. To avoid these concerns, the current study included a rare group of never-medicated, first-episode patients directly after their initial contact with psychiatric services.
Finally, to maximize the sensitivity for detecting disturbances of prefrontal function specific to schizophrenia, we used a context processing task. Although the term “context processing” has several connotations (for review, see reference 12), in this case context processing is the ability to represent and actively maintain information required to select and execute task-appropriate behavior (13). This function is a delimited executive process within the working memory model (14) and has been associated with activity in the middle frontal gyrus (15). Context processing deficits are prominent and persistent in chronic and first-episode schizophrenia patients (16, 17) and have been observed in their healthy siblings (18). These processes may also be involved in schizophrenic deficits in selective attention, such as those revealed on the Stroop task (19) and distractor span tasks (20). Context processing deficits are not prominent in nonpsychotic mood disorders (21) and are transient in patients with nonschizophrenia psychosis (17). Studies of context processing have typically involved medicated patients or previously medicated patients in later stages of illness. In the current study, we used an expectancy variant of the AX continuous performance task that included conditions selectively sensitive to context processing to test the following three hypotheses in never-medicated, first-episode patients who reported psychotic symptoms:
| 1. | Context processing, or the ability to represent and maintain task-relevant information, is related to middle frontal gyrus function, and context processing deficits in schizophrenia are associated with an inability to activate this region. | ||||
| 2. | Both context processing deficits and middle frontal gyrus dysfunction are specific to schizophrenia and do not occur in patients with nonschizophrenia psychosis. | ||||
| 3. | Middle frontal gyrus dysfunction is associated with both context processing deficits and disorganization symptoms. | ||||
Method
Participants
Participants were 30 never-medicated patients with schizophrenia and nonschizophrenia psychosis who underwent functional magnetic resonance imaging (fMRI) during an acute psychotic episode and 28 comparison participants. The patient participants were part of a larger study and were recruited because they reported experiencing some type of psychotic symptom and had not previously contacted psychiatric services. Rule-out criteria included 1) age >40 years or <14 years, 2) WAIS-R full-scale IQ <70, 3) non-English native language, 4) diagnosis of substance dependence or substance use disorder within 6 months of testing, 5) neurological disorders or family history of hereditary neurological disorder, or 6) pregnancy. This larger study group and performance measures outside the scanner on the expectancy AX task (see the next section) are described in detail elsewhere (17). After a complete description of the study to the subjects, written informed consent was obtained.
All patients were followed longitudinally, and their diagnoses were confirmed 6 months after their index hospitalization in diagnostic case conferences that included a review of information from the patients’ chart and from the Structured Clinical Interview for DSM-III-R (22) administered by trained research personnel. Forty-five patient participants were considered appropriate for the study and consented to be scanned in the current protocol (51% of consecutive participants in the larger study). There were no significant differences between scanned and unscanned participants in terms of demographic characteristics (gender, age, race, education, or parental education), out-of-scanner behavioral performance on the task of interest (expectancy AX task, described in the next section), or degree of psychotic psychopathology (including reality distortion, disorganization, and negative symptoms). Fifteen patient participants were eliminated from the final fMRI analysis for a number of reasons, including technical or equipment problems (N=9), a pattern of behavior that suggested they did not perform the task appropriately (N=4), movement of more than 3.75 mm (approximate 1 voxel) in any direction while being scanned (N=1), or premature withdrawal from the scanning session (N=1). Table 1 summarizes the characteristics of the 18 patient participants who completed the study and had a diagnosis of schizophrenia (N=16) or schizoaffective disorder (N=2) at follow-up, hereafter referred to as the schizophrenia patients. There were also 12 participants who received a diagnosis of another psychotic disorder (six with psychotic affective disorder, three with delusional disorder, and three with psychosis not otherwise specified), hereafter referred to as the nonschizophrenia psychosis patients. A more homogeneous subgroup of nonschizophrenia psychosis patients was not used because 1) selection of a subgroup would have limited the power to detect group differences and 2) all patients shared psychotic symptoms and changes in functioning that led to psychiatric hospitalization. Ratings from the Global Assessment Scale, Brief Psychiatric Rating Scale (23), Scale for the Assessment of Positive Symptoms (24), and Scale for the Assessment of Negative Symptoms (25) were used to measure symptom severity in terms of overall functioning and along three major dimensions of schizophrenia-related psychosis: reality distortion, disorganization, and negative symptoms (17). As shown in Table 1, the patient groups showed similar levels of dysfunction and nonpsychotic symptoms. However, the schizophrenia patients showed significantly more reality distortion (t=2.40, df=26, p=0.02) and disorganization (t=2.84, df=26, p=0.009) at index assessment, compared to the nonschizophrenia psychosis patients, and also showed more negative symptoms, although this difference did not reach significance (t=2.02, df=26, p=0.053).
