Obstetric Complications and Schizophrenia: Historical and Meta-Analytic Review
Abstract
OBJECTIVE: This paper reviews the literature on obstetric complications as a risk factor for schizophrenia. The authors trace the evolution of this literature through different methods and carry out a quantitative review of the results from prospective, population-based studies. METHOD: Relevant papers were identified by a MEDLINE search, by examination of reference lists of published papers, and through personal contact with researchers in the field. Studies were grouped in chronological order according to common themes or methods. Meta-analytic techniques were used to summarize the findings of prospective population-based studies. RESULTS: The meta-analytic synthesis of the prospective population-based studies revealed that three groups of complications were significantly associated with schizophrenia: 1) complications of pregnancy (bleeding, diabetes, rhesus incompatibility, preeclampsia); 2) abnormal fetal growth and development: (low birthweight, congenital malformations, reduced head circumference), and 3) complications of delivery (uterine atony, asphyxia, emergency Cesarean section). Pooled estimates of effect sizes were generally less than 2. CONCLUSIONS: Current methods of investigating the relationship between obstetric complications and schizophrenia are reaching the limit of their usefulness. Lack of statistical power to measure small and interactive effects and lack of detailed information about the prenatal period are major problems with current approaches. A combination of disciplines and approaches will be needed to elucidate the mechanisms underlying these small but important associations.
The much-investigated association between obstetric complications and schizophrenia has provided crucial support for developmental and nongenetic etiological models of the disorder. But does such an association really exist? This review outlines the history of the substantial literature examining this association and provides a quantitative synthesis of selected population-based studies. The limitations of the research methods that are currently used are discussed, and possible new lines of inquiry are suggested.
The first mention of an association between birth complications and schizophrenia occurred in the American Journal of Psychiatry in 1934. Rosanoff and colleagues (1) published “The Etiology of So-Called Schizophrenic Psychoses,” based on detailed case reports of 142 pairs of twins concordant and discordant for schizophrenia. The authors concluded that schizophrenia could be regarded (at least in part) as a “decerebration syndrome which may result from birth trauma.” Somewhat surprisingly, nothing further was published on this topic until 1956 when Pasamanick and colleagues (2) proposed their now-classic thesis of a “continuum of reproductive casualty,” whereby pregnancy and birth complications can lead to a gradient of injury extending from fetal and neonatal death through cerebral palsy, epilepsy, mental deficiency, and behavior disorder. The paper by Pasamanick and colleagues initially had its greatest impact on the field of child psychiatry. In the early 1960s, there were reports of significant associations between pregnancy complications (particularly toxemia, bleeding, and severe maternal illness) and childhood psychosis (3–6). However, diagnostic uncertainty about the classification of childhood psychosis seems to have halted research in this area. Even though a review in 1966 concluded that “the need for further research…is strongly indicated by these findings” (7), there was a gap of 10 years before a study by Torrey and colleagues (8) reported an association between bleeding in pregnancy and childhood psychosis.
Research on Low Birth Weight (1966–1970)
In 1966 attention shifted to adult schizophrenia when Lane and Albee (9) reported that the birth weights of 52 hospitalized schizophrenic adults were significantly lower than that of their siblings. Although the difference between the mean birth weights of the patients and their siblings was significant, it was quite small (in the region of 175 g), and few of the patients actually met the criteria for the “low birth weight” category (<2500 g). Rather, there appeared to be a “shift in distribution” of birth weight within a population of cases compared with noncases. In 1967 Stabenau and Pollin (10) published an analysis of birth histories of 100 pairs of monozygotic twins discordant for schizophrenia and reported that significantly more of the schizophrenic (index) twins had been the lighter of the two at birth and had experienced birth complications, particularly asphyxia. However, other investigators subsequently failed to find significant differences in birth weight between schizophrenic subjects and siblings or comparison subjects (11–13). It is likely that these studies were underpowered to find an effect, as the mean difference in birth weights between groups was actually very similar to that originally reported by Lane and Albee (9). The epidemiological concept of a “population shift” did not come to attention again until recent years (14).
Studies of High-Risk Groups (1970–1980)
The next phase of the literature was prompted by an analysis of obstetric complication data from the Copenhagen High-Risk Study (15, 16). The “high-risk” design examined the characteristics of a group of offspring of schizophrenic parents who were at 10–15 times higher risk of developing schizophrenia than individuals from the general population. Mednick (16) found that 70% of the “high-risk” children who were psychiatrically ill by their early 20s had suffered one or more serious pregnancy or birth complications, compared with 15% of the high-risk group who remained well and 33% of the comparison group (i.e., offspring of parents who did not suffer with schizophrenia). Mednick speculated that, given a subject’s genetic predisposition, schizophrenia would appear only if the hippocampus was selectively injured by anoxia at birth. Analyses of other high-risk study groups revealed an excess of unexplained fetal and neonatal deaths (17–19), bleeding and swelling during pregnancy (20), and neonatal problems (21). However the research strategy focusing on high-risk children was dealt a severe blow by a series a negative findings (22–25) and, in particular, by two reports that failed to find any differences between the birth histories of schizophrenic women and those of women with other psychiatric disorders (26, 27). A review concluded that there was little evidence for an excess of obstetric complications in births to parents with schizophrenia (28). Although this conclusion was later challenged (29), the era of research focused on high-risk offspring was effectively over by 1980. Indeed, only a handful of papers on the topic of obstetric complications and schizophrenia were published over the next few years (30–32), and interest in the topic seemed to have waned. The development of new brain imaging techniques provided the impetus for the next phase of the literature.
Brain Imaging Studies (1984–1987)
The finding of cerebral ventricular enlargement in schizophrenia was originally thought to reflect neurodegeneration (33). However, investigators found that ventricular enlargement was already present at the onset of the illness (34) and was positively correlated with a history of obstetric complications in groups of high-risk and schizophrenic subjects (35, 36). Murray and colleagues (37) suggested that intraventricular hemorrhage in the newborn infant might be one cause of the ventricular enlargement found in schizophrenia and proposed a distinction between “familial” and “sporadic” forms of the disorder. Andreasen and colleagues (38) found decreased cerebral and cranial size in schizophrenia and suggested that “some type of early developmental abnormality, such as complications of delivery” may be responsible for the findings. A “neurodevelopmental hypothesis” of schizophrenia began to take shape (39–41). This hypothesis or model proposed that schizophrenia was associated with a subtle, static brain lesion that was caused by a combination of genetic or environmental factors and that interacted with the normal maturational processes of the brain. Evidence for an association between obstetric complications and schizophrenia provided important support for the neurodevelopmental hypothesis.
Case-Control Studies (1987–1997)
In 1987, Lewis and Murray (42) published a case-control study reporting that patients with schizophrenia were more likely to have a history of obstetric complications recorded in their case notes than patients with other psychiatric disorders. In another paper (43), the authors divided the overall patient group on the basis of family history, age at onset of illness, premorbid personality, and cerebral ventricular size and compared rates of obstetric complications between the subgroups. The quest for “subgroups” and the search for correlates of obstetric complications were themes that would recur many times during the years that followed. Another consequence of this study was the introduction of the so-called “Lewis-Murray” scale for rating retrospective information on obstetric complications (43). The Lewis-Murray scale could be used for rating information on obstetric complications from case notes, birth records, and maternal interviews. It was derived from a consensus of six previous scales and consisted of 15 complications with thresholds for rating some as “definite” or “equivocal.”
