Are There Differences Between Women’s and Men’s Antidepressant Responses?
Abstract
OBJECTIVE: The study examined a large data set to determine whether patients’ sex affected the outcome of antidepressant treatment. METHOD: Data for 1,746 patients aged 18–65 years who had been treated with tricyclic antidepressants, monoamine oxidase inhibitors (MAOIs), fluoxetine, or placebo were examined in a retrospective analysis to determine whether men and women differed in their responses to antidepressants. To examine the effect of menopausal status in the absence of data on individual patients’ menopausal status, results for female patients younger or older than age 50, 52, 54, and 56 were compared. RESULTS: Men and women both younger and older than age 50 had equivalent response rates to tricyclics and fluoxetine. Women had a statistically superior response to MAOIs. Placebo response was equivalent across all groups. CONCLUSIONS: Neither sex nor menopausal status may be relevant in antidepressant treatment of adult depressed patients up to 65 years of age. Although women had a statistically superior response to MAOIs, this difference may not be clinically relevant.
Are there differences in the responses of men and women to antidepressants? A recent study suggested that women have a more favorable response to sertraline than to imipramine and men a more favorable response to imipramine (1, 2), but not all studies have supported this observation (3, 4). Clarifying the relevance of these sex-by-drug interactions is difficult because most research reports examining the effects of antidepressants do not discuss this issue. It is unclear if the dearth of reports reflects a failure to test for sex differences or underreporting of negative results. In addition to sex differences, menopausal status has also been suggested as a factor influencing antidepressant responsivity (1, 5).
The purpose of this study was to examine a large enough data set to study sex differences in antidepressant response. Research has suggested some theoretical reasons for suspecting that male-female differences in antidepressant response exist. First, the clearly established differences between men and women in the prevalence of depressive disorders suggest the possibility of other sex-related differences (6). Second, ovarian hormones alter imipramine binding sites, and this action may alter serotonin uptake (7). Furthermore, a relative difference between men and women in right and left brain function may be related to depression and antidepressant response (8). Therefore, apparent sex differences in cognitive styles that may reflect differences in right and left brain function (9) or less well-lateralized function in women (10) might also contribute to difference in antidepressant response.
We analyzed data from 1,746 patients to determine if sex and age (as a surrogate for menopausal status) affected response to tricyclic antidepressants, monoamine oxidase inhibitors (MAOIs), or fluoxetine. Since the issue of speed of response had also been raised (e.g., women respond more slowly to imipramine [11]), the timing of response was also examined.
The study addressed the following questions:
| 1. | Are there differences in the proportions of men and women who respond to antidepressants in 6- to 12-week trials? | ||||
| 2. | Are there sex differences in the speed of onset of response? | ||||
| 3. | Is there evidence that postmenopausal women respond to antidepressants differently than premenopausal women? | ||||
Method
Data collected over a 20-year period in an outpatient depression research clinic were examined. A total of 1,746 patients took part in the nine different studies (12–19, unpublished 2002 manuscript of Stewart et al.) that provided data for this report. Because 59 patients who received placebo in a three-arm study (phenelzine versus imipramine versus placebo) were included in both the tricyclic antidepressant contrasts and the MAOI contrasts, summing the number of patients across all comparisons suggests that 1,805 patients were included in these studies.
Most of these data and the details of the individual studies’ design and outcomes have been previously published; one study is being prepared for publication (unpublished 2002 manuscript of Stewart et al.). Eight studies were double-blind, randomized 6-week trials with fixed, flexible dose schedules (12–18, unpublished 2002 manuscript of Stewart et al.). All but one study (12) was placebo controlled. The ninth study was a five-site, open-label fluoxetine treatment study, the purpose of which was to identify fluoxetine responders. Fluoxetine responders were randomly assigned to receive the drug or placebo in a subsequent discontinuation study (19). Because of the absence of a placebo arm and blind ratings in the acute phase, the results of this 12-week fluoxetine study must be interpreted with caution, and thus they are presented separately. The potential role of bias in this study is addressed later in the paper. The other eight studies investigated the effects of tricyclics (desipramine, imipramine) and MAOIs (phenelzine, tranylcypromine, L-deprenyl).
Outpatients, aged 18 to 65 years, who gave written informed consent were evaluated by using the Research Diagnostic Criteria (20) or the DSM-III or DSM-III-R criteria (depending on the year the study was conducted), the 21-item Hamilton Depression Rating Scale, the Clinical Global Impression (CGI) scale, and the SCL-90 (21). All studies were approved by the clinic’s institutional review board. In the fluoxetine study (19), the 17-item Hamilton depression scale was used.