Comparison participants were recruited from the community through advertisements in local newspapers and notices. In addition to the exclusion criteria described for the patients, exclusion criteria for the comparison participants included 1) having a history of axis I disorder (26), 2) having a first-degree relative with a psychotic disorder, and 3) having been treated with any psychotropic medication within past 6 months. The three groups did not differ significantly in gender, age, race, education, parental education (a proxy measure of socioeconomic status), or handedness (Table 1).
Data from an fMRI study that included a partially overlapping group of 10 of the schizophrenia patients and seven of the comparison participants have been previously reported (27).
Context Processing and In-Scanner Performance
Participants performed a version of the expectancy AX task (21) in which a series of single letters in a large font appeared one at a time on a screen inside the MR scanner. The task is represented schematically in Figure 1. Participants were instructed to respond quickly and accurately with their dominant hand by pushing the target button whenever they observed an X (probe) after an A (cue) and by pushing the nontarget button for every other stimulus. Thus, participants had to represent and maintain the cue as the context for evaluating the subsequent probe to respond selectively to targets. In this modification of the AX continuous performance task, 70% of the randomly presented trials were AX cue-probe sequences, 10% were AY, 10% were BX, and 10% were BY (where B could be any non-A cue, and Y any non-X probe). The task was presented in 12 blocks of 20 stimuli (10 trials) each. The stimulus durations were 500 msec. To probe the ability to actively maintain context information, half the blocks had a long delay of 8000 msec between cue and probe and a 1000-msec delay until the next trial. The other blocks had a short delay of 1000 msec between cue and probe and an 8000-msec delay until the next trial. All subjects practiced the task extensively before scanning.
The expectancy AX task allows for the evaluation of a specific deficit in context processing. When a high proportion of AX trials is used, the BX condition is difficult if the context provided by the B cue is degraded. The degradation of this context is reflected in both a relatively higher number of false alarms (inappropriate target responses) and relatively slower reaction times on correct responses to the subsequent X. The AY condition is difficult if the context provided by the A cue leads to the expectation of a valid probe. The representation and maintenance of the context provided by the A cue is reflected in a relatively higher number of false alarms and relatively slower reaction times on correct responses to the subsequent Y. Generalized deficits can lead to a greater number of errors across all conditions. Thus, this task can produce a double-dissociation in performance between conditions of intact context processing (poorer AY performance or slower AY reaction times, relative to AX reaction times), disturbances of context processing (poorer BX performance or slower BX reaction times, relative to AX reaction times), or a pattern of generalized deficits (e.g., poorer performance across conditions).
In terms of in-scanner performance, there was no significant effect of group or delay on percentage of errors. However, as illustrated in Figure 2, there was a significant interaction of group and trial type (F=2.71, df=4.5, 120, p<0.03, with Greenhouse-Geisser correction). A priori contrasts between AY and BX trials (collapsed across delay) showed a disordinal interaction between the schizophrenia patients and the comparison subjects (F=8.70, df=1, 53, p=0.005) and between the schizophrenia patients and the nonschizophrenia psychosis patients (F=4.04, df=1, 53, p<0.05). Context representations are not all or nothing. Because the imaging analysis included only correct trials, it is useful to demonstrate that schizophrenia patients still suffer from degraded context representations even when they respond accurately. Figure 2 illustrates reaction time interference (18), that is, context processing on correct trials for the AY and BX conditions with the dominant (AX) reaction time subtracted (again collapsed across delay conditions, for which there was no differential effect). A priori contrasts showed a disordinal interaction between the schizophrenia patients and the comparison subjects (F=4.95, df=2, 53, p<0.04) but not between the schizophrenia patients and the nonschizophrenia psychosis patients (F=0.75, df=1, 27, p=0.39). Thus, these behavioral results were consistent with results for performance outside the scanner in a superset of this study group (17) and in other studies (18), although power and the number of critical trials inside the scanner were lower in the current study.
Neuroimaging Method
Acquisition
Functional scans were acquired by using a 1.5-T GE Signa (General Electric Medical Systems, Milwaukee) whole-body scanner with a standard head coil. Sixteen 3.8-mm-thick axial slices with 3.75 mm2 in-plane resolution were obtained beginning at the anterior commissure-posterior commissure line. Scans used a two-shot T2-weighted spiral scanning pulse sequence (TR=1250, TE=35 msec, flip angle=60°, field of view=24 cm), allowing full image acquisition every 2.5 seconds. As illustrated in Figure 1, four fMRI scans were acquired during each 10-second trial. Structural images were obtained before and in the same plane as the functional images by using a standard T1-weighted pulse sequence.