The combination of three factors—1) the theoretical framework provided by the “neurodevelopmental hypothesis,” 2) the possibility of using maternal recall and case notes as sources of information about obstetric complications, and 3) the availability of an easy-to-use rating scale—gave rise to a veritable flood of case-control studies investigating the association between obstetric complications and schizophrenia during the early and mid-1990s. Due to limitations of methods, however, this enormous research effort served to confuse rather than elucidate the association. Some studies found a significant overall effect for obstetric complications (44–48), others did not (49, 50). Some studies had no normal comparison group (51–53), while others included a sibling comparison group (44, 47, 49, 50, 54). Different variations of the Lewis-Murray scale were used, but the results were still presented as total scores. Although some studies clearly attempted to use population-based methods (28, 44, 45, 48), the final samples were selected to varying degrees and were prone to bias. Many studies relied solely on maternal recall as the source of information about the exposure (46, 49, 52). Subgroup analyses were common but yielded inconsistent results. Obstetric complications were examined in relation to family history (49, 51, 55–57), premorbid adjustment (51), imaging abnormalities (53, 58), age at onset of illness (45, 55, 59, 60), gender (45, 56, 57, 59), neurological abnormalities (49, 55), ethnicity (61), and season of birth (56), among others. The search for environmental risk factors for schizophrenia had become an example of “circular epidemiology,” namely “the tendency to perseverate at one level of evidence, for example, on one type of study design without moving forward” (62).
In 1995, Geddes and Lawrie (63) carried out a meta-analysis of published results from 16 case-control studies and two cohort studies. They concluded that there was a modest relationship between the broad category of obstetric complications and later schizophrenia, with a pooled odds ratio of 2.0 (95% confidence interval [CI]=1.6–2.4). However, they pointed out two important caveats: 1) there was evidence for selection and publication bias in the literature and 2) there was significant heterogeneity between studies with case-control and cohort designs. To examine specific complications, Geddes and colleagues (64) obtained raw data from the investigators of 12 case-control studies that had used the Lewis-Murray scale and carried out a meta-analysis using the data from the individual patients. This analysis, based on data from 700 schizophrenic subjects and 835 comparison subjects, found that the following obstetric complications were significantly associated with schizophrenia: premature rupture of membranes, prematurity, use of resuscitation or incubator, birthweight <2500 g, preeclampsia (birth record data only), and forceps delivery (maternal recall only). However, the problems with selection and information bias among the component studies threatened the validity of the meta-analysis. More robust, population-based methods were needed.
Population-Based Studies (1997–present)
This phase of the literature on obstetric complications and schizophrenia began in earnest in 1997 and continues to date. Studies are characterized by large samples drawn from population-based hospital or case registers, with comparison subjects drawn from the same population, and use of standardized, prospective obstetric data from birth records or registers. Investigators usually report odds ratios for individual obstetric complications and control for demographic confounders by matching or statistical adjustment.
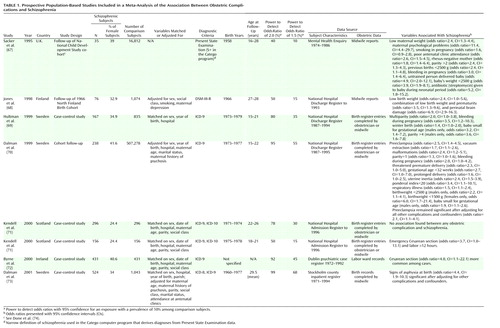
It was hoped (indeed expected!) that these large, methodologically robust studies would provide clear, consistent answers about the relationship between individual obstetric complications and schizophrenia, but this has not proved to be the case. As Table 1 shows, the findings from the population-based studies were mostly negative and surprisingly contradictory. Rather than dealing with each of these studies separately, we have elected to carry out a meta-analytic synthesis of the results. Possible reasons for the discrepancies between studies will be discussed after presentation of the findings from the meta-analysis. The standardized fashion of reporting results and the similarities in the methods of the population-based studies lend themselves to a meta-analytic approach. Meta-analysis provides a method for integrating quantitative data from multiple studies by using a weighted average of the results in which larger studies have more influence than smaller studies. It improves the estimates of effect size, increases the statistical power, and helps to make sense out of studies with conflicting conclusions (65, 66).
The limitations of using a meta-analytic approach for observational studies should be mentioned. Observational studies may yield estimates of association that are influenced to a greater or lesser degree by confounding or bias, and meta-analysis in itself is no defense against such factors (“bias in equals bias out”). The population-based studies in this analysis were relatively free from bias, and the odds ratios were adjusted for confounders such as sex, hospital where the birth took place, and social class. However the samples were drawn from different populations, in terms of geography, age at illness onset, and cohort and period effects, and all these differences could provide sources of confounding factors. Obstetric practices and methods for recording information about different complications vary between countries and over time.
Meta-Analytic Review of Population-Based Studies
Method
Inclusion criteria
Studies were included in the meta-analysis if they fulfilled the following a priori set of criteria: 1) inclusion of a well-defined sample of cases drawn from population-based registers or cohorts, 2) use of standardized, prospectively collected obstetric information from birth records or registers, 3) inclusion of comparison subjects drawn from the general population with information on obstetric complications collected from the same source, and 4) use of a standardized format for presentation of data on individual obstetric complications, allowing for comparisons between studies. The search strategies used were 1) computerized MEDLINE search, 2) cross-referencing of original studies, and 3) contact with other researchers in the field. We identified eight studies that fulfilled all four criteria (67–73). Two of these studies were reported within one scientific paper (71). The characteristics of the eight studies and a summary of their findings are presented in Table 1. We identified a further five studies that fulfilled the first three criteria but not the fourth—the obstetric data were presented in aggregate form and could not be used in the meta-analysis (74–78). These five studies will be mentioned in the discussion of the results of the meta-analysis where appropriate.
Statistical analysis
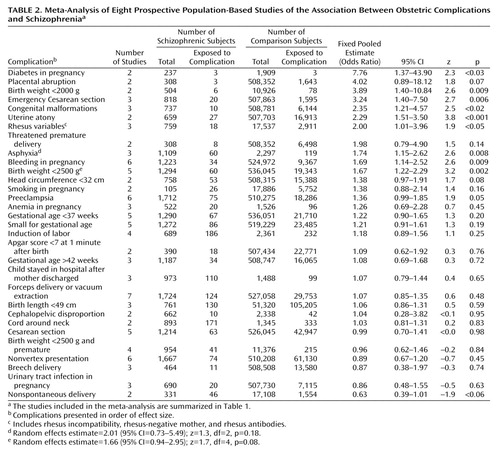
Individual obstetric complications were included in the analysis if more than two studies reported on that complication in a comparable way. Two of the studies (69, 70) report on the same data set, although with different sampling frames. Therefore, when both studies report on the same obstetric complication, data from only the larger study (70) were used. A funnel plot showed no evidence for publication bias. Adjusted odds ratios for the associations between individual obstetric complications and later schizophrenia with 95% CIs were extracted from each paper and used as the measures of effect size and the variance of the effect size, respectively. A pooled estimate was calculated for each association by using Woolf’s method, with 95% CIs and a test that the true pooled effect was zero (65). The analysis used a fixed-effects model, which assumes that variability between studies is due solely to random variation (66). A homogeneity statistic Q was calculated for each association (79). A significant value for Q indicates that there is heterogeneity between the studies for that complication. Where significant heterogeneity was detected, we calculated a random-effects estimate for the association. The random-effects model assumes a different underlying effect for each study and leads to wider CIs than the fixed-effects model (79). However since the maximum number of contributory studies never exceeded six, there was insufficient power to formally investigate potential sources of heterogeneity (such as age at onset, gender, or period effects) in this analysis. All analyses were performed with STATA 6.0 (Stata Corp., College Station, Tex.)
