Psychotic Depression and Mortality
Abstract
OBJECTIVE: Major depressive disorder is associated with elevated mortality rates that increase with the severity of depression. The authors hypothesized that patients with psychotic depression would have higher mortality rates than patients with nonpsychotic depression. METHOD: Survival analytic techniques were used to compare the vital status of 61 patients with psychotic major depression with that of 59 patients with nonpsychotic major depression up to 15 years after hospital admission. Medical status was assessed with the Cumulative Illness Rating Scale. Dexamethasone suppression test (DST) data were available for 101 patients. RESULTS: The mortality rate for subjects with psychotic depression was significantly greater than that for those with nonpsychotic depression, with 41% versus 20%, respectively, dying within 15 years after hospital admission. A proportional hazards model with age and medical status entered as covariates confirmed a significantly higher mortality rate in patients with psychotic depression (hazards ratio=2.31). A positive DST result was associated with psychotic depression but was not related to vital status. CONCLUSIONS: Patients with psychotic depression have a two-fold greater risk of death than do patients with severe, nonpsychotic major depression.
Major depressive disorder is associated with greater mortality (1–4) even after the effects of age, sex, and coexisting medical illness are controlled (5, 6). Mortality rates increase with an increasing number of depressive symptoms (7).
Unipolar psychotic depression is distinct from nonpsychotic depression (8, 9) and is associated with severe symptoms, prolonged course, poor response rates, more residual symptoms, frequent relapses (8–11), and higher rates of hypercortisolemia or dexamethasone nonsuppression (12). Given the severity of this disorder, we hypothesized that the mortality rates would be higher in subjects with psychotic depression than in those with nonpsychotic depression. We also hypothesized that hypercortisolemia might be associated with greater mortality.
Method
The Yale University Human Investigation Committee approved the research protocol. Inpatients who had participated in prior studies of psychotic depression or cortisol hypersecretion at Yale New Haven Hospital from 1977 to 1990 (8, 12–14) were selected and reviewed. All patients met RDC (15), DSM-III, or DSM-III-R (16) criteria for major depressive disorder. The distinction between psychotic and nonpsychotic depression was based on the presence of clear delusions or hallucinations. Patients with bipolar disorder, schizophrenia, schizoaffective disorder, current substance abuse, or acute medical illness were excluded. The attending psychiatrist (J.C.N.) established the psychiatric diagnosis at the time of admission. All of the patients with psychotic depression from this group were included. A comparable number of patients with nonpsychotic depression who were given a standard dexamethasone suppression test (DST) during their hospitalization were selected. For patients with multiple hospitalizations, the first admission was chosen as the index admission.
To assess medical comorbidity, medical records of the index hospitalization were reviewed using the Cumulative Illness Rating Scale (17). A single rater blind to diagnosis (M.V.) performed the Cumulative Illness Rating Scale assessment.
All of the nonpsychotic and 42 of the 61 patients with psychotic depression had a standard DST during their hospital stay. Administration of the DST has been described previously (13, 14). Briefly, 1 mg of dexamethasone was given at 11:00 p.m., and two cortisol samples (8:00 a.m. and 4:00 p.m. or 8:00 p.m.) were drawn the next day.
Death status was investigated by entering a subject’s name, date of birth, and social security number into a computerized search of national databases (U.S. Record Search); death certificates were obtained for those who had died. To standardize duration, we analyzed mortality data only up to 15 years following index hospital admission.
Between-group differences in dichotomous variables were assessed by using chi-square analyses with Yates’s correction. For continuous variables, t tests were used. Between-group differences in mortality rates were assessed by survival analytic techniques. Time to death, in years, was defined as the difference between year of death and year of index hospital admission. Observation times, also assessed in years, were terminated at either the number of years between index admission and 1999 (the final year in which death records were obtained), or 15 years, whichever was smaller. Relationships between patient variables and time to death were evaluated using chi-square tests based on Wilcoxon statistics derived from the analysis of Kaplan-Meier survival curves. The independent association of psychotic depression and mortality was assessed using a proportional hazards model that controlled for age and medical status.
Results
Sixty-one patients with psychotic depression and 59 patients with nonpsychotic depression were selected. The patients with psychotic depression were older than those with nonpsychotic depression (mean=62.8 years [SD=9.7] versus 57.6 years [SD=12.6], respectively; t=2.51, df=118, p=0.01) and, of those surviving, had been followed longer (mean=14.6 years [SD=1.0] versus 13.1 years [SD=1.1]; t=5.97, df=81, p<0.001). However, patients with nonpsychotic depression had higher Cumulative Illness Rating Scale scores (mean=4.6, [SD=2.8]) than did patients with psychotic depression (mean=3.8 [SD=2.9] t=1.64, df=118, p=0.10). The percentage of women within the group did not differ significantly between the patients with psychotic depression (59%, N=36) and those with nonpsychotic depression (64%, N=38).