Preprocessing
Functional images were reconstructed, and movement was estimated and corrected by using Automated Image Registration (28). After applying a maximum movement criterion for study inclusion (3.75 mm or degrees), multivariate analysis of variance results indicated no significant differences between groups in absolute movement from the reference scan or in scan-to-scan incremental movement (Wilks’s lambda=0.51, p=0.12). A 12-parameter automated algorithm (29) was used to align each participant’s structural and functional images to a reference brain image. Registered functional data were smoothed (8 mm full width at half maximum) to increase the signal.
Statistical Analysis
Analyses were performed in two stages: first, statistical maps were calculated for each individual, and then the values of these maps were grouped for hypothesis testing. Specifically, five independent variables were used to account for variance in the MR signal from trial to trial on an event-related basis. Two variables, cue and probe, were associated with perceiving and responding to stimuli. Three other variables associated with context processing included the occurrence of B cues (versus A cues), long delays (versus short delays for both A and B cues), and the interaction of B cues with long delays (versus B cues at the short delay and A cues at either delay). To calculate regressors, each variable was convolved with a canonical, double gamma hemodynamic response function (30, 31). These predictors were then entered simultaneously into a general linear model implemented by using AFNI software (32) to generate each participant’s partial correlation map (that is, a map of regions in which each variable correlated either positively or negatively with the hemodynamic response function and accounted for unique variance). For example, on a B cue in the long delay condition, there would be a hemodynamic response function associated with four regressors: cue, B cue, delay, and the interaction of B cue and delay. Because variables were dummy-coded, activity associated with each regressor was interpretable as a positive or a negative deflection from zero (which included the noncoded conditions). To avoid confounds associated with collinearity, we adopted the conservative approach of evaluating only partial correlations (unique variance) associated with each regressor. To control for the influence of task performance, hemodynamic response functions and MR data from error trials and no-response trials were removed from the analyses. To examine activity within groups, we compared participants’ partial r values, which are normally distributed, to zero for each regressor by using analysis of variance (ANOVA) with subject as a random variable. Images were thresholded at an alpha of p<0.01, with a contiguity criterion of eight voxels to correct for multiple comparisons (33). By using the same significance threshold, between-group differences were derived from ANOVAs for each regressor, with subject as a random variable, group as an independent variable, and partial r values as the dependent variable. Context processing likely involves a distributed network that includes motor areas, inferior parietal cortex, and visual processing areas, but because of the nature of our hypotheses, for simplicity we present data for frontal regions (y > 0 mm) with activation associated with the B cue, delay, and the interaction of B cue and delay. (Data for other regressors and more posterior activations [y < 0 mm] are available upon request.) In addition, post hoc analyses were conducted by using the Games-Howell formula, which does not assume equal sample sizes or variances. For schizophrenia patients, we tested correlations between individual differences in brain activity, task performance, and symptoms. Differences between correlations of symptoms with brain activity were tested by using Meng’s z test (34).
Results
Within-Group Analyses
Table 2 summarizes data for frontal regions with significant activation as defined by the correlation with task-related regressors in each group. Results for the first of these regressors, cue type (B versus A cue), indicated the regions associated with the representation of information required to select and execute task-appropriate behaviors in either delay condition. B cue activation was observed in the left inferior frontal cortex in the schizophrenia patients and the comparison subjects and in the right inferior frontal cortex in both patient groups. The nonschizophrenia psychosis patients and the comparison subjects also showed frontal activation in the left middle frontal gyrus, among other regions. Although the centroids of these activations were in different regions, the regions were largely overlapping (Figure 3). Next, activation related to the long delay condition (for both B and A cues) was observed only in the comparison subjects in the left middle frontal gyrus (Brodmann’s area 8). The last regressor, representing the interaction of cue type with delay (long delay with B cue versus other trial types), indicated regions associated with maintaining the context representation needed to overcome a prepotent response. Both patient groups showed inferior frontal activation, and the schizophrenia patients also showed activation in the right Brodmann’s area 9/10.
Thus, across analyses, the three groups showed overlapping areas of activation in the prefrontal cortices. Posterior regions, such as the bilateral inferior parietal cortex, also showed consistent activation across groups.
Between-Group Analyses
Table 3 summarizes the data for frontal regions with significantly different activation between groups across the three regressors of interest and reports results of post hoc analyses describing the direction of the difference.
First, the comparison of cue type revealed group differences in activation in the right middle frontal gyrus centered in Brodmann’s area 9, bordering Brodmann’s area 10 (Figure 4). This region showed lower levels of activation in the schizophrenia patients after the B cue, relative to the A cue, and no differences between the comparison subjects and the nonschizophrenia psychosis patients. Among the schizophrenia patients, lower levels of activation correlated with higher BX error rates (r=–0.49, N=18, p=0.04) and higher levels of disorganization (r=–0.47, N=18, p=0.05). The correlation between activation and disorganization was greater than the correlation between activation and either reality distortion or negative symptoms (Meng’s z values ≥1.62, p≤0.05). Activation in the two other regions of the prefrontal cortex that showed group differences was not associated with symptoms or performance.