Lacking data on menopausal status, we divided the female subjects into groups by using the arbitrary cutoff point of age 50. Obviously there is no fixed menopausal age. Stanford et al. (22) reported a median age of 51 years. In a study of 2,783 women, Cramer and Xu (23) found the mean age at menopause was 47.3 years (SD=3.5). They also noted that women with a history of depression go through menopause earlier. In the Results section, we demonstrate that there is no change in the study’s outcome if the female subjects were grouped with the cutoff point at age 52, 54, or 56, instead of age 50. Men were similarly grouped to assess the relevance of age for men’s response to antidepressants.
Since we were interested in the speed of onset of response to antidepressants, persistent improvement was most relevant. Fluctuating improvement would have less clinical relevance. Patients were judged to be responders at the point they received a score of 1 (very much improved) or 2 (much improved) on the change scale of the CGI and maintained that improvement for the duration of the study.
In organizing the data, we wished to have a large enough number of subjects and power to detect differences of 10%. At first, we combined data from the tricyclic antidepressant and MAOI studies (all were 6-week trials). A logistic regression analysis of the relationship between response and class of drug suggested that the outcome of the tricyclic antidepressant studies differed from that of the MAOI studies (χ2=13.91, df=1, p=0.002). Thus, separate examination of the data by drug class was required. The results of the tricyclic antidepressant studies are presented separately from those of the MAOI studies. Data from the fluoxetine study (a 12-week trial) are presented separately.
To determine if data from all studies within each drug class could be combined for analysis, the studies for each drug class were compared separately by using Cox proportional hazard analysis. When no differences in outcome by study by sex were found (i.e., similar to a finding of the absence of a site effect in a multisite study), data from studies utilizing antidepressants from the same class were combined. Cox proportional hazard analysis showed no differences in outcome in a five-by-two interaction examining the MAOI studies (χ2=5.42, df=4, p=0.25) and in a six-by-two interaction examining the tricyclic antidepressant studies (χ2=4.81, df=5, p=0.31). In all studies with a placebo control (13–18, unpublished 2002 manuscript of Stewart et al.), there were clear drug-placebo differences, indicating that some patients had drug-responsive disorders.
Kaplan-Meier estimates of time to response were computed and graphically presented to show potential sex differences. In these analyses, time to survival is equivalent to time to onset of response to medication. A shorter survival time, as shown by a steeper survival curve, indicates a greater response to the medication. Hazard ratios were used to contrast the speed of onset of the drug effect between sexes and age groups (24). The hazard estimate gives the conditional probability of becoming a responder during a time interval, provided that the individual has not responded at the beginning of the time interval. Thus, hazard is a function of survival time. The cumulative hazard at a given time is the hazard integrated over the whole time interval until the given time point. The cumulative hazard for a response estimate for men and women was calculated each week. If the difference between groups in hazard is statistically significant, one group is improving more rapidly than the other up to that point. With correction for multiple comparisons, p<0.008 was required for significance in the analyses for each of the 6 weeks in the MAOI and tricyclic antidepressant studies, and p<0.007 was required in the analysis of the fluoxetine study.
To determine if differences in response between women and men were attributable to a drug effect (e.g., whether the difference between drug and placebo effects for women is greater than that for men), Cox proportional hazards regression analysis was used. The parameter of interest was whether the sex-by-treatment interaction was significantly different from zero.
Results
Demographic Characteristics
The data were collected over a 20-year period in nine different studies. Owing to some variation in protocols, not all data were available for all patients.
The effects of tricyclic antidepressants were examined in seven double-blind, placebo-controlled trials involving a total of 602 subjects, 357 of whom were women. Of the subjects in the tricyclic studies, approximately 86% were Caucasian, 30% were married, and 57% were employed. The mean age was 38 years (SD=11). Seventy percent had a diagnosis of major depression, 27% had dysthymia, and 3% had depression not otherwise specified. Onset of the illness occurred after age 20 in 30% of subjects. At baseline, the subjects had a mean score of 15.7 (SD=4.6) on the 21-item Hamilton depression scale and 3.9 (SD=0.6) on the CGI severity scale. The mean age of the women older than age 50 (mean=56 years, SD=5) did not differ from that of the men older than age 50 (mean=56 years, SD=5).
