DST Studies in Psychotic Depression: A Meta-Analysis
Abstract
OBJECTIVE: Although hypersecretion of cortisol has frequently been reported in psychotic depression, the findings have been mixed. The authors performed a meta-analysis of the existing studies to determine the significance of differences in nonsuppression of cortisol across studies. METHOD: Fourteen studies that compared dexamethasone suppression test (DST) results in psychotic and nonpsychotic patients were examined, and a Mantel-Haenszel meta-analysis was performed. For comparison purposes, 19 studies of the DST with respect to melancholic/nonmelancholic and inpatient/outpatient distinctions were similarly reviewed. RESULTS: This analysis indicated a highly significant probability that a greater rate of cortisol nonsuppression occurs in psychotic depression. The nonsuppression rate was substantially higher in patients with psychotic depression (64%) than in nonpsychotic patients (41%). In the 19 studies of melancholia, nonsuppression was less frequent (36%), and when inpatient/outpatient status was controlled, melancholic depression was not significantly associated with nonsuppression. In nonmelancholic outpatients with major depression, the nonsuppression rate was low (12%). CONCLUSIONS: Among patients with major depression, psychotic depression is the subtype that is most closely associated with nonsuppression of cortisol on the DST. Hypercortisolemia is usually present in psychotic depression and may be important in understanding the pathophysiology of this syndrome. (Am J Psychiatry 1997; 154:1497–1503)
Several lines of evidence suggest that psychotic depression is a distinct disorder. Recurrence of psychotic depressed episodes in patients with psychotic depression suggests that the disorder runs “true to form” (1–7). Two family studies (8, 9), using direct interviews of family members, found an increased incidence of psychotic depression in first-degree relatives of probands with psychotic depression. Twelve treatment studies, reviewed elsewhere (10), indicate that patients with major depression and psychosis are much less likely to respond to tricyclic antidepressants alone than their nonpsychotic depressed counterparts.
A variety of biological variables have been examined in psychotic depression (11). Cortisol secretion is the most frequently studied measure, and the dexamethasone suppression test (DST) (12) is the method most frequently used. Although hypersecretion of cortisol has been reported in psychotic depression, results have been mixed, with several studies failing to find a significant difference in rates of nonsuppression of cortisol on the DST. The current study was undertaken to review these studies, perform a meta-analysis of the findings, and determine the status of the DST in psychotic depression. Because nonsuppression of cortisol on the DST has also been associated with melancholic depression and this association might explain the findings in psychotic depression, we also reviewed studies of the DST in patients with melancholic or endogenous depression.
METHOD
We reviewed the literature to find studies reporting DST results in psychotic and nonpsychotic patients. These studies were examined to determine that 1) the studies included independent, nonoverlapping groups of patients, 2) the criteria for major depression were specified, and 3) the methods used to assess the psychotic or nonpsychotic status of the patients were clear. We also reviewed these studies to determine the method of DST administration and whether patients for whom the test was inappropriate were excluded (12). Although there was some variability in the actual DST methods used, the methods were valid and relatively comparable.
Studies of the DST in melancholic depression were also reviewed. Again we determined that the groups of patients were independent, that the melancholic group was compared with patients who had nonmelancholic major depression (rather than those with other psychiatric disorders or normal control subjects), that a systematic method of DST administration was described, that appropriate exclusions were used, and that the number of patients who were nonsuppressors was presented for each group. The studies varied in the manner in which melancholic or endogenous depression was defined. The DSM-III criteria for melancholia and the Research Diagnostic Criteria (RDC) (13) for endogenous depression were the most common methods, but in one case ICD-8 criteria were used, and another study used clinical criteria described by the investigators.
We used the Mantel-Haenszel test (14) for the meta-analyses, following the recommendation of Emerson (15). It was previously used by the American Psychiatric Association's Task Force on Laboratory Tests in Psychiatry (16) and is the most common meta-analytic method used for dichotomous data in the medical literature. To examine whether the results were consistent from study to study, we determined the homogeneity of the effect size in the samples with the original Mantel-Haenszel method (14). This refers to the variability of the difference between rates of nonsuppression in psychotic and nonpsychotic patients from study to study. If the effect sizes differed significantly, we used the DerSimonian and Laird method (17), which does not assume a fixed effect size.