Delay-related activation differences were observed in the superior frontal cortex, where both patient groups showed more activation than the comparison subjects. Activation in this region was not significantly associated with symptoms or performance.
In comparing the interaction of cue type with delay across groups, the schizophrenia patients showed greater activation in the right middle frontal gyrus centered in Brodmann’s area 10 and bordering Brodmann’s area 9. This region was inversely related to the overlapping region centered in the right Brodmann’s area 9 identified in the comparison of cue types. For the schizophrenia patients, greater activation in this region correlated with more BX errors (r=0.57, N=18, p=0.01) and more disorganization (r=0.58, N=18, p=0.01), a correlation that was significantly greater than that with reality distortion or negative symptoms (Meng’s z values ≥2.20, p≤0.01). The schizophrenia patients also showed more activation in the bilateral inferior frontal cortices, but this activation was not significantly related to symptoms or performance.
Differential power, scanner drift, uncorrected movement, or large anatomical differences (compared to the reference brain) could result in greater variation in MR scan values that might produce systematic differences in signal-to-noise ratio across individuals. However, we found no significant difference in the number of analyzed trials across groups (F=1.85, df=2, 54, p=0.17) nor in the interaction of cue type (A versus B) and group (F=0.80, df=2, 54, p=0.46). These results suggested that our strategy of evaluating only correct trials did not unduly affect the power to detect effects across groups. Second, the signal-to-noise ratio for the regions listed in Table 3 was calculated by dividing each individual’s mean MR signal intensity value for each pixel by its variance over time. No regions showed a significant group difference (ANOVA, p>0.11 for all regions of interest). Internal activation standards could also be derived from activation associated with perceiving and responding to any cue or probe. Across all groups, cue- and probe-related activity was associated with activation in the left inferior parietal cortex. The consistency of these observations weighs against the possibility that observed group differences were due to measurement artifact. We also tested whether the group differences were similar in only right-handed subjects. All group differences remained significant when data for left-handed subjects were removed, except for the activation difference in the left middle frontal cortex (Brodmann’s area 10) associated with B-cue representation (F=3.10, df=2, 51, p=0.054).
Discussion
The finding of both lower and higher levels of hemodynamic activity in the prefrontal cortex in schizophrenia patients comes at a time of renewed debate about the nature of frontal abnormalities in this illness. Hypofrontality, or lower levels of activity, in the prefrontal cortex was observed in two positron emission tomography (PET) and five fMRI studies with various versions of the continuous performance task (18, 27, 35–39). However, two PET studies and one xenon study that included such tasks did not show hypofrontality in schizophrenia patients (40–42). Hypofrontality has also been observed in several fMRI studies that included working memory paradigms, such as the N-back task (8, 10, 43, 44), which requires both storage and executive functions. This pattern of findings is contested by researchers who have reported hyperfrontality, or higher levels of activity in the prefrontal cortex, in studies based on simple working memory storage paradigms (45–47) and low working memory loads on the N-back task (48). In the following discussion we focus on the two regions of the right middle frontal gyrus that showed both hypo- and hyperfrontality and on correlations of activation with external variables, such as performance and symptoms.
One of the primary task demands of the expectancy AX task is the representation of the context of the B cues so as to control, or overcome, the prepotent target response to the X probe. Consistent with previous studies (17, 21), we found that higher proportions of errors in the BX condition relative to the AY condition, suggesting that the schizophrenia patients, but not the nonschizophrenia psychosis patients, were specifically impaired in this task demand. In contrast, a broader impairment in response inhibition would have led to more errors in both the BX and the AY trials, whereas an impairment in storage would have led to more errors in the BX trials with delay, neither of which was observed. The finding that the right middle frontal gyrus (Brodmann’s area 9) was less active in schizophrenia patients than in the comparison subjects or the nonschizophrenia psychosis patients is consistent with the context processing model (13), which predicts that this region is an important component of a top-down control network that facilitates the representation and maintenance of context. The current study found that right hypofrontality was correlated with impaired performance, which has been reported (39), but left hypofrontality has also been correlated with impaired performance on this task (49), raising the possibility that hypofrontality during context representation is bilateral in schizophrenia. Previous studies have also reported that the degree of hypofrontality is correlated with the level of schizophrenia patients’ disorganization symptoms (8). The current data extend this finding by showing that it is not an artifact of significance thresholding, but instead that disorganization symptoms are significantly more correlated with dysfunction in this region than are other symptom types. Although this region is impaired, it is clear that patients perform the task far better than they would by chance; thus it may be that the region that showed group differences is not necessary for control. There are a number of explanations that may account for this finding. Not all of the regions that comparison subjects activated in the cue condition (Table 2) showed group differences (Table 3). Thus, some schizophrenia patients may have activated parts of these same regions, albeit at levels that did not show group-level significance. Another possibility is that schizophrenia patients use a different strategy when representing the context of the B cues.