According to a survival analysis, the mortality rate in subjects with psychotic major depression was significantly greater than in those with nonpsychotic major depression (χ2=3.99, df=1, p<0.05), with 41% (N=25) versus 20% (N=12), respectively, dying within 15 years after hospital admission. Age at entry (χ2=9.79, df=1, p=0.002) and Cumulative Illness Rating Scale score (χ2=10.84, df=1, p=0.001) were also significantly associated with mortality rate. Sex was not significantly associated with mortality. A proportional hazards model, in which age and medical status were entered as covariates, demonstrated a significantly higher hazard for death in patients with psychotic depression than for patients with nonpsychotic depression (Figure 1).
Patients with psychotic depression were more likely to have a positive DST (69%, N=29 of 42) than were those with nonpsychotic depression (41%, N=24 of 59) (χ2=6.82, df=1, p=0.009), and the highest mean postdexamethasone value was higher in the psychotic depression group (mean=11.9 [SD=5.8] versus 7.9 [SD=6.8]; t=3.13, df=99, p=0.002). However, DST status was not significantly associated with mortality rate in the survival analysis.
Cause of death was determined from death certificates for 34 of the 37 patients. In 30 of the patients, death resulted from medical causes. Three of the deaths were due to suicide. One motor vehicle death was suspicious for suicide. Two of the definite suicides were in the psychotic group, and one was from the nonpsychotic group. One of the 53 DST-positive patients committed suicide versus two of the 48 DST-negative patients. Group differences in suicide, either between patients with nonpsychotic versus psychotic depression or between patients with positive versus negative DST results, were not statistically significant.
Discussion
During a 15-year period following hospital admission, patients with psychotic depression were twice as likely to die as were their counterparts with nonpsychotic depression after controlling for age and medical illness. The difference in mortality rates might have been greater had subjects with psychotic depression been compared with depressed outpatients or a nondepressed sample. This finding provides further support for the distinct nature of psychotic depression.
The higher mortality among patients with psychotic depression was not explained by a higher number of suicides. Most of the deaths (30 of 34) were from medical causes. All of the suicides (including the probable case) were male patients, were violent deaths (three gunshot wounds to the head and one car crash), and occurred within 2 years of the index admission. Although it is possible that the suicide rate was underreported on the death certificates, chronic medical illness was usually present and appeared causally related.
We predicted a positive correlation between DST status and death on the basis of prior reports of cortisol’s deleterious effects on the immune system, glucose metabolism, bone, hippocampal neurons, and memory (18–20). A recent report (21) confirmed an association between DST nonsuppression and suicide risk in a mixed group of patients with affective disorders. However, in our study hypercortisolemia was not related to risk of death in depressed patients.
The present study does not explain the causes behind greater mortality in psychotic depression. It is possible that patients with psychotic depression are substantially less insightful about their illness than nonpsychotic patients, and therefore less likely to follow-up with treatment. A 10-year prospective study of psychotic depression found that these patients were more symptomatic and had more psychosocial impairment (22). This greater morbidity may be associated with greater all-cause mortality.
The findings of this study are limited by the group size and the lack of prospective monitoring of clinical status and treatment after the index admission. Nevertheless, the findings clearly show a higher mortality rate among patients with psychotic depression. The possibility that aggressive treatment might reduce mortality rates in patients with psychotic depression is intriguing and deserves further investigation.
Received Jan. 24, 2002; revision received Aug. 22, 2002; accepted Sept. 24, 2002. From the Department of Psychiatry, Yale University School of Medicine, New Haven, Conn. Address reprint requests to Dr. Vythilingam, National Institute of Mental Health, 15K North Dr., Room 111, MSC 2670, Bethesda, MD 20892-2670; [email protected] (e-mail). The authors thank Ryan Nearing for assistance with manuscript preparation.
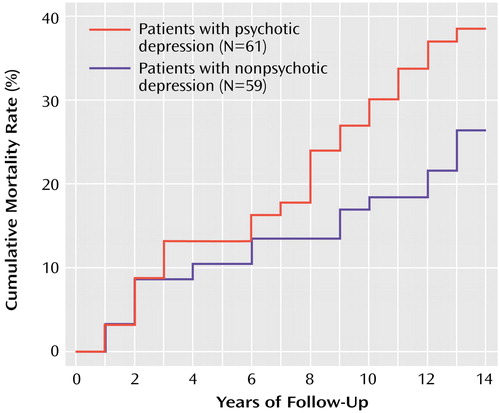
Figure 1. Mortality Rates Among Subjects With Psychotic Depression and Nonpsychotic Depression up to 15 Years After Their Index Hospital Admissiona
aThe proportional hazards model, with age and medical status (from the Cumulative Illness Rating Scale) entered as covariates, confirmed a significantly higher mortality rate among subjects with psychotic depression (hazard ratio=2.31; χ2=5.31, df=1, p=0.02). Cumulative mortality rates differ from overall mortality rates because the survival analysis accounts for early subject dropouts (i.e., the total N on which the percentages are based varies as subjects drop out of the study).