Results
Results of the meta-analysis for the individual complications are presented in Table 2. Significant differences between schizophrenic subjects and comparison subjects were found for the following variables in order of effect size: diabetes in pregnancy, birth weight <2000 g, emergency Cesarean section, congenital malformations, uterine atony, rhesus variables (comprising rhesus incompatibility, rhesus-negative mother, rhesus antibodies), asphyxia, bleeding in pregnancy, birth weight <2500 g, and preeclampsia. Significant heterogeneity was detected for two of these complications: asphyxia (Q=10.41, df=2, p=0.005) and birthweight <2500 g (Q=12.56, df=4, p<0.02). The random-effects estimate for asphyxia was 2.01 (95% CI=0.73–5.49; z=1.3, df=2, p=0.18) and for birth weight <2500 g was 1.66 (95% CI=0.94–2.95; z=1.7, df=4, p=0.08). The following three complications just missed formal statistical significance: placental abruption, head circumference <32 cm, and a negative association with nonspontaneous delivery.
Discussion
The significant estimates from the meta-analysis appear to group into three main categories: 1) complications of pregnancy (bleeding, preeclampsia, diabetes, and rhesus incompatibility), 2) abnormal fetal growth and development (low birth weight, congenital malformations, and small head circumference), and 3) complications of delivery (asphyxia, uterine atony, and emergency Cesarean section). The results will be discussed in these categories within the context of relevant findings from other studies that may elucidate these results.
Complications of pregnancy
Bleeding and preeclampsia in pregnancy have been associated with psychosis since the earliest days of such research. Pasamanick and colleagues (2) singled out the “anoxia-producing complications of pregnancy such as toxemia and bleeding” as most likely to be associated with behavior problems.
Preeclampsia came to particular attention in 1996 when Kendell and colleagues (80) reported a very strong association between preeclampsia and later schizophrenia, but, in their attempt to extend the study group and replicate this finding, a flaw in the original study design was uncovered, and a retraction was published (71). The revised analysis found no significant effect for preeclampsia. However, in the largest single population-based study to date, preeclampsia was the only obstetric risk factor that remained significant after the analysis controlled for all potentially confounding factors (70). What could be the mechanism of action of this association? The most popular theory at present involves the mechanism of abnormal fetal blood flow resulting in chronic fetal hypoxia or malnutrition (75).
Bleeding during pregnancy has many causes. Implantation bleeding and abbreviated menses are common in the first month, and placenta praevia and premature separation of the placenta are frequent causes in the last month. The causes of mid-pregnancy bleeding are less well understood. In one study, two-thirds of the cases were found to be due to premature separation, placenta praevia, hydatiform moles, incompetent cervices, and other identifiable causes, while in one-third of the cases, the cause could not be determined (81). In severe cases of bleeding, the pathogenic effect on the fetus is thought to be anoxia, but, in many cases, the amount of bleeding may be slight (8, 20), and anoxic brain damage is unlikely. Another explanation is that bleeding can represent a threatened spontaneous abortion. This is consistent with the striking and as yet unexplained findings of an excess of stillbirths and neonatal deaths among schizophrenic women (17–19). Rieder and colleagues (20) hypothesized that bleeding was “the result of rather than the cause of injury. In other words the process of uterine rejection could have begun but been interrupted.” Genetic or autoimmune factors may play a part in such a process.
The association between diabetes in pregnancy and later schizophrenia, although strong, is based on only two studies in this analysis, neither of which provided information on the type of diabetes. Indirect support for the association comes from one report that high prepregnancy body mass increases the risk of schizophrenia in the offspring (82), since high maternal body mass index is associated with non-insulin-dependent diabetes and gestational diabetes. The effects on the developing brain of altered glucose metabolism are not well understood (83). Poorly controlled maternal diabetes is associated with an increased risk of congenital anomalies and impaired intellectual and psychomotor development in offspring (reviewed in reference 82). Insulin-dependent diabetes mellitus has been found to be more common among the first-degree relatives of patients with schizophrenia than among comparison subjects (84, 85), indicating that an autoimmune process might be involved. Autoimmune mechanisms could also be implicated in the association between rhesus incompatibility and later schizophrenia. Rhesus hemolytic disease of the newborn is an illness with neurological consequences secondary to effects of a maternal antibody (86, 87). Hemolytic disease can lead to early spontaneous abortion, chronic fetal hypoxia, neonatal asphyxia and pulmonary edema, and neonatal hyperbilirubinemia and kernicterus (86). The association has independent support from a cohort study that found a twofold increase in relative risk of schizophrenia among men from rhesus-incompatible pregnancies (88) and a report that neonatal hyperbilirubinemia is a risk factor for later mental illness (89).
Abnormal fetal growth and development
Low birth weight has been associated with schizophrenia throughout each era of investigation (1, 9, 21, 29, 43, 52), but this association is not invariably found (71–73, 90), and there was significant heterogeneity in the estimate for low birth weight (<2500 g) in this meta-analysis. The concept of a “population shift” in birth weight may account for this heterogeneity, and investigators using an arbitrary cutoff for low birth weight (i.e., <2500 g) will find inconsistent associations depending on the power of the study. Using a quantitative approach, Wahlbeck et al. (91) found that the risk of schizophrenia decreases in a linear fashion with increasing birth weight, increasing length at birth, and increasing placental weight. Low birth weight is usually due to prematurity or intrauterine growth retardation. The lack of a significant association between risk of schizophrenia and prematurity in our meta-analysis indicates that the low birth weight is most likely due to intrauterine growth retardation (although there was no association between risk of schizophrenia and being small for gestational age). However, almost any factor adversely affecting the fetus will retard its growth, so these findings do not appreciably narrow our search. Low birth weight may be a proxy variable for some other adverse influence or influences on the developing fetus, whether of genetic or environmental origin. Women with schizophrenia or who later develop schizophrenia have been shown to be at increased risk of behaviors during pregnancy—such as smoking, taking medication, or poor attendance at antenatal clinics—that are associated with low birth weight outcomes (21, 29, 92). The increased prevalence of congenital malformations found in this meta-analysis echoes the extensive literature on minor physical abnormalities and schizophrenia (93, 94) and further implicates pregnancy as a time when potential etiological factors may be operating.