One possible alternative strategy is suggested by the other region that showed significant group differences and correlations with external variables. The Brodmann’s area 10 region of interest was located near a region that was previously found to be more active in schizophrenia patients during performance of a working memory paradigm (48), a finding that was interpreted in terms of cortical “inefficiency.” One might speculate that activity here and in the bilateral inferior frontal cortices reflects the use of episodic memory to encode information that is ordinarily actively maintained by the prefrontal cortex. This strategy is less efficient, as it requires subsequent retrieval of this information at the time of response (see reference 50 for a discussion of the relative advantages and efficiencies of memory mechanisms that use active maintenance versus synaptic modification). This hypothesis is consistent with findings suggesting that Brodmann’s area 10 is involved in episodic memory encoding and retrieval (51). The use of alternate, less efficient strategies (such as episodic memory and/or rehearsal) for representing and maintaining information about the cue may account for the degraded performance (slower reaction times and more errors on BX trials) and for the opposite relationship between performance and brain activity observed in areas of hyperactivity (e.g., Brodmann’s area 10) versus the areas of hypoactivity (e.g., Brodmann’s area 9) observed in patients with schizophrenia. This interpretation suggests that schizophrenia may be associated with a selective deficit in specific regions of the prefrontal cortex and is also consistent with the result of previous studies (27).
Conclusions
The study provides additional support for the context processing theory (13) regarding the relationship between middle frontal gyrus dysfunction, cognitive deficits, and the symptoms of disorganization in schizophrenia. This study was based on data from first-episode, never-medicated patients, which eliminated the possibility that these associations were due to effects of medication or chronicity of illness. The comparison of schizophrenia patients to nearly equally dysfunctional psychosis patients deemed not to have schizophrenia speaks strongly to the diagnostic specificity of these disturbances. Of course, many important questions remain regarding the relationship between regional activity, pathophysiology, cognitive disturbances, and the symptoms of schizophrenia. Of great interest is whether the methods we used and our findings can be used to predict response to medication, functional outcomes, or both. Furthermore, our study focused specifically on frontal cortex function and the cognitive processes it is thought to support. There is little doubt that schizophrenia is associated with disturbances of other brain areas and the cognitive functions that they support. However, we hope that our findings provide a useful impetus for the application of a theoretically driven approach to the study of other aspects of schizophrenia and to the study of other neuropsychiatric disorders.
 |
 |
 |
Data from the study were presented at the 9th Annual International Congress of Schizophrenia Research, Colorado Springs, Colo., March 29–April 2, 2003. Received Jan. 13, 2004; revision received March 31, 2004; accepted April 12, 2004. From the Department of Psychology, University of Minnesota; the Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh; the Department of Radiology, University of Pittsburgh Medical Center, Pittsburgh; the Department of Psychology, Washington University, St. Louis; and the Center for the Study of Brain, Mind, and Behavior and the Department of Psychology, Princeton University, Princeton, N.J. Address correspondence and reprint requests to Dr. MacDonald, Department of Psychology, University of Minnesota, N219 Elliott Hall, 75 E. River Rd., Minneapolis, MN 55455, [email protected] (e-mail). Supported by NIMH grant 5P50 MH-45156. The authors thank Macheri Keshavan, M.D., Gretchen Haas, Ph.D., Elizabeth Romney, Ph.D., and Debra Montrose for help with recruitment and demographic and clinical assessment of the study participants; Howard Aizenstein, M.D., Greg Siegle, Ph.D., Kristi Clark, B.S., Brittany Lourea, B.A., Grace Nah, B.A., Greg Nickliss, B.A., and the UPMC Magnetic Resonance Research Center staff for assistance in conducting the study; and Christine Belanger, B.S., for help with the graphics.
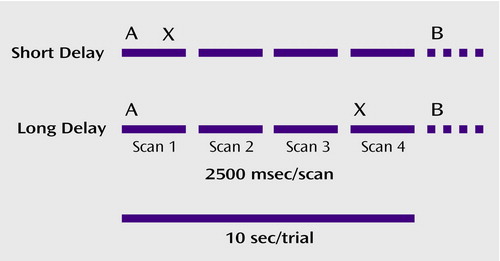
Figure 1. Schematic Representation of Expectancy AX Context Processing Task Showing Relationship Between Task Variables and Image Acquisitiona
aIn this variant of the traditional AX context processing task, participants responded with either the target button for X probes following A cues and the nontarget button for every other stimulus (including all cues, Y probes following A cues, and X probes following B cues, where B could be any non-A cue, and Y any non-X probe). Twelve blocks were completed, each consisting of seven AX, one AY, one BX, and one BY pair. Thus, the A cue was associated with a more automatic response, whereas the B cue indicated a greater need to represent and maintain the context. Half the blocks had a short delay (1000 msec) between the cue and probe, and half had a long (8000 msec) delay.
