Association of Risperidone Treatment Response With a Polymorphism in the 5-HT2A Receptor Gene
Abstract
OBJECTIVE: This study investigated the effect of the 102-T/C polymorphism in the 5-HT2A receptor gene on risperidone efficacy. METHOD: One hundred Han Chinese patients with acutely exacerbated schizophrenia were given risperidone for up to 42 days. The patients were genotyped for 5-HT2A polymorphisms. Psychopathology was measured biweekly with the Positive and Negative Syndrome Scale while the patients were taking risperidone. Generalized estimating equation methods were used to analyze the effects of treatment duration, T/C genotypes, and other prognostic factors on Positive and Negative Syndrome Scale performance. RESULTS: Patients with the C/C genotype had lower total scores, negative subscale scores, and general psychopathology scores but not positive subscale scores on the Positive and Negative Syndrome Scale than patients with the 102-T/C genotype. Patients with the T/C and T/T genotypes had comparable total and subscale scores. The number of previous hospitalizations and the dose of risperidone also affected Positive and Negative Syndrome Scale total scores. CONCLUSIONS: These results suggest that variations in the 5-HT2A receptor gene may influence individual responses to risperidone.
The human 5-HT2A receptor gene has been regarded as a candidate gene for susceptibility to schizophrenia (1), although there are inconsistent findings (2, 3). Studies regarding associations between this gene’s polymorphisms and response to clozapine (the prototype of atypical antipsychotics) also yielded conflicting results (3–5). Risperidone is a newer atypical agent with efficacy for both positive and negative symptoms of schizophrenia. Its affinity in vitro is 20 times higher for 5-HT2A receptors than for D2 receptors; in vivo it occupies 5-HT2A receptors at a rate 10 times lower than it does D2 receptors (6) Clozapine differs from risperidone in its relatively weak 5-HT2A antagonism, its very weak D2 antagonism, and its rather high affinities for several other receptors (6). A balance of full 5-HT2A receptor occupancy and partial dopamine D2 receptor occupancy probably underlies the beneficial therapeutic effect of risperidone, especially on negative symptoms (6). However, the contribution of 5-HT2 activity to risperidone efficacy is still debatable (7). The present study investigates the influence of the 102-T/C polymorphism in the 5-HT2A receptor gene on risperidone’s effectiveness.
Method
All newly hospitalized Han Chinese patients with acutely exacerbated schizophrenia entered this study if they satisfied inclusion criteria described elsewhere (8). After a complete description of the study to the subjects, written informed consent was obtained.
The subjects initially received a placebo for 1–7 days. Administration of risperidone was then titrated to the target dose of 6 mg/day (or lower, in the case of intolerance) within 1 week. From day 8 to day 42 of risperidone treatment, the dose remained the same as that used on day 7 or could be reduced on day 14 or day 28 to curtail side effects. This dosing strategy was based on our recent work (8). No other centrally acting drugs were permitted except lorazepam and benztropine.
Clinical assessments were conducted on days 0, 14, 28, and 42. The psychopathology instrument was the Positive and Negative Syndrome Scale (9), including the positive, negative, and general psychopathology subscales. Drug safety was evaluated by physical examinations, laboratory tests, the Extrapyramidal Symptom Rating Scale (10), and the Udvalg for Kliniske Undersøgelser (UKU) Side Effect Rating Scale (11). Patients were genotyped for 5-HT2A polymorphisms by polymerase chain reaction amplification of DNA and digestion with MspI for detection of 102-T/C (2).
Positive and Negative Syndrome Scale total and subscale scores were used as a measure of response to risperidone. Potential prognostic factors included T/C genotype as well as treatment duration, baseline Positive and Negative Syndrome Scale score, gender, age, education level, diagnosis subtype, age at illness onset, duration of illness, number and total duration of previous hospitalizations, and endpoint risperidone dose. Because there were repeated assessments, multiple linear regression with the generalized estimating equation method was used to adjust the within-subject dependence. Statistical significance was defined as p<0.05.