1.. Bruce ML, Leaf PJ, Rozal GP, Florio L, Hoff RA: Psychiatric status and 9-year mortality data in the New Haven Epidemiologic Catchment Area Study. Am J Psychiatry 1994; 151:716-721Link, Google Scholar
2.. Jorm AF, Henderson AS, Kay DWK, Jacomb PA: Mortality in relation to dementia, depression and social integration in an elderly community sample. Int J Geriatr Psychiatry 1991; 6:5-11Crossref, Google Scholar
3.. Pulska T, Pahkala K, Laippalla P, Kivela SL: Major depression as a predictor of premature deaths in elderly people in Finland: a community study. Acta Psychiatr Scand 1998; 97:408-411Crossref, Medline, Google Scholar
4.. Rabins PV, Harvis K, Koven S: High fatality rates of late-life depression associated with cardiovascular disease. J Affect Disord 1985; 9:165-167Crossref, Medline, Google Scholar
5.. Koenig HG, Shelp F, Goli V, Cohen HJ, Blazer DG: Survival and health care utilization in elderly medical inpatients with major depression. J Am Geriatr Soc 1989; 37:599-606Crossref, Medline, Google Scholar
6.. Murphy E, Smith R, Lindesay J, Slattery J: Increased mortality rates in late-life depression. Br J Psychiatry 1988; 152:347-353Crossref, Medline, Google Scholar
7.. Whooley MA, Browner WS (Study of Osteoporotic Fractures Research Group): Association between depressive symptoms and mortality in older women. Arch Intern Med 1998; 158:2129-2135Crossref, Medline, Google Scholar
8.. Charney DS, Nelson JC: Delusional and nondelusional unipolar depression: further evidence for distinct subtypes. Am J Psychiatry 1981; 138:328-333Link, Google Scholar
9.. Schatzberg AF, Rothschild AJ: Psychotic (delusional) major depression: should it be included as a distinct syndrome in DSM-IV? Am J Psychiatry 1992; 149:733-745Link, Google Scholar
10.. Chan CH, Janicak PG, Davis JM, Altman E, Andriukaitis S, Hedeker D: Response of psychotic and nonpsychotic depressed patients to tricyclic antidepressants. J Clin Psychiatry 1987; 48:197-200Medline, Google Scholar
11.. Coryell W, Endicott J, Keller M, Andreasen NC: Phenomenology and family history in DSM-III psychotic depression. J Affect Disord 1985; 9:13-18Crossref, Medline, Google Scholar
12.. Nelson JC, Davis JM: DST studies in psychotic depression: a meta-analysis. Am J Psychiatry 1997; 154:1497-1503Link, Google Scholar
13.. Miller KB, Nelson JC: Dexamethasone nonsuppression and EEG abnormalities. Biol Psychiatry 1987; 22:1151-1155Crossref, Medline, Google Scholar
14.. Miller KB, Nelson JC: Does the dexamethasone suppression test relate to subtypes, factors, symptoms, or severity? Arch Gen Psychiatry 1987; 44:769-774Crossref, Medline, Google Scholar
15.. Spitzer RL, Endicott J, Robins E: Research Diagnostic Criteria (RDC) for a Selected Group of Functional Disorders, 3rd ed. New York, New York State Psychiatric Institute, Biometrics Research, 1978Google Scholar
16.. Spitzer RL, Williams JBW, Gibbon M, First MB: Structured Clinical Interview for DSM-III-R—Patient Version (SCID-P). New York, New York State Psychiatric Institute, Biometrics Research, 1990Google Scholar
17.. Linn BS, Linn MW, Gurel L: Cumulative Illness Rating Scale. J Am Geriatr Soc 1968; 16:622-626Crossref, Medline, Google Scholar
18.. Cizza G, Ravn P, Chrousos GP, Gold PW: Depression: a major, unrecognized risk factor for osteoporosis? Trends Endocrinol Metab 2001; 12:198-203Crossref, Medline, Google Scholar
19.. Ligier S, Sternberg EM: Neuroendocrine host factors and inflammatory disease susceptibility. Environ Health Perspect 1999; 107(suppl 5):701-707Google Scholar
20.. Sapolsky RM: Depression, antidepressants, and the shrinking hippocampus. Proc Natl Acad Sci USA 2001; 98:12320-12322Crossref, Medline, Google Scholar
21.. Coryell W, Schlesser M: The dexamethasone suppression test and suicide prediction. Am J Psychiatry 2001; 158:748-753Link, Google Scholar
22.. Coryell W, Leon A, Winokur G, Endicott J, Keller M, Akiskal H, Solomon D: Importance of psychotic features to long-term course in major depressive disorder. Am J Psychiatry 1996; 153:483-489Link, Google Scholar