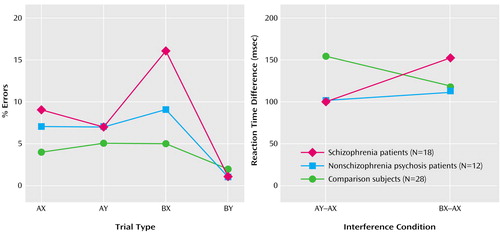
Figure 2. Accuracy on Expectancy AX Context Processing Task Trial Types and Interference Reaction Time for Correct Trials of Patients With Schizophrenia, Patients With Nonschizophrenia Psychosis, and Healthy Comparison Subjectsa
aSee Figure 1 and Method section for explanation of context processing task parameters. Interference reaction time reflects context processing for the AY and BX conditions with the dominant (AX) reaction time subtracted.
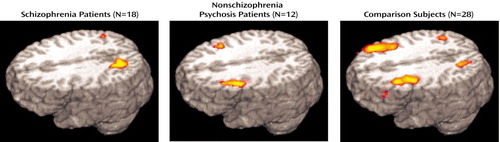
Figure 3. Regions of Increased Activation Associated With the B Cue Type in the Expectancy AX Context Processing Task in Patients With Schizophrenia, Patients With Nonschizophrenia Psychosis, and Healthy Comparison Subjectsa
aSee Figure 1 and Method section for explanation of context processing task parameters. Posterior activations represented in the figure are not reported in the tables.
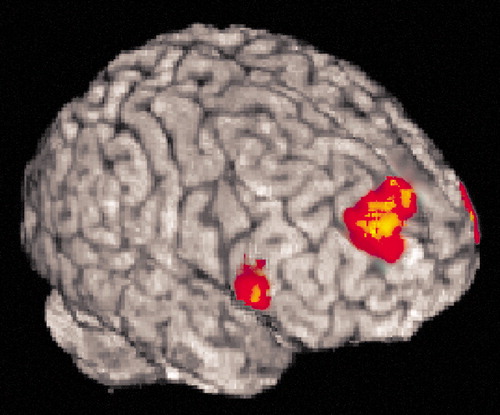
Figure 4. Region of Increased Activation Associated With Group Differences Related to the B Cue Type in the Expectancy AX Context Processing Taska
aSee Figure 1 and Method section for explanation of context processing task parameters. Group differences between patients with schizophrenia, patients with nonschizophrenia psychosis, and healthy comparison subjects were found in the right middle frontal gyrus (dorsolateral prefrontal cortex, Brodmann’s area 9).
1. Andreasen NC, Rezai K, Alliger R, Swayze VW II, Flaum M, Kirchner P, Cohen G, O’Leary DS: Hypofrontality in neuroleptic-naive patients and in patients with chronic schizophrenia: assessment with Xenon 133 single-photon emission computed tomography and the Tower of London. Arch Gen Psychiatry 1992; 49:943–958Crossref, Medline, Google Scholar
2. Liddle PF: Inner connections within domain of dementia praecox: role of supervisory mental processes in schizophrenia. Eur Arch Psychiatry Clin Neurosci 1995; 245:210–215Crossref, Medline, Google Scholar
3. Berman KF, Doran AR, Pickar D, Weinberger DR: Is the mechanism of prefrontal hypofunction in depression the same as in schizophrenia? regional cerebral blood flow during cognitive activation. Br J Psychiatry 1993; 162:183–192Crossref, Medline, Google Scholar
4. Curtis VA, Dixon TA, Morris RG, Bullmore ET, Brammer MJ, Williams SC, Sharma T, Murray RM, McGuire PK: Differential frontal activation in schizophrenia and bipolar illness during verbal fluency. J Affect Disord 2001; 66:111–121Crossref, Medline, Google Scholar
5. Barch D, Sheline YI, Csernansky J, Snyder A: Working memory and prefrontal cortex dysfunction: specificity to schizophrenia compared with major depression. Biol Psychiatry 2003:376–384Google Scholar
6. O’Connell RA, Van Heertun RL, Luck D, Yudd AP, Cueva JE, Billick SB, Cordon DJ, Gersh RJ, Masdeu JC: Single-photon emission computed tomography of the brain in acute mania and schizophrenia. J Neuroimaging 1995; 5:101–104Crossref, Medline, Google Scholar
7. Wood FB, Flowers DL: Hypofrontal vs hypo-sylvian blood flow in schizophrenia. Schizophr Bull 1990; 16:413–424Crossref, Medline, Google Scholar
8. Perlstein WM, Carter CS, Noll DC, Cohen JD: Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. Am J Psychiatry 2001; 158:1105–1113Link, Google Scholar
9. Heckers S, Goff D, Schacter DL, Savage CR, Fischman AJ, Alpert NM, Rauch SL: Functional imaging of memory retrieval in deficit vs nondeficit schizophrenia. Arch Gen Psychiatry 1999; 56:1117–1123Crossref, Medline, Google Scholar
10. Menon V, Anagnoson RT, Mathalon DH, Glover GH, Pfefferbaum A: Functional neuroanatomy of auditory working memory in schizophrenia: relation to positive and negative symptoms. Neuroimage 2001; 13:433–446Crossref, Medline, Google Scholar
11. Gur RC, Gur RE: Hypofrontality in schizophrenia: RIP. Lancet 1995; 345:1338–1340Crossref, Google Scholar
12. Phillips WA, Silverstein SM: Convergence of biological and psychological perspectives on cognitive coordination in schizophrenia. Behav Brain Sci 2003; 26:65–138Crossref, Medline, Google Scholar