Results
One hundred patients completed the assessments on day 0 and day 14 (the first posttreatment visit) and were eligible for data analysis. The numbers of patients actively participating on days 28 and 42 were 95 and 81, respectively. The mean age of the 100 patients was 34.0 years (SD=9.7); 55 were men, and 45 were women. The mean age at illness onset was 24.4 years (SD=8.2), the mean illness duration was 114.8 months (SD=91.0), the mean number of previous hospitalizations was 1.9 (SD=2.5), the mean duration of previous hospitalizations was 23.7 weeks (SD=42.7), and the mean level of education was 10.7 years (SD=3.1). The distribution of schizophrenia subtypes was 66 paranoid, 15 disorganized, and 19 undifferentiated. The mean risperidone dose at endpoint was 4.2 mg/day (SD=1.4). The genotype distribution did not deviate significantly from the Hardy-Weinberg equilibrium: 102-T/T in 43 patients, C/C in 10, and T/C in 47. The allele frequency of 102-T was similar to that in the earlier study enrolling Han Chinese (2) but higher than that in Western populations (1, 3).
The mean Positive and Negative Syndrome Scale total scores declined during risperidone treatment: 87.4 (SD=14.1) at baseline and 70.2 (SD=15.0) at endpoint. The mean positive subscale scores declined from 23.2 (SD=4.1) to 17.1 (SD=5.0). The mean negative scores declined from 25.9 (SD=5.4) to 22.6 (SD=5.6), respectively, and the general psychopathology scores declined from 38.5 (SD=8.1) to 30.7 (SD=7.1). However, the distributions of the Positive and Negative Syndrome Scale total and subscale scores were skewed to the right (data not shown) and unsuitable for regression analyses. The value of each Positive and Negative Syndrome Scale total or subscale score was thus transformed to its natural logarithm to obtain normal distributions (data not shown).
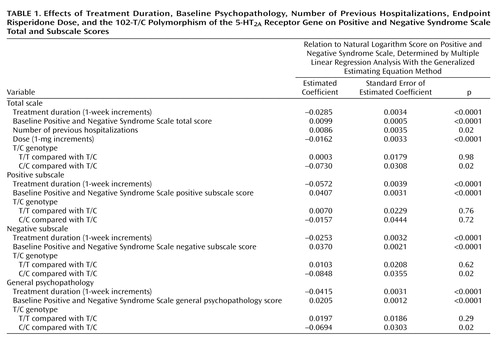
Table 1 shows that, after adjustment for the effects of treatment duration and patient-related variables, the 102-C/C genotype was related to better clinical performance. Compared with patients who had the T/C genotype, the Positive and Negative Syndrome Scale total scores of patients with the C/C genotype were 7.04% lower (e-0.0730=0.9296; 1–0.9296=0.0704), their negative subscale scores were 8.13% lower (e-0.0848=0.9187; 1–0.9187=0.0813), their general psychopathology scores were 6.70% lower (e-0.0694=0.9330; 1–0.9330=0.0670), but their positive subscale scores were not lower. Patients with the T/C and T/T genotypes did not differ significantly in any subscale score or the total score. In addition, each one-time increment in number of previous hospitalizations increased the Positive and Negative Syndrome Scale total score by 0.86% (e-0.0086=1.0086; 1.0086–1=0.0086), and each 1-mg increment in the daily dose of risperidone decreased the Positive and Negative Syndrome Scale total score by 1.61% (e-0.0162=0.9839; 1–0.9839=0.0161). Other potential prognostic factors did not significantly influence clinical performance.
Discussion
Our findings suggest that the 5-HT2A receptor 102-C/C genotype may predict superior risperidone response (particularly for negative symptoms rather than positive symptoms) in patients with acutely exacerbated schizophrenia. However, it has been shown that the 102-C/C genotype is more frequent among clozapine nonresponders than responders (3). Several reasons may contribute to this discrepancy. First, the subjects in the clozapine studies were treatment-resistant patients (3), but those in the present study were acutely ill patients. Second, clozapine is distinguished from risperidone in pharmacological profile (6). Third, subject ethnicity and study methodology differed between risperidone and clozapine studies.