MAOIs were studied in five double-blind, placebo-controlled trials that included a total of 363 subjects, 210 of whom were women. Approximately 86% of all subjects in the MAOI studies were Caucasian, 29% were married, and 58% were employed. The mean age was 38 years (SD=10). Seventy percent had a diagnosis of major depression, 27% had dysthymia, and 3% had depression not otherwise specified. Onset of the illness occurred after age 20 in 27% of the subjects. At baseline, the subjects had a mean score of 15.6 (SD=4.6) on the 21-item Hamilton depression scale and 3.9 (SD=0.6) on the CGI severity scale. The mean age of the women older than age 50 (mean=54 years, SD=5) did not differ significantly from that of the men older than age 50 (mean=57 years, SD=4).
The fluoxetine lead-in study was an open trial that had 840 subjects, 574 of whom were women. Approximately 91% of the subjects were Caucasian, and their mean age was 39 years (SD=11). Data on employment and marital status were not available for this study. All patients met the criteria for major depressive disorder. At baseline, they had a mean score of 22.3 (SD=4.0) on the 17-item Hamilton depression scale and 4.2 (SD=0.7) on the CGI severity scale. The mean age of the women older than age 50 (mean=56 years, SD=4) did not differ from that of the men older than age 50 (mean=56 years, SD=4).
Age and Response
To test the effect of different age cutoff points on women’s outcome, women were grouped by whether they were older or younger than age 50, 52, 54, and 56 years. In the groups older than age 50, 52, 54, and 56, the proportions of women responding to tricyclic antidepressants were 66% (N=19 of 29 women), 63% (N=15 of 24), 65% (N=11 of 17), and 60% (N=9 of 15), respectively. For MAOIs, the proportions of women responding were 79% (N=15 of 19 women), 79% (N=11 of 14), 86% (N=6 of 7), and 83% (N=5 of 6), respectively. For SSRIs, the proportions were 62% (N=59 of 95 women), 61% (N=45 of 74), 56% (N=31 of 55), and 55% (N=22 of 40), respectively. The differences in proportions of responders determined by using different cutoff ages did not appear to be clinically relevant. Therefore, we used a cutoff of age 50 to distinguish younger from older subjects.
Response to Tricyclics
The tricyclic trials included a total of 602 subjects, 312 of whom received the medication and 290 of whom received placebo (12–14, 16–19). The drugs studied in these trials included imipramine and desipramine. Placebo response rates were 24% (N=37 of 155) for the younger women, 23% (N=19 of 83) for the younger men, 22% (N=6 of 27) for the older women, and 28% (N=7 of 25) for the older men. A survival analysis suggested that the four groups did not differ in time to onset of response for placebo-treated patients (χ2=0.33, df=3, p=0.96, log-rank test). Therefore, to simplify examination of the data, only the survival curves representing time to onset of response for patients who received the study medication are presented.
The outcomes of tricyclic antidepressant treatment for the four age/sex groups were quite similar, and the four groups’ curves for time to onset of response overlapped (Figure 1). (To facilitate examination, curves for subjects younger than age 50 and for those age 50 or older are presented separately.) No differences were found for men (N=109) and women (N=146) younger than age 50 (χ2=0.02, df=1, p=0.88, log-rank test) or for men (N=28) and women (N=29) age 50 or older (χ2=1.56, df=1, p=0.21, log-rank test). The response of older women was superior to that of younger women (χ2=3.87, df=1, p<0.05, log-rank test). Younger and older men had no difference in outcome (χ2=0.02, df=1, p=0.68, log-rank test). The findings can be summarized as follows: older women > younger women = younger men = older men.
A Cox proportional hazards regression analysis relating time to response to treatment (tricyclic antidepressant versus placebo), sex, and age showed that none of the interaction terms were significant (three-way interaction χ2=2.03, df=1, p=0.16; two-way interaction terms χ2=2.21, df=3, p=0.053). In addition, in the model with main effects, sex and age factors were not significant (t=1.52, df=602, p=0.13 and t=0.52, df=602, p=0.60, respectively). In this group of subjects, age and sex did not appear to affect the chance of response.