13. Cohen JD, Servan-Schreiber D: Context, cortex and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychol Rev 1992; 99:45–77Crossref, Medline, Google Scholar
14. Baddeley AD, Hitch GJ: Working memory, in The Psychology of Learning and Motivation. Edited by Bower G. San Diego, Academic Press, 1974, pp 47–90Google Scholar
15. MacDonald AW III, Cohen JD, Stenger VA, Carter CS: Dissociating the role of dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 2000; 288:1835–1838Crossref, Medline, Google Scholar
16. Javitt DC, Shelley AM, Silipo G, Lieberman JA: Deficits in auditory and visual context-dependent processing in schizophrenia. Arch Gen Psychiatry 2000; 57:1131–1137Crossref, Medline, Google Scholar
17. Barch D, Carter C, MacDonald A, Braver T, Cohen J: Context processing deficits in schizophrenia: diagnostic specificity, four-week course, and relationships to clinical symptoms. J Abnorm Psychol 2003; 112:132–143Crossref, Medline, Google Scholar
18. MacDonald AW III, Pogue-Geile MF, Johnson MK, Carter CS: A specific deficit in context processing in the unaffected siblings of patients with schizophrenia. Arch Gen Psychiatry 2003; 60:57–65Crossref, Medline, Google Scholar
19. Heinrichs RW, Zakzanis KK: Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology 1998; 12:426–445Crossref, Medline, Google Scholar
20. Oltmanns TF, Neale JM: Schizophrenic performance when distractors are present: attentional deficit or differential task difficulty? J Abnorm Psychol 1975; 84:205–209Crossref, Medline, Google Scholar
21. Servan-Schreiber D, Cohen JD, Steingard S: Schizophrenic deficits in the processing of context: a test of a theoretical model. Arch Gen Psychiatry 1996; 53:1105–1112Crossref, Medline, Google Scholar
22. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R, Version 1.0 (SCID). Washington, DC, American Psychiatric Press, 1990Google Scholar
23. Overall JE, Gorham DR: The Brief Psychiatric Rating Scale. Psychol Rep 1962; 10:799–812Crossref, Google Scholar
24. Andreasen NC: Scale for the Assessment of Positive Symptoms (SAPS). Iowa City, University of Iowa, 1983Google Scholar
25. Andreasen NC: Scale for the Assessment of Negative Symptoms (SANS). Iowa City, University of Iowa, 1983Google Scholar
26. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R—Non-Patient Edition (SCID-NP, Version 1.0). Washington, DC, American Psychiatric Press, 1990Google Scholar
27. Barch DM, Carter CS, Braver TS, Sabb FW, MacDonald AW III, Noll DC, Cohen JD: Selective deficits in prefrontal cortex function in medication-naive patients with schizophrenia. Arch Gen Psychiatry 2001; 58:280–288Crossref, Medline, Google Scholar
28. Woods RP, Cherry SR, Mazziotta JC: Rapid automated algorithm for aligning and re-slicing PET images. J Comput Assist Tomogr 1992; 16:620–633Crossref, Medline, Google Scholar
29. Woods RP, Grafton ST, Holmes CJ, Cherry SR, Mazziotta JC: Automated image registration, I: general methods and intrasubject, intramodality validation. J Comput Assist Tomogr 1998; 22:139–152Crossref, Medline, Google Scholar
30. Dale AM, Buckner RL: Selective averaging of rapidly presented individual trials using fMRI. Hum Brain Mapp 1997; 5:329–340Crossref, Medline, Google Scholar
31. Friston K, Holmes A, Worsley K, Poline J, Frith C, Frackowiak R: Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 1995; 2:189–210Crossref, Google Scholar
32. Cox R: AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 1996; 29:162–173Crossref, Medline, Google Scholar
33. Foreman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC: Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med 1995; 33:636–647Crossref, Medline, Google Scholar
34. Meng XL, Rosenthal R, Rubin DB: Comparing correlated correlation coefficients. Psychol Bull 1992; 111:172–175Crossref, Google Scholar