RESULTS
Fourteen studies (18–31) that involved independent groups of psychotic and nonpsychotic depressed patients met our criteria and were included in the analysis (table 1). All of these studies used either the RDC (13), the Feighner criteria (32), or the DSM-III criteria for major depression. Most study groups consisted of inpatients, but two studies (22, 30) included both inpatients and outpatients. All studies observed exclusions for the DST, usually those described by Carroll and associates (12). The dexamethasone dose and timing of cortisol samples varied across studies (table 1).
These studies included a total of 276 psychotic and 708 nonpsychotic depressed patients. The overall rates of nonsuppression of cortisol were 64% for the psychotic depressed patients and 41% for the nonpsychotic group. The Mantel-Haenszel analysis of the 14 studies demonstrated a highly significant probability that the greater rate of nonsuppression in the psychotic depressed patients was not a chance finding (χ2=47.43, df=1, p<0.001; odds ratio=3.0; 95% confidence interval=2.2–4.1). An analysis of the homogeneity of the effect size was nonsignificant (χ2=11.39, df=15, p=0.58), indicating that the effect size did not differ significantly from study to study.
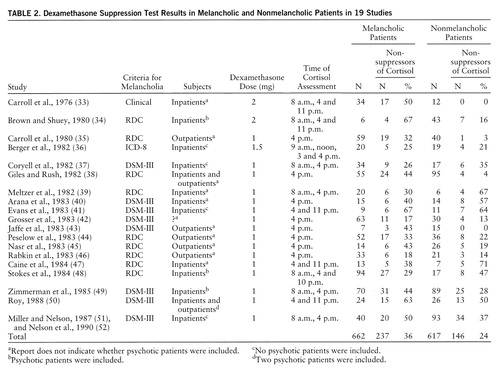
Nineteen studies (33–52) that compared the DST in melancholic and nonmelancholic patients were identified (table 2). (Two study groups [51, 52] that partially overlapped were combined to eliminate duplication and were subsequently treated as a single group.) Of the 662 melancholic patients, 36% were nonsuppressors of cortisol on the DST; of the 617 nonmelancholic patients, 24% were nonsuppressors. An analysis of the homogeneity of the findings among the studies was significant (χ2=44.44, df=18, p<0.001), indicating that the effect size varied significantly from study to study. Thus, we examined these data using the DerSimonian and Laird method. This analysis indicated that nonsuppression of cortisol was significantly more likely in melancholic depressed patients, but the magnitude of the probability was substantially lower than that for patients with psychotic depression (χ2=4.84, df=1, p<0.03; odds ratio=2.0; 95% confidence interval=1.5–2.6).
The 19 studies of melancholia included both inpatients and outpatients, and rates of nonsuppression on the DST differed for these groups. The overall rate of nonsuppression in the five studies of outpatients only was 22% (N=68 of 303), while in the 11 studies of inpatients only, the rate was 36% (N=244 of 683). The difference between inpatients and outpatients was similar to that observed for melancholic and nonmelancholic patients. If inpatient/outpatient status was accounted for, melancholia was not associated with nonsuppression of cortisol on the DST. A DerSimonian and Laird meta-analysis of rates of nonsuppression limited to the 11 inpatient studies indicated that rates of nonsuppression did not differ significantly in melancholic and nonmelancholic patients: 38% (N=136 of 355) and 33% (N=108 of 328), respectively (χ2=0.11, df=1, p=0.74). Alternatively, among the melancholic patients, rates of nonsuppression in inpatients and outpatients showed a similar difference (38% versus 31%). It appeared that both melancholic status and inpatient status were associated with a moderate increase in cortisol nonsuppression; however, the combination of melancholia and inpatient status did not appear to significantly or substantially increase the rate of nonsuppression once either single variable was present. In nonmelancholic outpatients, the nonsuppression rate was low, 12% (N=17 of 138).
DISCUSSION
Although less than one-half of the studies of the DST in psychotic depression individually found significant differences between patient groups, all but one of the studies found a higher rate of nonsuppression of cortisol in psychotic depressed patients than in nonpsychotic depressed patients. Our meta-analysis demonstrated a very substantial probability that nonsuppression occurs more frequently in psychotic depression than in nonpsychotic depression. Further, the actual rate in psychotic depression, 64%, indicates that hypercortisolemia is a characteristic of most patients with psychotic depression. These findings support the distinction between psychotic and nonpsychotic major depression and are consistent with the hypothesis, advanced by Schatzberg and colleagues (53, 54), that elevated cortisol levels may play a role in the pathophysiology of psychotic depression.
