Normal P50 Gating in Unmedicated Schizophrenia Outpatients
Abstract
OBJECTIVE: The hypothesis of a sensory gating defect in schizophrenia has been supported by studies demonstrating deficient auditory P50 gating in patients. P50 gating is the relative attenuation of P50 amplitude in the auditory evoked potential following the second auditory stimulus of a stimulus pair. METHOD: Auditory evoked potentials of 12 unmedicated male patients with schizophrenia and 24 healthy men were recorded during three runs of 40 click pairs. Three alternative waveform-processing strategies were used to analyze the data. RESULTS: Regardless of strategy used, the differences between subject groups regarding P50 amplitude and gating were nonsignificant. CONCLUSIONS: The P50 gating in the patient group was normal. The results do not support the concept of the P50 gating defect as a general trait marker of schizophrenia.
Deficiency in auditory P50 gating has supported the theory of sensory gating defects in patients with schizophrenia. In the setting of a larger, cross-modal investigation of sensory gating in schizophrenia, we recorded auditory P50 gating in a group of unmedicated schizophrenia patients and healthy comparison subjects.
Method
Patients with schizophrenia (N=12) were included if they were not currently receiving medication and had no history of substance abuse with the exception of tobacco. DSM-IV diagnoses (preceding 4 weeks and lifetime) were confirmed with the Schedules for Clinical Assessment in Neuropsychiatry (1). Healthy comparison subjects (N=24) were also assessed with the Schedules for Clinical Assessment in Neuropsychiatry interview; no family history of psychiatric disease was allowed. The Scale for the Assessment of Positive Symptoms and Scale for the Assessment of Negative Symptoms were used to derive the scores within the three symptom dimensions of schizophrenia (2). All subjects gave informed written consent as approved by the Ethics Committee, and they were paid to participate in the experiment.
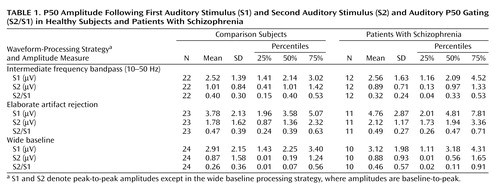
During recording, the subjects were seated comfortably upright with closed eyes in dim light and with background masking low level white noise. Subjects were allowed to smoke during predetermined study breaks. Among other tests, three runs of the auditory gating paradigm were recorded within 6 hours in a fixed order. The three recording runs of 40 paired stimuli, the paradigm, the equipment, set-up, and peak detection were similar to earlier recordings in our laboratory (3). Sweeps were rejected when the electro-oculogram recordings exceeded ±70 μV, baseline-corrected with the mean of samples –40 to 0, and filtered with a bandpass of 10–50 Hz (rolloff of 12 dB/octave) (4). Two alternative waveform-processing strategies were applied to the data, one that included a very elaborate range of artifact rejections (5, 6) and the other which used a wide baseline, causing detrending of the waveform (7). Mann-Whitney U tests were used to test for significance of difference by group, and Spearman correlations (significance level p<0.01) were used to determine the relationship between gating and subject variables.
Results
Twelve male schizophrenia patients (catatonic type [295.20]: N=1; paranoid type [295.30]: N=3; residual type [295.60]: N=1; undifferentiated type [295.90]: N=5; schizophreniform disorder, provisional [295.40]: N=1; and schizoaffective disorder, bipolar type [295.70]: N=1) and 24 healthy men were included. The mean age of the entire sample was 30.7 years; there was no difference between groups. However, the groups differed regarding the number of cigarettes smoked on the day of recording (healthy subjects: mean=1.3 [SD=2.7]; schizophrenia patients: mean=4.5 [SD=3.8]; Mann-Whitney test for independent samples: z=–2.74, p=0.01) but not regarding habitual smoking. Two of the patients were drug naïve, and the others had been without medication between 1 and 210 months (mean=54, SD=72). Illness duration ranged from 0.5 to 23 years (mean=9.5, SD=8.7), and cumulated total duration of former antipsychotic treatment ranged from 0 to 28 months (mean=8.8, SD=9.7). Mean symptom scores were 4.3 (SD=2.3) for positive symptoms, 6.8 (SD=4.5) for negative symptoms, 2.9 (SD=2.6) for disorganized symptoms, and 14.0 (SD=4.0) for total sum of scores.
The healthy comparison subjects had a gating ratio of 0.40 (SD=0.30), and the patients with schizophrenia had a gating ratio of 0.32 (SD=0.24). When the two alternative processing strategies were followed, the lack of between-group differences was also evident (Table 1).
No significant correlations were found between gating and age or consumption of cigarettes. Likewise, in the patients with schizophrenia, no correlations with gating were seen for the aforementioned illness parameters.
Discussion
The expected deficiency in P50 gating in the patient group was not observed. Most published studies have reported significantly increased gating ratios between 0.75–0.90 in schizophrenic patients (5–9). A negative finding has only been published once (10), and it was criticized for the stimulus being 10 msec long.
Smoking affects gating differentially in comparison subjects and patients (11). This was a major concern in the planning of the experiment, but no correlation between smoking and gating was seen across or within groups.