Complications of delivery
The common mechanism for the delivery complications of asphyxia, uterine atony, and emergency Cesarean section found in our meta-analysis appears to be fetal hypoxia or anoxia. This is by no means a new idea, as anoxia has been mentioned since the earliest days of this literature (2). Geddes and colleagues (64) concluded that the underlying mechanism for all the complications associated with schizophrenia in their meta-analysis was likely to involve fetal hypoxia but that a “more specific measure of exposure to hypoxia” was required. The pooled estimate for asphyxia in our meta-analysis demonstrated significant heterogeneity, possibly due to the different definitions of asphyxia used. Three population-based studies that could not be included in the meta-analysis but that fulfilled three of the four inclusion criteria (76–78) provide additional support for the involvement of fetal hypoxia or asphyxia in the etiology of schizophrenia . Individuals from the National Collaborative Perinatal Project with three or more hypoxia-related obstetric complications were more than five times more likely to develop schizophrenia than individuals with no hypoxia-related obstetric complications (76), and this finding was particularly marked among patients with early-onset schizophrenia (77). Siblings of schizophrenic subjects were no more likely to experience these complications than were comparison subjects (76, 77). Cannon and colleagues (76, 77) suggest that these results are consistent with a model of schizophrenia involving interaction of genetic vulnerability and obstetric complications (95, 96) and that the neurotoxic effect of fetal hypoxia leads to an early onset of schizophrenia through premature cortical synaptic pruning (77). Putative hypoxia-related complications have been related to brain structural abnormalities among schizophrenic patients (95, 97).
The major difficulty with proposing hypoxic-ischemic damage as a causal risk factor for schizophrenia is the establishment of independence—it may be related to pregnancy complications, preexisting problems with the fetus (98, 99), or maternal behaviors (29, 92). Although some initial investigations have not found empirical support for this notion (100), few studies have had sufficient power to examine the interrelationships between various obstetric complications in the same individuals.
Why Is It So Difficult to Study Obstetric Complications and Schizophrenia?
Statistical Power
The effect sizes for the relationship between obstetric risk factors and later schizophrenia are generally small, with odds ratios of less than 2 (Table 1 and Table 2). This is the sort of effect size reported for the relationship between passive smoking and lung cancer (101) or the risk of breast cancer among users of the oral contraceptive pill (102). Such small effects are usually controversial (103) and are some way from indicating strong causality. One may be dealing with proxy effects for some other lifestyle or socioeconomic factors or interactive effects with as-yet-unknown genetic or epigenetic factors. The study of individual obstetric risk factors for schizophrenia could be conceptualized as the search for rare risk factors for a rare disease and is therefore truly suitable neither for the classic cohort design nor for case-control designs. The power of even the largest population-based studies to detect odds ratios of 1.5 was less than 70% (Table 1). Methods such as the “nested” case-control design (69, 73) are useful, but, even so, the lack of independence of individual obstetric complications (discussed earlier) and possible interactive effects further erode statistical power. Some studies have tried to overcome low statistical power by examining only one exposure on the basis of a prior hypothesis. This has proved a relatively fruitful endeavor, showing significant effects for the putative risk-increasing mechanism of hypoxic and ischemic damage (73, 75–78), but each set of researchers has used different combinations of exposures, hindering replication and comparison or pooling of the results between studies.
Definition of Obstetric Complications
No one realized initially just how common the broadly defined Lewis-Murray obstetric complications are in the general population—about 25%–30% of births involve at least one Lewis-Murray complication (see references 68 and 104). So many discrete exposures are wrapped up in the term “obstetric complication” that it is essentially meaningless to consider them as one risk factor (105). Before beginning to judge whether any association should be interpreted as causal, researchers must consider many distinct associations in terms of chance, bias, or confounding. If the field is to progress, advances are needed not only in population-based methods but also in sharpening the definitions of the exposures under scrutiny. A broad definition of obstetric complications should no longer be used as it will not improve our understanding of the field any further. More careful definition of exposures, such as prenatal measurement of maternal antibodies, and more extensive use of quantitative measures, including birth weight or head circumference, are likely to show larger and more consistent effects.
Information on the Prenatal Period
Birth records tend to include detailed information about the delivery and neonatal period, but information about the prenatal period is less reliable (106). Details about the course of the pregnancy are often recorded at the time of admission to the labor ward. Major pregnancy complications, such as preeclampsia, are usually mentioned, but there may be no record of problems such as prenatal stress or there may be insufficient detail about timing of infection or bleeding. Nevertheless, the current meta-analysis shows that many prenatal factors (even with the less-than-optimal data available) are significantly associated with later schizophrenia. Even stronger effects may have been found if detailed, prospective data on the prenatal period were available. Ecological data, cunningly exploited, have provided clues of the existence of other prenatal risk factors for schizophrenia, including prenatal infection (for a review, see references 14, 107, 108), prenatal malnutrition (109), and prenatal stress (110, 111). Such exposures should ideally be incorporated into future studies of obstetric complications and schizophrenia. Another approach would be to follow up a cohort of individuals who have suffered definite specific prenatal (or perinatal) complications and assess a range of outcomes during development—a return to the position advocated by Pasamanick and colleagues many decades ago (2). This approach has already been useful in elucidating some rare prenatal exposures such as rubella infection (112) and rhesus incompatibility (88) and is currently being applied to follow-up studies of subjects with low birth weight and premature birth (113). This approach could also address the issue of specificity of obstetric risk factors for schizophrenia.
Interactive or Subgroup Effects
There has long been interest in whether the effect of obstetric complications may be confined to a subgroup of patients with schizophrenia, such as those with a family history, or male sex, or early onset, but most studies have not had sufficient power to examine this issue reliably. Verdoux and colleagues (114), in an individual-patient data meta-analysis, found a relationship between age at onset of schizophrenia and obstetric complications—the earlier the age at onset of schizophrenia, the more likely a history of obstetric complications—but found no relationship with family history of schizophrenia or gender. Cannon and colleagues (76, 77) also found a relationship between asphyxia and schizophrenia among patients with early-onset schizophrenia. However, in general, the recent population-based studies have tended not to examine subgroups within the population of patients with schizophrenia.
The study of gene-environment interactions is beginning to be applied to schizophrenia (115). There is increasing awareness that “hidden” genetic factors can have a substantial influence on the effect of environmental exposures, such as obstetric complications. An exposure that has a small effect on schizophrenia in general may have a large effect in those with a specific genetic make-up. In the coming decade we may see the first reports of studies that examine precisely measured genetic and environmental causes of schizophrenia in the same population (116). However, to adequately model interactive effects involving rare environmental risk factors (such as individual obstetric complications) and genes of small effect, sample sizes of tens of thousands will be required (14). We also have to take into account the dynamic interplay between genes and environment in utero (117).
Need for Collaborative Approaches
Current methods for studying the relationship between obstetric complications and schizophrenia merely allow us to report associations and, as a result, we have become stuck at the point of reporting risk factors of “vanishingly small effect” over and over again (62, 118). Research in this area will need to move beyond the domain of epidemiology and involve other disciplines, such as developmental biology, neuropathology, and genetics (119, 120). Innovative approaches include the use of animal models to study effects of prenatal infection (121, 122) and the analysis of large cohorts with detailed prenatal data and stored prenatal serum (116, 123). As we enter what some researchers have called a new age of epidemiology for schizophrenia (116, 124), the combination of larger cohorts, new paradigms, and modern statistical and molecular techniques provides the opportunity to discover how obstetric complications contribute to the causation of schizophrenia.
 |
 |
Received Jan. 19, 2001; revision received Oct. 1, 2001; accepted Oct. 10, 2001. From the Division of Psychological Medicine, Institute of Psychiatry; and the Department of Psychiatry, University of Cambridge, Cambridge, U.K. Address reprint requests to Dr. Cannon, Division of Psychological Medicine, P063, Institute of Psychiatry, De Crespigny Park, London SE5 8AF, UK; [email protected] (e-mail). Supported by a Wellcome Trust Advanced Research Fellowship to Dr. Cannon, the EJLB Foundation, and by the Theodore and Vada Stanley Foundation. The authors thank Dr. Christina Dalman, Dr. Hollie Thomas, Dr. Paul Fearon, Prof. Glyn Lewis, and Prof. Robert Kendell for their help in preparing the paper.
