EEG Synchronization to Modulated Auditory Tones in Schizophrenia, Schizoaffective Disorder, and Schizotypal Personality Disorder
Abstract
OBJECTIVE: The authors tested whether neural synchronization deficits were present in subjects with schizophrenia and schizotypal personality disorder. METHOD: Amplitude-modulated tones were used to evaluate auditory steady-state evoked potential entrainment in a combined group of 21 subjects with schizophrenia or schizoaffective disorder, 11 subjects with schizotypal personality disorder, and 22 nonpsychiatric comparison subjects. RESULTS: The schizophrenia or schizoaffective disorder group exhibited decreased power compared to the schizotypal personality disorder and nonpsychiatric comparison groups. There were no differences between groups in N100 amplitude. CONCLUSIONS: Subjects with schizophrenia but not subjects with schizotypal personality disorder have deficits in steady-state responses to periodic stimuli, despite an intact response to sensory-evoked potentials (N100). These deficits reflect aberrant neural synchronization or resolution and may contribute to disturbed perceptual and cognitive integration in schizophrenia.
Neural synchronization in the beta (12 to 25 Hz) and gamma (greater than 25 Hz) ranges may be associated with perceptual binding and cognitive integration in animals and humans (1). Several investigators (2, 3) have suggested that disturbed neural synchronization may contribute to the failures of perceptual and cognitive integration observed in schizophrenia. The capacity of sensory-neural circuits to synchronize at specific frequencies can be tested using steady-state evoked-potential paradigms (4). Kwon et al. (5) found reduced gamma range power to click stimuli at 40 Hz but not at lower rates of stimulation in schizophrenia.
It is not known whether these disturbances of synchronization are specific to schizophrenia or might also appear in related disorders, such as schizotypal personality disorder. Schizotypal personality disorder is associated with symptoms, cognitive deficits, familial risk, and neurobiological abnormalities similar to those observed in schizophrenia (6). In the present study, amplitude-modulated tones were used to elicit the auditory steady-state evoked potential (4) across a wide range of frequencies (11 to 82 Hz) in patients with schizophrenia or schizoaffective disorder, schizotypal personality disorder, and nonpsychiatric comparison subjects.
Method
We tested 21 subjects with schizophrenia or schizoaffective disorder (three women), 11 subjects with schizotypal personality disorder (eight women), and 22 nonpsychiatric comparison subjects (nine women). Exclusion criteria for all subjects included serious head injury, treatment with ECT, neurological disorders, and current substance abuse or dependency. Written informed consent was obtained from all subjects. Schizophrenia and schizoaffective patients were evaluated with the Structured Clinical Interview for DSM-IV Axis I Disorders and a review of clinical records to obtain a DSM-IV diagnosis. Eight of the schizophrenia and schizoaffective participants had a history of substance abuse. All but three patients were taking antipsychotic medication.
The subjects with schizotypal personality disorder and the comparison subjects were recruited through newspaper advertisements and were excluded from participation if they had a history of substance abuse or dependency. Neither group had first-order relatives with schizophrenia, met criteria for an axis I disorder, or were receiving pharmacological treatment at the time of testing. None of the nonpsychiatric subjects met criteria for an axis II disorder, while three of the subjects with schizotypal personality disorder met criteria for other cluster A personality disorders.
The combined schizophrenia and schizoaffective and nonpsychiatric groups did not significantly differ in age (subjects with schizophrenia or schizoaffective disorder: mean=45.6, SD=6.2; nonpsychiatric comparison subjects: mean=39.7, SD=12.6). The subjects with schizotypal personality disorder did not differ significantly in age from the nonpsychiatric subjects (schizotypal personality disorder: mean=32.0, SD=11.3); however, they were significantly younger than the subjects with schizophrenia and schizoaffective disorder (t=4.39, df=30, p<0.01).
Eight amplitude-modulated tones were presented at an average intensity of 86 dB. Two tones were presented simultaneously, one to each ear, and administered in four randomly ordered blocks. Tone-modulation rates were paired as follows: left ear, 11 Hz; right ear, 62 Hz; left ear, 31 Hz; right ear, 82 Hz; left ear, 51 Hz; right ear, 22 Hz; left ear, 71 Hz; and right ear, 42 Hz. Stimuli to the left ear were delivered with a 1000-Hz carrier pitch and stimuli to the right ear with a 500-Hz carrier pitch. Tones were presented for 1024 msec, with a 200-msec interstimulus interval. Electrode placement followed the 10–20 system, with use of a 16-channel electrode cap with a left-ear reference. All impedances were below 10 kΩ . EEG was filtered with 1-Hz high-pass and 200-Hz low-pass filters and were sampled at 1000 Hz. EEG data were segmented into 1024-msec epochs, with no prestimulus interval. After artifact rejection, fast Fourier transformation was used to compute power at the frequency of stimulation in each condition at midline electrode sites (Fz, Cz, and Pz). Log10-EEG power values were used for analyses.
N100 amplitude measurements were taken from averaged waveforms. The data were filtered with a zero-phase-shifted, 24-Hz, low-pass filter (24 dB/octave), and the most negative voltage between 50 and 250 msec after stimulus onset was recorded at Fz, Cz, and Pz.