The long hours of continuous experimental recording have not been encountered before in studies of P50 gating. Arousal increment due to a painful stimulus or an embarrassing task decreases gating (4, 12, 13), and while for a short time in a neurophysiological laboratory, the patients could be more aroused than the comparison subjects. This difference is more likely to be evened out across several hours of recording.
Only a few studies have included patients with a drug-free period slightly resembling the long time seen in the present patient group (6, 7). The patients were disabled by their illness, but they were not severely disorganized, aggressive, or paranoid patients. However, a difference in symptoms does not seem to explain the normal gating, since stable unmedicated patients (6) and schizotypical personality disorder patients (14) have shown a gating impairment. Further studies are necessary to investigate if the gating defect serves as marker of a specific subgroup within the schizophrenia spectrum or if the state sensitivity of the P50 gating measure should lead to the prescription of highly standardized experimental procedures to sustain the findings of deficits in schizophrenia.
 |
Presented in part at the fifth World Congress of Biological Psychiatry, Copenhagen, June 13–16, 2002; and the Multidisciplinary Summer School on Schizophrenia, Kuopio, Finland, June 28–July 2, 2002. Received March 27, 2003; revision received July 3, 2003; accepted July 10, 2003. From the Department of Psychiatry, Bispebjerg Hospital, University Hospital of Copenhagen; and the Human Brain Mapping and Cortical Imaging Laboratory, Aalborg University, Denmark. Address reprint requests to Dr. Arnfred, Department of Psychiatry, Bispebjerg Hospital, University Hospital of Copenhagen, Bispebjerg Bakke 23, DK-2400 København NV, Denmark; [email protected] (e-mail).The study was supported by a Ph.D. grant to Dr. Arnfred from the Faculty of Health Sciences, University of Copenhagen, as well as by unrestricted grants from The Lundbeck Foundation, The Ivan Nielsen Foundation for Rare Psychiatric Disorders, The Schizophrenia Foundation of 1986, and the Danish Hospital Foundation for Medical Research–Region of Copenhagen, The Faeroe Islands, and Greenland.
1. Wing JK, Sartorius N, Üstün TB (eds): Diagnosis and Clinical Measurement in Psychiatry: A Reference Manual for SCAN/PSE-10. New York, Cambridge University Press, 1998Google Scholar
2. Andreasen NC, Arndt S, Alliger R, Miller D, Flaum M: Symptoms of schizophrenia: methods, meanings, and mechanisms. Arch Gen Psychiatry 1995; 52:341–351Crossref, Medline, Google Scholar
3. Arnfred SM, Eder DN, Hemmingsen RP, Glenthoj BY, Chen AC: Gating of the vertex somatosensory and auditory evoked potential P50 and the correlation to skin conductance orienting response in healthy men. Psychiatry Res 2001; 101:221–235Crossref, Medline, Google Scholar
4. White PM, Yee CM: Effects of attentional and stressor manipulations on the P50 gating response. Psychophysiology 1997; 34:703–711Crossref, Medline, Google Scholar
5. Nagamoto HT, Adler LE, Waldo MC, Freedman R: Sensory gating in schizophrenics and normal controls: effects of changing stimulation interval. Biol Psychiatry 1989; 25:549–561Crossref, Medline, Google Scholar
6. Myles-Worsley M: P50 sensory gating in multiplex schizophrenia families from a Pacific Island isolate. Am J Psychiatry 2002; 159:2007–2012Link, Google Scholar
7. Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R: Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiatry 1982; 17:639–654Medline, Google Scholar
8. Freedman R, Adler LE, Waldo MC, Pachtman E, Franks RD: Neurophysiological evidence for a defect in inhibitory pathways in schizophrenia: comparison of medicated and drug-free patients. Biol Psychiatry 1983; 18:537–551Medline, Google Scholar
9. Freedman R, Adler LE, Gerhardt GA, Waldo M, Baker N, Rose GM, Drebing C, Nagamoto H, Bickford-Wimer P, Franks R: Neurobiological studies of sensory gating in schizophrenia. Schizophr Bull 1987; 13:669–678Crossref, Medline, Google Scholar
10. Guterman Y, Josiassen RC: Sensory gating deviance in schizophrenia in the context of task related effects. Int J Psychophysiol 1994; 18:1–12Crossref, Medline, Google Scholar
11. Adler LE, Hoffer LD, Wiser A, Freedman R: Normalization of auditory physiology by cigarette smoking in schizophrenic patients. Am J Psychiatry 1993; 150:1856–1861Link, Google Scholar
12. Johnson MR, Adler LE: Transient impairment in P50 auditory sensory gating induced by a cold-pressor test. Biol Psychiatry 1993; 33:380–387Crossref, Medline, Google Scholar
13. Yee CM, White PM: Experimental modification of P50 suppression. Psychophysiology 2001; 38:531–539Crossref, Medline, Google Scholar
14. Cadenhead KS, Light GA, Geyer MA, Braff DL: Sensory gating deficits assessed by the P50 event-related potential in subjects with schizotypal personality disorder. Am J Psychiatry 2000; 157:55–59Link, Google Scholar