1. Rosanoff AJ, Handy LM, Plesset IR, Brush S: The etiology of so-called schizophrenic psychoses: with special reference to their occurrence in twins. Am J Psychiatry 1934; 91:247-286Link, Google Scholar
2. Pasamanick B, Rogers ME, Lilienfield AM: Pregnancy experience and the development of behavior disorder in children. Am J Psychiatry 1956; 112:613-618Link, Google Scholar
3. Hinton GG: Childhood psychosis or mental retardation: a diagnostic dilemma, II: paediatric and neurologic aspects. Can Med Assoc J 1963; 89:1020-1024Medline, Google Scholar
4. Taft L, Goldfarb W: Prenatal and perinatal factors in childhood schizophrenia. Dev Med Child Neurol 1964; 6:32-43Medline, Google Scholar
5. Terris M, Lapouse R, Monk MA: The relation of prematurity and previous fetal loss to childhood schizophrenia. Am J Psychiatry 1964; 121:476-481Link, Google Scholar
6. Voster D: An investigation of the part played by organic factors in childhood schizophrenia. J Ment Sci 1960; 106:494-522Crossref, Google Scholar
7. Pollack M, Woerner MG: Pre- and perinatal complications and “childhood schizophrenia”: a comparison of five controlled studies. J Child Psychol Psychiatry 1966, 7:235-242Google Scholar
8. Torrey EF, Hersch SP, McCabe KD: Early childhood psychosis and bleeding during pregnancy. J Autism Child Schizophr 1975; 5:287-297Crossref, Medline, Google Scholar
9. Lane EA, Albee GW: Comparative birth weights of schizophrenics and their siblings. J Psychol 1966; 64:227-231Crossref, Google Scholar
10. Stabenau JR, Pollin W: Early characteristics of monozygotic twins discordant for schizophrenia. Arch Gen Psychiatry 1967; 17:723-734Crossref, Medline, Google Scholar
11. Pollack M, Woerner MG, Goodman W, Greenberg I: Childhood developmental patterns of hospitalized adult schizophrenic patients and non-schizophrenic patients and their siblings. Am J Orthopsychiatry 1966; 36:510-517Crossref, Medline, Google Scholar
12. Woerner MG, Pollack M, Klein DF: Birth weight and length in schizophrenics, personality disorders and their siblings. Br J Psychiatry 1971; 118:461-464Crossref, Medline, Google Scholar
13. Woerner MG, Pollack M, Klein DF: Pregnancy and birth complications in psychiatric patients: a comparison of schizophrenic and personality disorder patients with their siblings. Acta Psychiatr Scand 1971; 49:712-721Crossref, Google Scholar
14. Jones PB: Longitudinal approaches to the search for the causes of schizophrenia: past, present and future, in Search for the Causes of Schizophrenia, vol IV: Balance of the Century. Edited by Gattaz WF, Hafner H. New York, Springer-Verlag, 1999, pp 91-119Google Scholar
15. Mednick SA, Schulsinger F: Some premorbid characteristics related to breakdown in children with schizophrenic mothers. J Psychiatr Res 1968; 6(suppl):267Google Scholar
16. Mednick SA: Breakdown in individuals at high risk for schizophrenia: possible predispositional perinatal factors. Ment Hyg 1970; 54:51-63Google Scholar
17. Sobel D: Infant malformations and mortality in children of schizophrenic parents. Psychiatr Q 1961; 35:60-64Crossref, Google Scholar
18. Rieder RO, Rosenthal D, Wender P, Blumenthal H: The offspring of schizophrenics, I: fetal and neonatal deaths. Arch Gen Psychiatry 1975; 32:200-211Crossref, Medline, Google Scholar
19. Modrzewska K: The offspring of schizophrenic parents in a North Swedish isolate. Clin Genet 1980; 17:191-201Crossref, Medline, Google Scholar
20. Rieder RO, Broman SH, Rosenthal D: The offspring of schizophrenics, II: perinatal factors and IQ. Arch Gen Psychiatry 1977; 34:789-799Crossref, Medline, Google Scholar
21. Wrede G, Mednick SA, Huttunen MO, Nilsson CG: Pregnancy and delivery complications in the births of an unselected series of Finnish children with schizophrenic mothers. Acta Psychiatr Scand 1980; 62:369-381Crossref, Medline, Google Scholar
22. McNeil TF, Kaij L: Obstetric complications and physical size of offspring of schizophrenic, schizophrenic-like and control mothers. Br J Psychiatry 1973; 123:341-381Crossref, Medline, Google Scholar
23. Mirdal GKM, Mednick SA, Schulsinger F, Fuchs F: Perinatal complications in children of schizophrenic mothers. Acta Psychiatr Scand 1974; 50:553-568Crossref, Medline, Google Scholar
24. Cohler BJ, Gallant DH, Grunebaum HU, Weiss JL, Gamer E: Pregnancy and birth complications among mentally ill and well mothers and their children. Soc Biol 1975; 22:269-278Crossref, Medline, Google Scholar
25. Hanson DR, Gottesman II, Heston LC: Some possible indicators of adult schizophrenia inferred from children of schizophrenics. Br J Psychiatry 1976; 129:142-154Crossref, Medline, Google Scholar
26. Sameroff AJ, Zax M: Perinatal characteristics of the offspring of schizophrenic women. J Nerv Ment Dis 1973; 157:191-199Crossref, Medline, Google Scholar
27. Zax M, Sameroff AJ, Babigian HM: Birth outcomes in the offspring of mentally disordered women. Am J Orthopsychiatry 1977; 47:218-230Crossref, Medline, Google Scholar
28. McNeil TF, Kaij L: Obstetric factors in the development of schizophrenia: complications in the births of preschizophrenics and in reproduction by schizophrenic parents, in The Nature of Schizophrenia: New Approaches to Research and Treatment. Edited by Wynne LC, Cromwell RL, Mathysse S. New York, John Wiley & Sons, 1978, pp 401-429Google Scholar
29. Sacker A, Done DJ, Crow TJ: Obstetric complications in children born to parents with schizophrenia: a meta-analysis of case-control studies. Psychol Med 1996; 26:279-287Crossref, Medline, Google Scholar
30. Jacobsen B, Kinney DK: Perinatal complications in adopted and non-adopted schizophrenics and their controls: preliminary results. Acta Psychiatr Scand Suppl 1980; 285:337-346Crossref, Google Scholar
31. Parnas J, Schulsinger F, Teasdale TW, Schulsinger H, Feldman PM, Mednick SA: Perinatal complications and clinical outcome within the schizophrenia spectrum. Br J Psychiatry 1982; 140:416-420Crossref, Medline, Google Scholar
32. DeLisi LE, Goldin LR, Maxwell E, Karuba DM, Gershon ES: Clinical features of illness in siblings with schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 1987; 44:891-896Crossref, Medline, Google Scholar