35. Cohen RM, Nordahl TE, Semple WE, Andreason P, Pickar D: Abnormalities in the distributed network of sustained attention predict neuroleptic treatment response in schizophrenia. Neuropsychopharmacology 1998; 19:36–47Crossref, Medline, Google Scholar
36. Schroeder J, Buchsbaum MS, Siegel BV, Geider FJ, Haier RJ, Lohr J, Wu J, Potkin SG: Patterns of cortical activity in schizophrenia. Psychol Med 1994; 24:947–955Crossref, Medline, Google Scholar
37. Volz H, Gaser C, Hager F, Rzanny R, Ponisch J, Mentzel H, Kaiser WA, Sauer H: Decreased frontal activation in schizophrenics during stimulation with the continuous performance test—a functional magnetic resonance imaging study. Eur Psychiatry 1999; 14:17–24Crossref, Medline, Google Scholar
38. Volz H-P, Nenadic I, Gaser C, Rammsayer T, Hager F, Sauer H: Time estimation in schizophrenia: an fMRI study at adjusted levels of difficulty. Neuroreport 2001; 12:313–316Crossref, Medline, Google Scholar
39. Perlstein W, Dixit N, Carter C, Noll D, Cohen J: Prefrontal cortex dysfunction mediates deficits in working memory and prepotent responding in schizophrenia. Biol Psychiatry 2003; 53:25–38Crossref, Medline, Google Scholar
40. Jernigan TL, Sargent T III, Pfefferbaum A, Kusubov N, Stahl SM: 18Fluorodeoxyglucose PET in schizophrenia. Psychiatry Res 1985; 16:317–329Crossref, Medline, Google Scholar
41. O’Leary DS, Andreasen NC, Hurtig RR, Kesler ML, Rogers M, Arndt S, Cizadlo T, Watkins GL, Boles Ponto LL, Kirchner PT, Hichwa RD: Auditory attentional deficits in patients with schizophrenia: a positron emission tomography study. Arch Gen Psychiatry 1996; 53:633–641Crossref, Medline, Google Scholar
42. Berman K, Zec R, Weinberger DR: Physiological dysfunction of dorsolateral prefrontal cortex in schizophrenia, II: role of neuroleptic treatment, attention, and mental effort. Arch Gen Psychiatry 1986; 43:126–135Crossref, Medline, Google Scholar
43. Callicott JH, Egan MF, Mattay VS, Bertolino A, Jones KM, Goldberg TE, Weinberger DR: Altered prefrontal cortical function in unaffected siblings of patients with schizophrenia (abstract). Neuroimage 1998; 7(suppl 2):S895Google Scholar
44. Meyer-Lindenberg A, Poline J-B, Kohn PD, Holt JL, Egan MF, Weinberger DR, Berman KF: Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry 2001; 158:1809–1817Link, Google Scholar
45. Manoach DS, Press DZ, Thangaraj V, Searl MM, Goff DC, Halpern E, Saper CB, Warach S: Schizophrenic subjects activate dorsolateral prefrontal cortex during a working memory task, as measured by fMRI. Biol Psychiatry 1999; 45:1128–1137Crossref, Medline, Google Scholar
46. Manoach DS, Gollub RL, Benson R, Searl MM, Goff DC, Halpern E, Saper CB, Rauch SL: Schizophrenia subjects show aberrant fMRI activation of dorsolateral prefrontal cortex and basal ganglia during working memory performance. Biol Psychiatry 2000; 48:99–109Crossref, Medline, Google Scholar
47. Quintana J, Wong T, Ortiz-Portillo E, Kovalik E, Davidson T, Marder SR, Mazziotta JC: Prefrontal-posterior parietal networks in schizophrenia: primary dysfunctions and secondary compensations. Biol Psychiatry 2003; 53:12–24Crossref, Medline, Google Scholar
48. Callicott JH, Bertolino A, Mattay V, Langheim FJP, Duyn J, Coppola R, Goldberg TE, Weinberger DR: Physiological dysfunction of the dorsolateral prefrontal cortex in schizophrenia revisited. Cereb Cortex 2000; 11:1078–1092Crossref, Google Scholar
49. MacDonald AW III, Carter CS: Event-related fMRI study of context processing in dorsolateral prefrontal cortex of patients with schizophrenia. J Abnorm Psychol 2003; 112:689–697Crossref, Medline, Google Scholar
50. O’Reilly RC, Munakata Y: Psychological function in computational models of neural networks, in Handbook of Psychology, Vol 3: Biological Psychology. Edited by Gallagher M, Nelson RJ, Weiner IB. New York, John Wiley & Sons, 2003, pp 637–654Google Scholar
51. Cabeza R, Dolcos F, Graham R, Nyberg L: Similarities and differences in the neural correlates of episodic memory retrieval and working memory. Neuroimage 2002; 16:317–330Crossref, Medline, Google Scholar