Several of the studies we reviewed noted that not only did the DST show nonsuppression of cortisol in psychotic depression, but the actual cortisol levels were quite high (22, 28–31). Some investigators (22, 28, 29, 55) have suggested that a higher threshold between 11 and 15 µg/dl might be more specific and predictive for psychotic depression.
Because psychotic depression is a severe disorder, it might be questioned whether the differences in rates of nonsuppression on the DST between psychotic and nonpsychotic patients is explained by severity. First, it should be noted that in most cases, the comparison groups in the 14 studies consisted of melancholic inpatients whose illness was also reasonably severe. In six of the studies (18, 20, 21, 26, 29, 30), the issue of severity was explicitly addressed. In each case, either severity was not associated with suppression/nonsuppression or multivariate analysis indicated that severity did not account for the difference in the rate of nonsuppression in psychotic and nonpsychotic depressed patients.
The studies of psychotic depressed subjects varied in the use of other subtyping restrictions (e.g., whether the subjects had endogenous, primary, or unipolar depression); however, in all but two studies, the comparison subjects were inpatients. The diagnostic issue that appeared to be most important was how schizoaffective disorder, other signs of schizophrenia, or mood-incongruent delusions were dealt with. In two of the studies with the lowest rates of cortisol nonsuppression (19, 24), schizoaffective patients or those with “subtle signs of schizophrenia” were not excluded. In the second of these studies, 64% of the psychotic patients had mood-incongruent delusions. Ayuso-Gutierrez and colleagues (27), in the only study with a lower rate of nonsuppression in the psychotic patients, noted that the eight patients with mood-incongruent delusions had a much lower rate of nonsuppression (12%) than the 18 patients with mood-congruent delusions (55%). The rate of nonsuppression in the patients with mood-congruent delusions was slightly higher than the rate in the nonpsychotic patients, consistent with the other 13 studies. Lower rates of nonsuppression have also been observed in schizophrenic and schizoaffective patients and patients with atypical psychotic features (53).
The meta-analysis of the differences between melancholic and nonmelancholic patients was clearly significant, but the effect size was not as large as it was for the psychotic/nonpsychotic distinction. Further, these studies varied with respect to inclusion of psychotic patients. Reports from four studies (34, 48, 49, 50) indicated that psychotic patients were included, four studies (36, 37, 41, 51, 52) excluded psychotic patients (or results were presented separately), and in 11 studies psychotic status was not specified. In the four studies that explicitly excluded psychotic patients (all were inpatient studies), rates of nonsuppression did not differ significantly in the 103 patients with endogenous depression (39%) and the 140 with nonendogenous depression (36%). It is possible that inclusion of psychotic patients (who are usually melancholic) contributed to the purported relationship between cortisol nonsuppression and melancholia. It seems even more likely that controlling for inpatient/outpatient status helps to explain the lack of difference between melancholic and nonmelancholic patients in these four studies.
Both inpatient status and melancholia were associated with a moderate increase in the rate of nonsuppression of cortisol, but because they covary, it was difficult to separate their independent associations with the DST results. It is probable that both variables are important, and both could reflect some underlying factor associated with hypercortisolemia.
In a study of 95 nonpsychotic inpatients, Miller and Nelson (51) examined this question. Cortisol nonsuppression occurred in 43% (N=41) of these inpatients but was not significantly correlated with DSM-III melancholia, RDC endogenous depression, or severity. Seven individual symptoms were significantly correlated with nonsuppression of cortisol. These symptoms were examined to determine whether there were common factors that were related to hypercortisolemia. No common factors were found. Miller and Nelson (51) reviewed 11 other studies (34, 56–65) that examined the relationship of individual symptoms to DST results. The most common symptom correlates of the DST were psychomotor change (both retardation and agitation) and sleep disturbance, followed by weight loss and somatic anxiety. Although these symptoms appear to be characteristic of melancholia, other symptoms also central to the concept of melancholia, such as loss of interest and lack of reactivity, were not frequently associated with nonsuppression. Further, these symptoms did not vary together, but were individually associated with the DST results. Thus, in our previous study (51), in our review (51), and in other reviews (58, 66, 67), it has not been possible to identify a diagnostic syndrome or a common factor other than psychotic depression that relates to nonsuppression.
It might be questioned whether the lack of homogeneity of effect size in the 19 melancholia studies suggests flawed methods in some of the studies. There are other explanations, however, that appear to provide a satisfactory explanation for the lack of homogeneity. These studies included inpatients and outpatients, different criteria for melancholia, and different methods for performing the DST (for example, usually only one cortisol sample was obtained from outpatients).