33. Johnstone EC, Crow TJ, Frith CD, Husband J, Kreel L: Cerebral ventricular size and cognitive impairment in chronic schizophrenia. Lancet 1976; 2:924-926Crossref, Medline, Google Scholar
34. Weinberger DR, DeLisi LE, Perman G, Targum S, Wyatt RJ: Computed tomography scans in schizophreniform disorder and other acute psychiatric disorders. Arch Gen Psychiatry 1982; 39:778-783Crossref, Medline, Google Scholar
35. Schulsinger F, Parnas J, Petersen ET, Schulsinger H, Teasdale TW, Mednick SA, Moller L, Silverton L: Cerebral ventricular size in the offspring of schizophrenic mothers—a preliminary study. Arch Gen Psychiatry 1984; 41:602-606Crossref, Medline, Google Scholar
36. Reveley AM, Reveley MA, Murray RM: Cerebral ventricular enlargement in non-genetic schizophrenia: a controlled twin study. Br J Psychiatry 1984; 144:89-93Crossref, Medline, Google Scholar
37. Murray RM, Lewis SW, Reveley AM: Towards an aetiological classification of schizophrenia. Lancet 1985; 1:1023-1026Crossref, Medline, Google Scholar
38. Andreasen N, Nasrallah HA, Dunn V, Olson SC, Grove WM, Ehrhardt JC, Coffman JA, Crossett JHW: Structural abnormalities in the frontal system in schizophrenia. Arch Gen Psychiatry 1986; 43:136-144Crossref, Medline, Google Scholar
39. Weinberger DR: The pathogenesis of schizophrenia: a neurodevelopmental theory, in The Neurology of Schizophrenia. Edited by Nasrallah HA, Weinberger DR. Amsterdam, Elsevier, 1986, pp 397-406Google Scholar
40. Murray RM, Lewis SW: Is schizophrenia a neurodevelopmental disorder? (editorial) Br Med J 1987; 295:681-682Crossref, Medline, Google Scholar
41. Weinberger DR: Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 1987; 44:660-669Crossref, Medline, Google Scholar
42. Lewis SW, Murray RM: Obstetric complications, neurodevelopmental deviance and risk of schizophrenia. J Psychiatr Res 1987; 21:413-421Crossref, Medline, Google Scholar
43. Lewis SW, Owen MJ, Murray RM: Obstetric complications and schizophrenia: methodology and mechanisms, in Schizophrenia: Scientific Progress. Edited by Schultz SC, Tamminga CA. New York, Oxford University Press, 1989, pp 56-68Google Scholar
44. Eagles JM, Gibson I, Bremner MH, Clunie F, Ebmeier KP, Smith NC: Obstetric complications in DSM-III schizophrenics and their siblings. Lancet 1990; 335:1139-1141Crossref, Medline, Google Scholar
45. O’Callaghan E, Gibson T, Colohan HA, Buckley P, Walshe DG, Larkin C, Waddington JL: Risk of schizophrenia in adults born after obstetric complications and their association with early onset of illness: a controlled study. Br Med J 1992; 305:1256-1259Crossref, Medline, Google Scholar
46. Verdoux H, Bourgeois M: A comparative study of obstetric history in schizophrenics, bipolar patients, and normal subjects. Schizophr Res 1993; 9:67-69Crossref, Medline, Google Scholar
47. Gunther-Genta F, Bovet P, Hohlfield P: Obstetric complications and schizophrenia: a case-control study. Br J Psychiatry 1994; 164:165-170Crossref, Medline, Google Scholar
48. Hultman CM, Öhman A, Cnattingius S, Wieselgren I-M, Lindström LH: Prenatal and neonatal risk factors for schizophrenia. Br J Psychiatry 1997; 170:128-133Crossref, Medline, Google Scholar
49. McCreadie RG, Hall DJ, Berry IJ, Robertson LJ, Ewing JI, Geals MF: The Nithsdale schizophrenia surveys, X: obstetric complications, family history and abnormal movements. Br J Psychiatry 1992; 160:799-805Crossref, Medline, Google Scholar
50. Heun R, Maier W: The role of obstetric complications in schizophrenia. J Nerv Ment Dis 1993; 181:220-226Crossref, Medline, Google Scholar
51. Foerster A, Lewis SW, Owen MJ, Murray R: Low birth weight and a family history of psychosis predict poor premorbid functioning in psychosis. Schizophr Res 1991; 5:13-20Crossref, Medline, Google Scholar
52. Rifkin L, Lewis S, Jones P, Toone B, Murray R: Low birth weight and schizophrenia. Br J Psychiatry 1994; 165:357-362Crossref, Medline, Google Scholar
53. Smith GN, Kopala LC, Lapointe JS, MacEwan GW, Altman S, Flynn SW, Schnieder T, Falkai P, Honer WG: Obstetric complications, treatment response and brain morphology in adult onset and early-onset males with schizophrenia. Psychol Med 1998; 28:645-653Crossref, Medline, Google Scholar
54. Willinger U, Heiden A, Mesaros K: Obstetric complications, premorbid and current cognitive functioning in schizophrenics and their same sex healthy siblings (abstract). Schizophr Res 1996; 18:100Crossref, Google Scholar
55. O’Callaghan E, Larkin C, Kinsella A, Waddington JL: Obstetric complications, the putative familial-sporadic distinction and tardive dyskinesia in schizophrenia. Br J Psychiatry 1990; 157:578-584Crossref, Medline, Google Scholar
56. McNeil TF, Cantor-Graae E, Nordstrom LG, Rosenlund T: Head circumference in “preschizophrenic” and control neonates. Br J Psychiatry 1993; 162:517-523Crossref, Medline, Google Scholar
57. Kunugi H, Takei N, Murray RM, Saito K, Nanko S: Small head circumference at birth in schizophrenia. Schizophr Res 1996; 20:165-170Crossref, Medline, Google Scholar
58. Owen MJ, Lewis SW, Murray RM: Obstetric complications and schizophrenia: a computed tomographic study. Psychol Med 1998; 18:331-339Crossref, Google Scholar
59. Kirov G, Jones PB, Harvey I, Lewis SW, Toone BK, Rifkin L, Sham P, Murray RM: Do obstetric complications cause the earlier age at onset in male than female schizophrenics? Schizophr Res 1996; 20:117-124Crossref, Medline, Google Scholar
60. Nicolson R, Malaspina D, Giedd JN, Hamburger S, Lenane M, Bedwell J, Fernandez T, Berman A, Susser E, Rapoport JL: Obstetrical complications and childhood-onset schizophrenia. Am J Psychiatry 1999; 156:1650-1652Link, Google Scholar
61. Hutchinson G, Takei N, Bhugra D, Fahy TA, Gilvarry C, Mallett R, Moran P, Leff J, Murray RM: Increased rate of psychosis among African-Caribbeans in Britain is not due to an excess of pregnancy and birth complications. Br J Psychiatry 1997; 171:145-147Crossref, Medline, Google Scholar
62. Kuller LH: Circular epidemiology. Am J Epidemiol 1999; 150:897-903Crossref, Medline, Google Scholar
63. Geddes JR, Lawrie SM: Obstetric complications and schizophrenia: a meta-analysis. Br J Psychiatry 1995; 167:786-793Crossref, Medline, Google Scholar