The preliminary results in this study support the hypothesis that the 5-HT2A receptor 102-T/C polymorphism or, alternatively, another genetic variation that is in linkage disequilibrium (3) may influence individual response to risperidone. However, we might have had a chance false positive finding. Further studies in other ethnic populations or in chronically ill patients are warranted. In addition, possible effects of other 5-HT2A receptor polymorphisms such as 452-His/Tyr (3–5) on risperidone efficacy also require investigation.
 |
Received Oct. 31, 2001; revision received March 18, 2002; accepted March 21, 2002. From the Department of Psychiatry, Tzu-Chi General Hospital and the Institutes of Neuroscience and Medical Science, Tzu-Chi University; the Department of Psychiatry, China Medical College and Hospital, Taichung, Taiwan; the Department of Mathematics, Tamkang University, Taipei, Taiwan; and the Laboratory of Biological Psychiatry, Taipei City Psychiatric Center, Taiwan. Address reprint requests to Dr. Wen-Ho Chang, Department of Psychiatry, Tzu-Chi University, Number 701, Section 3, Chung-Yan Road, Hualien City, Taiwan 970; [email protected] (e-mail). Supported by National Science Council grants NSC 90-2314-B-320-003 and NSC 90-2314-B-320-004 and by National Health Research Institutes grant NHRI-EX-91-9134PI (Taiwan). The authors thank Shaw-Mei Chao, M.S., and Xing-Ru Lin, B.S., for technical support.
1. Williams J, Spurlock G, McGuffin P, Mallet J, Nothen MM, Gill M, Aschauer H, Nylander PO, Macciardi F, Owen MJ (European Multicentre Association Study of Schizophrenia [EMASS] Group): Association between schizophrenia and T102C polymorphism of the 5-hydroxytryptamine type 2a-receptor gene. Lancet 1996; 347:1294-1296Crossref, Medline, Google Scholar
2. Chen CH, Lee YR, Wei FC, Koong FJ, Hwu HG, Hsiao KJ: Lack of allelic association between 102T/C polymorphism of serotonin receptor type 2A gene and schizophrenia in Chinese. Psychiatr Genet 1997; 7:35-38Crossref, Medline, Google Scholar
3. Arranz MJ, Munro J, Sham P, Kirov G, Murray RM, Collier DA, Kerwin RW: Meta-analysis of studies on genetic variation in 5-HT2A receptors and clozapine response. Schizophr Res 1998; 32:93-99Crossref, Medline, Google Scholar
4. Malhotra AK, Goldman D, Ozaki N, Breier A, Buchanan R, Pickar D: Lack of association between polymorphisms in the 5-HT2A receptor gene and the antipsychotic response to clozapine. Am J Psychiatry 1996; 153:1092-1094Link, Google Scholar
5. Masellis M, Basile V, Meltzer HY, Lieberman JA, Sevy S, Macciardi FM, Cola P, Howard A, Badri F, Nothen MM, Kalow W, Kennedy JL: Serotonin subtype 2 receptor genes and clinical response to clozapine in schizophrenia patients. Neuropsychopharmacology 1998; 19:123-132Crossref, Medline, Google Scholar
6. Leysen JE, Janssen PM, Megens AA, Schotte A: Risperidone: a novel antipsychotic with balanced serotonin-dopamine antagonism, receptor occupancy profile, and pharmacologic activity. J Clin Psychiatry 1994; 55(May suppl):5-12Google Scholar
7. Kapur S, Seeman P: Does fast dissociation from the dopamine D2 receptor explain the action of atypical antipsychotics? a new hypothesis. Am J Psychiatry 2001; 158:360-369Link, Google Scholar
8. Lane HY, Chiu WC, Chou JCY, Wu ST, Su MH, Chang WH: Risperidone in acutely exacerbated schizophrenia: dosing strategies and plasma levels. J Clin Psychiatry 2000; 61:209-214Crossref, Medline, Google Scholar
9. Kay SR, Fiszbein A, Opler LA: The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 1987; 13:261-276Crossref, Medline, Google Scholar
10. Chouinard G, Ross-Chouinard A, Annable L, Jones B: Extrapyramidal Symptom Rating Scale (abstract). Can J Neurol Sci 1980; 7:233Google Scholar
11. Lingjaerde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K: The UKU Side Effect Rating Scale: a new comprehensive rating scale for psychotropic drugs and cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand Suppl 1987; 334:1-100Crossref, Medline, Google Scholar