The question also arises whether reports of rates of cortisol nonsuppression might be inflated because of the tendency not to report negative results (the “file drawer” issue). Although this is possible, it seems relatively unlikely. The DST has been studied over many years. After the initial positive reports, negative reports became worthy of publication. In fact, five groups of investigators (37, 39, 40, 47, 48) reported negative results for the DST in melancholia (i.e., a higher rate of nonsuppression of cortisol in nonmelancholic patients). Further, most of the focus has been on the DST in melancholia; thus, reports on the relation of DST results to psychotic depression may be less biased. Given the strength of the association of nonsuppression on the DST with psychotic depression in the studies reviewed, we estimated that negative results from another 4,162 studies would have to be reported to render our findings statistically nonsignificant (68).
CONCLUSIONS
These findings indicate that psychotic depression is the subtype of depression associated with nonsuppression of cortisol following administration of dexamethasone, and that nonsuppression of cortisol occurs in two-thirds of these patients. Nonsuppression is found in about one-third of melancholic patients and of inpatients, but if inpatient status is accounted for, the significance of the association between melancholia and nonsuppression is lost. In outpatients with major depression, the nonsuppression rate is low. Individual symptoms have been associated with nonsuppression, but no other subtypes or factors that explain the association of these symptoms with hypercortisolemia have been identified.
From a clinical perspective, nonsuppression of cortisol on the DST is of limited diagnostic usefulness. Often the diagnosis of psychotic depression is obvious. In patients for whom the diagnosis is questionable, however, a substantially elevated cortisol level after dexamethasone would be another piece of evidence favoring the diagnosis of psychotic depression. This test might be particularly useful in “near-delusional” patients with depression. Two studies (69, 70) have noted that near-delusional patients respond less well to antidepressants given alone and may require combined antipsychotic and antidepressant treatment. In addition, this test may prove useful for assessing when a psychotic depressed patient has been adequately treated, because it has been suggested that the DST result reverts to normal with recovery of the patient and that failure to revert may predict suicide potential (16). Roose and associates (71) have reported that psychotic depressed patients are more likely to commit suicide, even when they appear to have improved. The use of the DST may help to determine whether a patient with psychotic depression has been adequately treated.
From a theoretical perspective, it has been suggested that hypercortisolemia may contribute to psychosis by enhancing dopamine activity (53, 54). Several animal studies (reviewed elsewhere [53]) and two human studies (72, 73) indicated that administration of glucocorticoids increases dopamine or dopamine metabolite levels in the CSF or plasma. Two studies of patients with psychotic depression (30, 74) found a specific pattern of response to dexamethasone which differed from that of other patients or control subjects. Increased dopamine activity, as evidenced by elevated plasma dopamine levels (30), elevated CSF homovanillic acid (HVA, the metabolite of dopamine) levels (75–78), and plasma HVA levels (79, 80), has been linked to psychosis in depressed patients. Two studies (81, 82) noted an increase in CSF HVA in depressed patients who became psychotic during antidepressant treatment, while in most patients HVA levels fell. These findings suggest that hypercortisolemia may enhance dopamine activity in some patients, and this change may contribute to psychosis. Further investigation of the role of hypercortisolemia in psychotic depression is warranted.
 |
 |
Received Jan. 5, 1996; revisions received April 16 and July 14, 1997; accepted July 16, 1997. From the Department of Psychiatry, Yale University School of Medicine, and the Department of Psychiatry, University of Illinois at Chicago. Address reprint requests to Dr. Nelson, Department of Psychiatry, Yale-New Haven Hospital, 20 York St., New Haven, CT 06517.