64. Geddes JR, Verdoux H, Takei N, Lawrie SM, Bovet P, Eagles JM, Heun R, McCreadie RG, McNeil TF, O’Callaghan E, Stöber G, Willinger U, Murray RM: Schizophrenia and complications of pregnancy and labor: an individual-patient data meta-analysis. Schizophr Bull 1999; 25:413-423Crossref, Medline, Google Scholar
65. Fleiss JL: The statistical basis of meta-analysis. Stat Methods Med Res 1993; 2:121-145Crossref, Medline, Google Scholar
66. Egger M, Smith GD, Phillips AN: Meta-analysis: principles and procedures. Br Med J 1997; 315:1533-1537Crossref, Medline, Google Scholar
67. Sacker A, Done DJ, Crow TJ, Golding J: Antecedents of schizophrenia and affective illness: obstetric complications. Br J Psychiatry 1995; 166:734-741Crossref, Medline, Google Scholar
68. Jones PB, Rantakallio P, Hartikainen A-L, Isohanni M, Sipila P: Schizophrenia as a long-term outcome of pregnancy, delivery, and perinatal complications: a 28-year follow-up of the 1966 North Finland general population birth cohort. Am J Psychiatry 1998; 155:355-364Link, Google Scholar
69. Hultman CM, Sparen P, Takei N, Murray RM, Cnattingius S: Prenatal and perinatal risk factors for schizophrenia, affective psychosis, and reactive psychosis of early onset: case-control study. Br Med J 1999; 318:421-426Crossref, Medline, Google Scholar
70. Dalman C, Allebeck P, Cullberg J, Grunewald C, Köster M: Obstetric complications and the risk of schizophrenia: a longitudinal study of a national birth cohort. Arch Gen Psychiatry 1999; 56:234-240Crossref, Medline, Google Scholar
71. Kendell RE, McInneny K, Jusczak E, Bain M: Obstetric complications and schizophrenia: two case-control studies based on structured obstetric records. Br J Psychiatry 2000; 174:516-522Crossref, Google Scholar
72. Byrne M, Browne R, Mulryan N, Scully A, Morris M, Kinsella A, Takei N, McNeil T, Walsh D, O’Callaghan E: Labour and delivery complications and schizophrenia: case-control study using contemporaneous labour ward records. Br J Psychiatry 2000; 176:531-536Crossref, Medline, Google Scholar
73. Dalman C, Thomas HV, David AS, Gentz J, Lewis G, Allebeck P: Signs of asphyxia at birth and risk of schizophrenia: population-based case-control study. Br J Psychiatry 2001; 179:415-416Crossref, Medline, Google Scholar
74. Done DJ, Johnstone EC, Frith CD, Golding J, Shepherd PM, Crow TJ: Complications of pregnancy and delivery in relation to psychosis in adult life: data from the British Perinatal Mortality Survey sample. Br Med J 1991; 302:1576-1580Crossref, Medline, Google Scholar
75. Buka SL, Tsuang MT, Lipsitt LP: Pregnancy/delivery complications and psychiatric diagnosis: a prospective study. Arch Gen Psychiatry 1993; 50:151-156Crossref, Medline, Google Scholar
76. Cannon TD, Rosso IM, Hollister JM, Bearden CE, Sanchez, Hadley T: A prospective cohort study of genetic and perinatal influences in the etiology of schizophrenia. Schizophr Bull 2000; 26:249-256Crossref, Medline, Google Scholar
77. Rosso IM, Cannon TD, Huttunen T, Huttunen MO, Lönnqvist J, Gasperoni TL: Obstetric risk factors for early-onset schizophrenia in a Finnish birth cohort. Am J Psychiatry 2000; 157:801-807Link, Google Scholar
78. Zornberg GL, Buka SL, Tsuang MT: Hypoxic-ischemia-related fetal/neonatal complications and risk of schizophrenia and other nonaffective psychoses: a 19-year longitudinal study. Am J Psychiatry 2000; 157:196-202Link, Google Scholar
79. DerSimonian R, Laird N: Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177-188Crossref, Medline, Google Scholar
80. Kendell RE, Juszczack E, Cole SK: Obstetric complications and schizophrenia: a case-control study based on standardised obstetric records. Br J Psychiatry 1996; 168:556-561Crossref, Medline, Google Scholar
81. Scott JR: Vaginal bleeding in the midtrimester of pregnancy. Am J Obstet Gynecol 1972; 113:329-334Crossref, Medline, Google Scholar
82. Schaefer C, Brown AS, Wyatt RJ, Kline J, Begg MD, Bresnahan MA, Susser ES: Maternal prepregnant body mass and risk of schizophrenia in the offspring. Schizophr Bull 2000; 26:275-286Crossref, Medline, Google Scholar
83. Eriksson UJ: The pathogenesis of congenital malformations in diabetic pregnancy. Diabetes Metab Rev 1995; 11:63-82Crossref, Medline, Google Scholar
84. Wright P, Sham PC, Gilvarry CM, Jones PB, Cannon M, Sharma T, Murray RM: Autoimmune diseases in the pedigrees of schizophrenic and control subjects. Schizophr Res 1996; 20:261-267Crossref, Medline, Google Scholar
85. Gilvarry CM, Sham PC, Jones PB, Cannon M, Wright P, Lewis SW, Bebbington P, Toone BK, Murray RM: Family history of autoimmune diseases in psychosis. Schizophr Res 1996; 19:33-40Crossref, Medline, Google Scholar
86. Hollister JM, Brown AS: Rhesus incompatibility and schizophrenia, in Prenatal Exposures in Schizophrenia. Edited by Susser ES, Brown AS, Gorman JM. Washington, DC, American Psychiatric Press, 1999, pp 197-214Google Scholar
87. Hollister JM, Kohler C: Schizophrenia: a long-term complication of haemolytic disease of the fetus and newborn? Int J Ment Health 2001; 29:38-61Google Scholar
88. Hollister JM, Laing P, Mednick SA: Rhesus incompatibility as a risk factor for schizophrenia in male adults. Arch Gen Psychiatry 1996; 53:19-24Crossref, Medline, Google Scholar
89. Dalman C, Cullberg J: Neonatal hyperbilirubinemia—a vulnerability factor for mental disorder? Acta Psychiatr Scand 1999; 100:469-471Crossref, Medline, Google Scholar
90. Ichiki M, Kunugi H, Takei N, Murray RM, Baba H, Arai H, Oshima I, Okagami K, Sato T, Hirose T, Nanko S: Intra-uterine physical growth in schizophrenia: evidence confirming excess of premature birth. Psychol Med 2000; 30:597-604Crossref, Medline, Google Scholar
91. Wahlbeck K, Forsen T, Osmond C, Barker DJP, Eriksson JG: Association of schizophrenia with low maternal body mass index, small size at birth and thinness during childhood. Arch Gen Psychiatry 2001; 58:48-52Crossref, Medline, Google Scholar
92. Bennedsen BE: Adverse pregnancy outcome in schizophrenic women: occurrence and risk factors. Schizophr Res 1998; 33:1-26Crossref, Medline, Google Scholar