Results
A mixed-model analysis of covariance (ANCOVA) comparing the log10-EEG power and gender between groups at electrode sites Fz, Cz, and Pz, with age as a covariate, indicated a main effect of group (F=4.05, df=2, 47, p<0.03), a group-by-gender interaction (F=3.77, df=2, 47, p=0.03), and a group-by-frequency interaction (F=2.04, df=14, 336, p<0.02). Age was not a significant covariate (t=0.07, df=47, p=0.95). To investigate group differences further, additional analyses of variance (ANOVAs) were conducted between two groups only. The patients with schizophrenia or schizoaffective disorder showed reduced power across all frequencies of stimulation, as indicated by a main effect of group (F=5.26, df=1, 41, p<0.03), with no interactions. An ANOVA between the subjects with schizotypal personality disorder and the subjects with schizophrenia or schizoaffective disorder revealed a main effect of group (F=5.13, df=1, 30, p<0.04) and a group-by-frequency interaction (F=3.13, df=7, 210, p=0.004) (Figure 1).
In an effort to determine whether the group differences were obtained as a result of differences in resting EEG unrelated to stimulus frequency, a mixed-model ANCOVA comparing the log10-EEG power and the gender of the subjects with nonstimulated frequencies with age as a covariate was computed. Analyses revealed no main effect of group or interactions involving group factors, indicating that EEG power at nonstimulated frequencies did not differ between groups (Figure 1).
A mixed-model ANOVA measuring N100 amplitude indicated no significant differences between groups (F=1.32, df=2, 51, p=0.28), no group-by-frequency interaction (F=0.36, df=6, 153, p=0.91), and no group-by-site interaction (F=0.95, df=4, 102, p=0.44).
Discussion
Consistent with previous studies of neural synchronization in schizophrenia, patients exhibited lower power in response to steady-state auditory stimuli compared to nonpsychiatric subjects (5). This deficit may reflect less efficient local neural synchronization to external stimuli in the sensory cortex or in thalamic-sensory oscillations. In the present study, there were no group differences in amplitude of the N1 evoked related potential in response to amplitude-modulated tones. The N1 component reflects synchronous activation of large neuronal populations in the primary auditory cortex (3). Therefore, differences in neural entrainment to the amplitude-modulated tones were most likely not due to deficits of early auditory sensory processing in the patients with schizophrenia or schizoaffective disorder. In contrast to the patients with schizophrenia or schizoaffective disorder, the subjects with schizotypal personality disorder showed no deficits in synchronization at any frequency of stimulation. These findings suggest that steady-state synchronization deficits may only occur with the appearance of psychosis and may contribute to failures of perceptual and cognitive integration (1, 5). There are several limitations to this study that should be addressed in future research, including the role of medication, arousal, and genetic risk on neural synchronization.
Presented at the 57th meeting of the Society of Biological Psychiatry, Philadelphia, May 16–18, 2002, and the 41st meeting of the Society for Psychophysiological Research, Montreal, Quebec, Canada, Oct. 10–14, 2001. Received Nov. 16, 2002; revision received May 13, 2003; accepted May 15, 2003. From the Department of Psychology, Indiana University, Bloomington; and the Department of Psychiatry, Roudebush Veterans Administration Medical Center, Indianapolis, Ind. Address reprint requests to Dr. O’Donnell, Department of Psychology, Indiana University, 1101 East Tenth St., Bloomington, IN 47405; [email protected] (e-mail). Supported by NIMH grants MH-62150, MH-63112, and MH-17146.
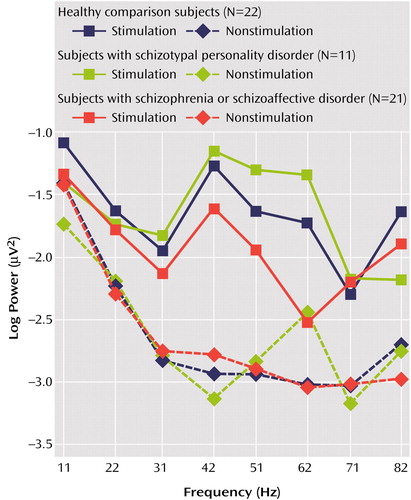
Figure 1. Log10-EEG Power at Electrode Site Fz During Stimulation and in the Absence of Stimulation for Each Frequency of Interest in Patients With Schizophrenia or Schizoaffective Disorder, Schizotypal Personality Disorder, and Healthy Comparison Subjects
1. Singer W: Neuronal synchrony: a versatile code for the definition of relations. Neuron 1999; 24:49–65Crossref, Medline, Google Scholar
2. Clementz BA, Blumenfeld LD, Cobb S: The gamma band response may account for poor p50 suppression in schizophrenia. Neuroreport 1997; 8:3889–3893Crossref, Medline, Google Scholar
3. Naatanen R, Winkler I: The concept of auditory stimulus representation in cognitive neuroscience. Psychol Bull 1999; 25:826–859Crossref, Google Scholar
4. Lins OG, Picton TW: Auditory steady-state responses to multiple simultaneous stimuli. Electroencephalogr Clin Neurophysiol 1995; 96:420–432Crossref, Medline, Google Scholar
5. Kwon JS, O’Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, Hasselmo ME, Potts GF, Shenton ME, McCarley RW: Gamma frequency range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry 1999; 56:1001–1005Crossref, Medline, Google Scholar
6. Siever LJ, Koenigsberg HW, Harvey P, Mitropoulou V, Laruelle M, Abi-Dargham A, Goodman M, Buchsbaum M: Cognitive band brain function in schizotypal personality disorder. Schizophr Res 2002; 54:157–167Crossref, Medline, Google Scholar