1. Charney DS, Nelson JC: Delusional and nondelusional unipolar depression: further evidence for distinct subtypes. Am J Psychiatry 1981; 138:328–333Link, Google Scholar
2. Lykouras E, Christodoulou GN, Malliaras D: Type and content of delusions in unipolar psychotic depression. J Affect Disord 1985; 9:249–252Crossref, Medline, Google Scholar
3. Coryell W, Endicott J, Keller M, Andreasen NC: Phenomenology and family history in DSM-III psychotic depression. J Affect Disord 1985; 9:13–18Crossref, Medline, Google Scholar
4. Miller F, Chabrier LA: Delusional and nondelusional unipolar depression: further evidence for distinct subtypes. Hosp Community Psychiatry 1986; 37:1157–1158Google Scholar
5. Kettering RL, Harrow M, Grossman L, Meltzer HY: The prognostic relevance of delusions in depression: a follow-up study. Am J Psychiatry 1987; 144:1154–1160Google Scholar
6. Baldwin RC: Delusional and nondelusional depression in late life: evidence for distinct subtypes. Br J Psychiatry 1988; 152:39–44Crossref, Medline, Google Scholar
7. Maj M, Pirozzi R, DeCaprio EL: Major depression with mood-congruent psychotic features: a distinct diagnostic entity or a more severe subtype of depression? Acta Psychiatr Scand 1990; 82:439–444Google Scholar
8. Leckman JF, Weissman MM, Prusoff BA: Subtypes of depression. Arch Gen Psychiatry 1984; 41:833–838Crossref, Medline, Google Scholar
9. Endicott J, Nee J, Coryell W, Keller M, Andreasen N, Croughan J: Schizoaffective, psychotic, and nonpsychotic depression: differential familial association. Compr Psychiatry 1986; 27:1–13Crossref, Medline, Google Scholar
10. Chan CH, Janicak PG, Davis JM: Response of psychotic and nonpsychotic depressed patients to tricyclic antidepressants. J Clin Psychiatry 1987; 48:197–200Medline, Google Scholar
11. Schatzberg AF, Rothschild AJ: Psychotic (delusional) major depression: should it be included as a distinct syndrome in DSM-IV? Am J Psychiatry 1992; 149:733–745Google Scholar
12. Carroll BJ, Feinberg M, Greden JF, Tarika J, Albala AA, Haskett RF, James NM, Kronfol Z, Lohr N, Steiner M, de Vigne JP, Young E: A specific laboratory test for the diagnosis of melancholia: standardization, validation, and clinical utility. Arch Gen Psychiatry 1981; 38:15–22Crossref, Medline, Google Scholar
13. Spitzer RL, Endicott J, Robins E: Research Diagnostic Criteria (RDC) for a Selected Group of Functional Disorders, 3rd ed. New York, New York State Psychiatric Institute, Biometrics Research, 1977Google Scholar
14. Mantel N, Haenszel W: Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959; 22:719–748Medline, Google Scholar
15. Emerson JD: Combining estimates of the odds ratio: the state of the art. Stat Methods Med Res 1994; 3:157–178Crossref, Medline, Google Scholar
16. APA Task Force on Laboratory Tests in Psychiatry: The dexamethasone suppression test: an overview of its current status in psychiatry. Am J Psychiatry 1987; 144:1253–1262Google Scholar
17. DerSimonian R, Laird N: Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188Crossref, Medline, Google Scholar
18. Mendlewicz J, Charles G, Franckson JM: The dexamethasone suppression test in affective disorder: relationship to clinical and genetic subgroups. Br J Psychiatry 1982; 141:464–470Crossref, Medline, Google Scholar
19. Coryell W, Gaffney G, Burkhardt PE: The dexamethasone suppression test and familial subtypes of depression—a naturalistic replication. Biol Psychiatry 1982; 17:33–40Medline, Google Scholar
20. Asnis GM, Halbreich U, Nathan RS, Ostrow L, Novacenko H, Endicott J, Sachar EJ: The dexamethasone suppression test in depressive illness: clinical correlates. Psychoneuroendocrinology 1982; 7:295–301Crossref, Medline, Google Scholar
21. Rudorfer MV, Hwu HG, Clayton PJ: Dexamethasone suppression testing in primary depression: significance of family history and psychosis. Biol Psychiatry 1982; 17:41–48Medline, Google Scholar