93. Lane A, Kinsella A, Murphy P, Byrne M, Keenan J, Colgan K, Cassidy B, Sheppard N, Horgan R, Waddington JL, Larkin C, O’Callaghan E: The anthropometric assessment of dysmorphic features in schizophrenia as an index of its developmental origins. Psychol Med 1996; 27:1155-1164Crossref, Google Scholar
94. McNeil T, Cantor-Graae E, Ishmail B: Obstetric complications and congenital malformations in schizophrenia. Brain Res Brain Res Rev 2000; 31:166-178Crossref, Medline, Google Scholar
95. Cannon TD, Mednick SA, Parnas J, Schulsinger F, Praestholm J, Vestergaard A: Developmental brain abnormalities in the offspring of schizophrenic mothers, I: contributions of genetic and perinatal factors. Arch Gen Psychiatry 1993; 50:551-564Crossref, Medline, Google Scholar
96. Cannon TD: On the nature and mechanisms of obstetric influences in schizophrenia: a review and synthesis. Int Rev Psychiatry 1997; 9:387-397Crossref, Google Scholar
97. McNeil TF, Cantor-Graae E, Weinberger DR: Relationship of obstetric complications and differences in size of brain structures in monozygotic twin pairs discordant for schizophrenia. Am J Psychiatry 2000; 157:203-212Link, Google Scholar
98. Goodman R: Are complications of pregnancy and birth causes of schizophrenia? Dev Med Child Neurol 1990; 30:391-406Crossref, Google Scholar
99. Nelson KB, Ellenberg JH: Antecedents of cerebral palsy: multivariate analysis of risk. N Engl J Med 1986; 315:81-86Crossref, Medline, Google Scholar
100. McNeil TF, Cantor-Graae E: Does pre-existing abnormality cause labor-delivery complications in fetuses who will develop schizophrenia? Schizophr Bull 1999; 25:425-435Crossref, Medline, Google Scholar
101. Wald NJ, Nanachal K, Thompson SG, Cuckle HS: Does breathing other people’s tobacco smoke cause lung cancer? Br Med J (Clin Res Ed) 1986; 293:1217-1222Crossref, Medline, Google Scholar
102. Collaborative Group on Hormonal Factors in Breast Cancer: Breast cancer and hormonal contraceptives: collaborative reanalysis of individual data of 53,297 women with breast cancer and 100,239 women without breast cancer from 54 epidemiological studies. Lancet 1996; 347:1713-1727Crossref, Medline, Google Scholar
103. Taubes G: Epidemiology faces its limits. Science 1995; 269:164-169Crossref, Medline, Google Scholar
104. Cannon M, Jones P, Gilvarry C, Rifkin L, McKenzie K, Foerster A, Murray RM: Premorbid social functioning in schizophrenia and bipolar disorder: similarities and differences. Am J Psychiatry 1997; 154:1544-1550Abstract, Google Scholar
105. Zornberg G, Buka SL, Tsuang MT: The problem of obstetrical complications and schizophrenia. Schizophr Bull 2000; 26:249-256Crossref, Medline, Google Scholar
106. McNeil TF Perinatal risk factors and schizophrenia: selective review and methodological concerns. Epidemiol Rev 1995; 17:107-112Crossref, Medline, Google Scholar
107. Bagalkote H, Pang D, Jones P: Maternal influenza and schizophrenia in the offspring. Int J Ment Health 2001; 29:3-21Google Scholar
108. Brown AS: Prenatal infection and adult schizophrenia: a review and synthesis. Int J Ment Health 2001; 29:22-37Google Scholar
109. Susser E, Lin SP: Schizophrenia after prenatal exposure to the Dutch Hunger Winter of 1944-1945. Arch Gen Psychiatry 1992; 49:983-988Crossref, Medline, Google Scholar
110. Huttunen M, Niskanen P: Prenatal loss of father and psychiatric disorders. Arch Gen Psychiatry 1978; 35:427-431Crossref, Google Scholar
111. Kinney DK: Prenatal stress and risk for schizophrenia. Int J Ment Health 2001; 29:62-71Google Scholar
112. Brown AS, Cohen P, Greenwald S, Susser E: Nonaffective psychosis after prenatal exposure to rubella. Am J Psychiatry 2000; 157:438-443Link, Google Scholar
113. Stewart AL, Rifkin L, Amess PN, Kirkbride V, Townsend JP, Miller DH, Lewis SW, Kingsley DPE, Moseley IF, Foster O, Murray RM: Brain structure and neurocognitive and behavioural function in adolescents who were born very preterm. Lancet 1999; 353:1653-1657Crossref, Medline, Google Scholar
114. Verdoux H, Geddes JR, Takei N, Lawrie SM, Bovet P, Eagles JM, Heun R, McCreadie RG, McNeil TF, O’Callaghan E, Stöber G, Willinger U, Wright P, Murray RM: Obstetric complications and age at onset in schizophrenia: an international collaborative meta-analysis of individual patient data. Am J Psychiatry 1997; 154:1220-1227Link, Google Scholar
115. Van Os J, Marcelis M: The ecogenetics of schizophrenia: a review. Schizophr Res 1998; 32:127-135Crossref, Medline, Google Scholar
116. Susser E, Schaefer C, Brown A, Begg M, Wyatt RJ: The design of the Prenatal Determinants of Schizophrenia Study (PDS). Schizophr Bull 2000; 26:257-274Crossref, Medline, Google Scholar
117. Kandel ER: A new intellectual framework for psychiatry. Am J Psychiatry 1998; 155:457-469Link, Google Scholar
118. Fearon P, Cannon M, Murray RM: A critique of the idea and science of risk-factor research in schizophrenia. Int J Ment Health 2001; 30:82-90Crossref, Google Scholar
119. Benes FM: Emerging principles of altered neural circuitry in schizophrenia. Brain Res Brain Res Rev 2000; 31:251-269Crossref, Medline, Google Scholar
120. Susser M, Susser E: Choosing a future for epidemiology, II: from black boxes to Chinese boxes and eco-epidemiology. Am J Public Health 1996; 86:674-677Crossref, Medline, Google Scholar
121. Cotter D, Takei N, Farrell M, Sham PC, Quinn P, Larkin C, O’Callaghan E: Does prenatal exposure to influenza in mice induce pyramidal cell disarray in the dorsal hippocampus? Schizophr Res 1995; 16:233-241Crossref, Medline, Google Scholar
122. Fatemi SH, Emamian ES, Kist D, Sidwell RW, Nakajima K, Akhter P, Shier A, Sheikh S, Bailey K: Defective corticogenesis and reduction in Reelin immunoreactivity in cortex and hippocampus of prenatally infected neonatal mice. Mol Psychiatry 1999; 4:145-154Crossref, Medline, Google Scholar
123. Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Bernstein D, Yolken RH: Maternal infections and subsequent psychosis among offsrping. Arch Gen Psychiatry 2001; 58:1032-1037Crossref, Medline, Google Scholar
124. Jones P, Cannon M: The new epidemiology of schizophrenia. Psychiatr Clin North Am 1998; 21:1-26Crossref, Medline, Google Scholar