22. Schatzberg AF, Rothschild AJ, Stahl JB, Bond TC, Rosenbaum AH, Lofgren SB, MacLaughlin RA, Sullivan MA, Cole JO: The dexamethasone suppression test: identification of subtypes of depression. Am J Psychiatry 1983; 140:88–91Link, Google Scholar
23. Caroff S, Winokur A, Rieger W, Schweizer E, Amsterdam J: Response to dexamethasone in psychotic depression. Psychiatry Res 1983; 8:59–64Crossref, Medline, Google Scholar
24. Coryell W, Pfohl B, Zimmerman M: The clinical and neuroendocrine features of psychotic depression. J Nerv Ment Dis 1984; 172:521–528Crossref, Medline, Google Scholar
25. Nelson WH, Khan A, Orr WW Jr: Delusional depression: phenomenology, neuroendocrine function, and tricyclic antidepressant response. J Affect Disord 1984; 6:297–306Crossref, Medline, Google Scholar
26. Rihmer Z, Arato M, Szadoczyk E, Revai K, Demeter E, Gyorgy S, Udvarhelyi P: The dexamethasone suppression test in psychotic versus nonpsychotic endogenous depression. Br J Psychiatry 1984; 145:508–511Crossref, Medline, Google Scholar
27. Ayuso-Gutierrez JL, Almoguera MI, Garcia-Camba E, Frias JO, Cabranes JA: The dexamethasone suppression test in delusional depression: further findings. J Affect Disord 1985; 8:147–151Crossref, Medline, Google Scholar
28. Evans DL, Nemeroff CB: The clinical use of the dexamethasone suppression test in DSM-III affective disorders: correlation with the severe depressive subtypes of melancholia and psychosis. J Psychiatr Res 1987; 21:185–194Crossref, Medline, Google Scholar
29. Levy AB, Stern SL: DST and TRH stimulation test in mood disorder subtypes. Am J Psychiatry 1987; 144:472–475Link, Google Scholar
30. Rothschild AJ, Schatzberg AF, Langlais PJ, Lerbinger JE, Miller MM, Cole JO: Psychotic and nonpsychotic depressions, I: comparison of plasma catecholamines and cortisol measures. Psychiatry Res 1987; 20:143–153Crossref, Medline, Google Scholar
31. Brown RP, Stoll PM, Stokes PE, Frances A, Sweeney J, Kocsis JH, Mann JJ: Adrenocortical hyperactivity in depression: effects of agitation, delusions, melancholia, and other illness variables. Psychiatry Res 1987; 23:167–178Crossref, Google Scholar
32. Feighner JP, Robins E, Guze SB, Woodruff RA Jr, Winokur G, Munoz R: Diagnostic criteria for use in psychiatric research. Arch Gen Psychiatry 1972; 26:57–63Crossref, Medline, Google Scholar
33. Carroll BJ, Curtis GC, Mendels J: Neuroendocrine regulation in depression: discrimination of depressed from nondepressed patients. Arch Gen Psychiatry 1976; 33:1051–1058Google Scholar
34. Brown WA, Shuey I: Response to dexamethasone and subtype of depression. Arch Gen Psychiatry 1980; 37:747–751Crossref, Medline, Google Scholar
35. Carroll BJ, Feinberg M, Greden JF, Haskett RF, James MN, Steiner M, Tarika J: Diagnosis of endogenous depression: comparison of clinical, research and neuroendocrine criteria. J Affect Disord 1980; 2:177–194Crossref, Medline, Google Scholar
36. Berger M, Doerr P, Lund R, Bronisch T, vonZerssen D: Neuroendocrinological and neurophysiological studies in major depressive disorders: are there biological markers for the endogenous subtype? Biol Psychiatry 1982; 17:1217–1242Google Scholar
37. Coryell W, Gaffney G, Burkhardt PE: DSM-III melancholia and the primary-secondary distinction: a comparison of concurrent validity by means of the dexamethasone suppression test. Am J Psychiatry 1982; 139:120–122Link, Google Scholar
38. Giles DE, Rush AJ: Relationship of dysfunctional attitudes and dexamethasone response in endogenous and nonendogenous depression. Biol Psychiatry 1982; 17:1303–1314Google Scholar
39. Meltzer HY, Fang VS, Tricou BJ, Robertson A, Piyaka SK: Effect of dexamethasone on plasma prolactin and cortisol levels in psychiatric patients. Am J Psychiatry 1982; 136:763–768Google Scholar
40. Arana GW, Barreira PJ, Cohen BM, Lipinski JF, Fogelson D: The dexamethasone suppression test in psychotic disorders. Am J Psychiatry 1983; 140:1521–1523Google Scholar
41. Evans DL, Burnett GB, Nemeroff CB: The dexamethasone suppression test in the clinical setting. Am J Psychiatry 1983; 140:586–589Link, Google Scholar
42. Grosser BI, Brainard J, Byerley B, Reimherr F, Wood D: Low sensitivity of the dexamethasone suppression test in melancholia, in 1983 Annual Meeting New Research Program and Abstracts. Washington, DC, American Psychiatric Association, 1983, p NR21Google Scholar
43. Jaffe K, Barnshaw HD, Kennedy ME: The dexamethasone suppression test in depressed outpatients with and without melancholia. Am J Psychiatry 1983; 140:492–493Link, Google Scholar
44. Peselow ED, Goldring N, Fieve RR, Wright R: The dexamethasone suppression test in depressed outpatients and normal control subjects. Am J Psychiatry 1983; 140:245–247Link, Google Scholar
45. Nasr SJ, Pandey G, Altman EG, Gibbons R, Gaviria FM, Davis JM: Symptom profile of patients with positive DST: a pilot study. Biol Psychiatry 1983; 18:571–574Medline, Google Scholar
46. Rabkin JG, Quitkin FM, Stewart JW, McGrath PJ, Puig-Antich J: The dexamethasone suppression test with mildly to moderately depressed outpatients. Am J Psychiatry 1983; 140:926–927Link, Google Scholar
47. Caine ED, Yerevanian BI, Bamford KA: Cognitive function and the dexamethasone suppression test in depression. Am J Psychiatry 1984; 141:116–118Link, Google Scholar
48. Stokes PE, Stoll PM, Koslow SH, Maas JW, Davis JM, Swann AC, Robins E: Pretreatment DST and hypothalamic-pituitary-adrenocortical function in depressed patients and comparison groups: a multicenter study. Arch Gen Psychiatry 1984; 41:257–267Crossref, Medline, Google Scholar
49. Zimmerman M, Coryell W, Pfohl B, Stangl D: Four definitions of endogenous depression and the dexamethasone suppression test. J Affect Disord 1985; 8:37–45Crossref, Medline, Google Scholar
50. Roy A: Cortisol nonsuppression in depression: relationship to clinical variables. J Affect Disord 1988; 14:265–270Crossref, Medline, Google Scholar
51. Miller KB, Nelson JC: Does the dexamethasone suppression test relate to subtypes, factors, symptoms, or severity? Arch Gen Psychiatry 1987; 44:769–774Google Scholar
52. Nelson JC, Mazure CM, Jatlow PI: Does melancholia predict response in major depression? J Affect Disord 1990; 18:157–165Google Scholar
53. Schatzberg AF, Rothschild AJ, Langlais PJ, Bird ED, Cole JO: A corticosteroid/dopamine hypothesis for psychotic depression and related states. J Psychiatr Res 1985; 19:57–64Crossref, Medline, Google Scholar
54. Schatzberg AF, Rothschild AJ: The roles of glucocorticoid and dopaminergic systems in delusional (psychotic) depression. Ann NY Acad Sci 1988; 537:462–471Crossref, Medline, Google Scholar
55. Rothschild AJ, Schatzberg AF, Rosenbaum AH, Stahl JB, Cole JO: The dexamethasone suppression test as a discriminator among subtypes of psychotic patients. Br J Psychiatry 1982; 141:471–474Crossref, Medline, Google Scholar
56. Klein HE, Bender W, Mayr H, Niederschweiberer A, Schmauss M: The DST and its relationship to psychiatric diagnosis, symptoms and treatment outcome. Br J Psychiatry 1984; 145:591–599Crossref, Medline, Google Scholar
57. Kasper S, Beckmann H: Dexamethasone suppression test in a pluridiagnostic approach: its relationship to psychopathological and clinical variables. Acta Psychiatr Scand 1983; 68:31–37Crossref, Medline, Google Scholar
58. Zimmerman M, Coryell W, Pfohl B: The validity of the dexamethasone suppression test as a marker for endogenous depression. Arch Gen Psychiatry 1986; 43:347–355Crossref, Medline, Google Scholar
59. Zimmerman M, Stangl D, Coryell W: The Research Diagnostic Criteria for endogenous depression and the dexamethasone suppression test: a discriminant function analysis. Psychiatry Res 1985; 14:197–208Crossref, Medline, Google Scholar
60. Nasr SJ, Gibbons RD: Depressive symptoms associated with dexamethasone resistance. Psychiatry Res 1983; 10:183–189Crossref, Medline, Google Scholar
61. Brown WA, Qualls CB: Pituitary-adrenal disinhibition in depression: marker of a subtype with characteristic clinical features and response to treatment? Psychiatry Res 1981; 4:115–128Google Scholar
62. Alessi NE, Robbins DR: Symptoms and subtypes of depression among adolescents distinguished by the dexamethasone suppression test: a preliminary report. Psychiatry Res 1984; 11:177–184Crossref, Medline, Google Scholar
63. Reus VI: Pituitary-adrenal disinhibition as the independent variable in the assessment of behavioral symptoms. Biol Psychiatry 1982; 17:317–326Medline, Google Scholar
64. Rubin AL, Price LH, Charney DS, Heninger GR: Noradrenergic function and the cortisol response to dexamethasone in depression. Psychiatry Res 1985; 15:5–15Crossref, Medline, Google Scholar
65. Krishnan KRR, France RD, Pelson S, McCann UD, Manepalli AN, Davidson JRT: What does the dexamethasone suppression test identify? Biol Psychiatry 1985; 20:957–964Google Scholar
66. Arana GW, Baldessarini RJ, Ornsteen M: The dexamethasone suppression test for diagnosis and prognosis in psychiatry. Arch Gen Psychiatry 1985; 42:1193–1204Google Scholar
67. Garvey MJ, Schaffer C, Schaffer L, Perry P: Is DST status associated with depression characteristics? J Affect Disord 1989; 16:159–165Google Scholar
68. Rosenthal R: The file drawer problem and tolerance for null results. Psychol Bull 1979; 86:638–641Crossref, Google Scholar
69. Janicak PG, Pandey GN, Davis JM, Boshes R, Bresnahan D, Sharma R: Response of psychotic and nonpsychotic depression to phenelzine. Am J Psychiatry 1988; 145:93–95Link, Google Scholar
70. Nelson JC, Mazure CM, Jatlow PI: Characteristics of desipramine-refractory depression. J Clin Psychiatry 1994; 55:12–19Medline, Google Scholar
71. Roose SP, Glassman AH, Walsh BT, Woodring S, Vital-Herne J: Depression, delusions, and suicide. Am J Psychiatry 1983; 140:1159–1162Google Scholar
72. Rothschild AJ, Langlais PJ, Schatzberg AF, Walsh FX, Cole JO, Bird ED: Dexamethasone increases plasma free dopamine in man. J Psychiatr Res 1984; 18:217–223Crossref, Medline, Google Scholar
73. Wolkowitz OM, Doran A, Breier A, Roy A, Jimerson DC, Sutton ME, Golden RN, Paul SM, Pickar D: The effects of dexamethasone on plasma homovanillic acid and 3-methoxy-4-hydroxyphenylglycol. Arch Gen Psychiatry 1987; 44:782–789Crossref, Medline, Google Scholar
74. Wolkowitz OM, Doran A, Breier A, Roy A, Pickar D: Specificity of plasma HVA response to dexamethasone in psychotic depression. Psychiatry Res 1989; 29:177–186Crossref, Medline, Google Scholar
75. Sweeney D, Nelson JC, Bowers MB, Maas J, Heninger G: Delusional versus non-delusional depression: neurochemical differences (letter). Lancet 1978; 2:100–101Crossref, Medline, Google Scholar
76. Aberg-Wistedt A, Wistedt B, Bertilsson L: Higher CSF levels of HVA and 5-HIAA in delusional compared to nondelusional depression (letter). Arch Gen Psychiatry 1985; 42:925–926Crossref, Medline, Google Scholar
77. Agren H, Terenius L: Hallucinations in patients with major depression: interactions between CSF monoaminergic and endorphinergic indices. J Affect Disord 1985; 9:25–34Crossref, Medline, Google Scholar
78. Brown RP: CSF monoamine and depressive subtypes, in 1987 Annual Meeting New Research Program and Abstracts. Washington, DC, American Psychiatric Association, 1987, p 28Google Scholar
79. Devanand DP, Bowers MB, Hoffman FJ, Nelson JC: Elevated plasma homovanillic acid in depressed females with melancholia and psychosis. Psychiatry Res 1985; 15:1–4Crossref, Medline, Google Scholar
80. Mazure CM, Bowers MB, Hoffman F, Miller KB, Nelson JC: Plasma catecholamine metabolites in subtypes of major depression. Biol Psychiatry 1987; 22:1469–1472Google Scholar
81. Bowers MB: Cerebrospinal fluid 5-hydroxyindoleacetic acid (5-HIAA) and homovanillic acid (HVA) following probenecid in unipolar depressives treated with amitriptyline. Psychopharmacology (Berl) 1972; 23:26–33Crossref, Google Scholar
82. Linnoila M, Karoum F, Potter WZ: Effects of antidepressant treatments on dopamine turnover in depressed patients. Arch Gen Psychiatry 1983; 40:1015–1017Google Scholar