Response to MAOIs
The MAOI trials included a total of 363 subjects, 210 of whom received the medication and 153 of whom received placebo (13–17). The drugs studied in these trials included phenelzine, tranylcypromine, and L-deprenyl. Placebo response rates were 21% (N=17 of 81) for younger women, 28% (N=15 of 53) for younger men, 17% (N=2 of 12) for older women, and 43% (N=3 of 7) for older men. A survival analysis of time to onset of response for placebo-treated patients showed no evidence for differences between groups in placebo response rates (χ2=3.04, df=3, p=0.39, log-rank test). Therefore, to simplify data examination, only the curves representing time to onset of response for subjects who received the study medication are presented.
Survival curves showing time to onset of response to the medication for younger and older subjects are presented separately (Figure 1). Younger women (N=98) had a superior response, compared to younger men (N=74) (χ2=4.97, df=1, p=0.03, log-rank test). There was no evidence of a difference in response between older men (N=19) and older women (N=19) (χ2=0.33, df=1, p=0.61, log-rank test). However, because only 19 subjects were included in each group, the analysis was underpowered. There were no differences in time to medication response between younger men and older men (χ2=1.18, df=1, p=0.28, log-rank test) or between younger women and older women (χ2=0.23, df=1, p=0.63, log-rank test). The findings can be summarized as follows: older women = younger women > younger men = older men.
To determine if differences between men and women in response were attributable to a true drug effect, Cox proportional hazards regression was used to test the interaction of sex (male versus female) and treatment (medication versus placebo). The results indicated a difference in efficacy of MAOIs between men and women, with women having a larger drug effect (beta=–0.98, exponential of beta=0.37, SE=0.37, z=–2.64, p<0.009).
In an attempt to understand younger women’s superior response, we modeled the odds for response as a function of various predictors using a logistic regression model. Sex, age, diagnostic subtype (i.e., atypical, melancholia), and chronicity (presence or absence), and interactions between these variables were used as predictors. Except for sex, all predictors and interactions were not significant (analysis not shown). The results suggest that the observed sex difference cannot be explained by another identifiable variable, e.g., by the presence of a greater proportion of women with a diagnosis of atypical depression.
Response to Fluoxetine
The open trial of fluoxetine included in this analysis had 840 subjects. Survival curves representing time to onset of response to medication for the younger and older men and women are presented in Figure 1. No overall differences were found between the younger men (N=208) and women (N=479) (χ2=0.22, df=1, p=0.70, log-rank test) or the older women (N=95) and men (N=58) (χ2=0.95, df=1, p=0.34, log-rank test). There were no differences between older and younger women (χ2=0.05, df=1, p=0.82, log-rank test) or between older and younger men (χ2=0.46, df=1, p=0.50, log-rank test).
Speed of Response
To examine speed of response, hazard ratios were used to compare onset of medication response between men and women for each medication group. Compared with the younger men who received MAOIs, younger women who received MAOIs had a more rapid onset of response, which was evident at week 3 (hazard ratio=0.32, p=.004). The comparisons between sexes for tricyclic antidepressants and fluoxetine were not significant (data not shown).
Dropouts
Analysis of the proportions of male and female dropouts within each drug group was used to determine whether findings suggesting a drug effect might be attributable to differential attrition. Dropout rates in the MAOI studies were 26% (N=25 of 98) for younger women, 8% (N=6 of 74) for younger men, 21% (N=4 of 19) for older women, and 21% (N=4 of 19) for older men, a significant difference (χ2=8.63, df=3, p<0.04). The dropout rate for younger men was lower than that for younger women (χ2=8.30, df=1, p<0.01). The Hamilton depression scale scores of the younger women who dropped out were compared with those of all remaining patients (at the time of dropout). F tests were done for each week contrasting the Hamilton depression scale score for female dropouts and for the remaining patients; none of the differences were significant (analysis not shown). If the women dropouts had low Hamilton depression scale scores, they may have gotten worse with time, which could have biased the last-observation-carried-forward analysis. However, this analysis does not appear flawed by this potential bias.
The dropout rates for the four age/sex groups in the tricyclic antidepressant studies and fluoxetine study were similar (data not shown).
Dose Effects
Mean daily doses of medication differed by sex for the MAOIs. For example, the mean daily phenelzine dose was 79 mg (SD=16) for younger men, 67 mg (SD=15) for younger women, 77 mg (SD=16), for older men, and 65 mg (SD=12) for older women (F=5.84, df=3, 105, p=0.001). Younger men had a significantly higher dose than younger women (t=3.84, df=91, p=0.001); there was no difference in the doses of older men and older women (t=1.82, df=14, p=0.09). Therefore, women’s more favorable response to MAOIs was not explained by dose.
Doses were similar for men and women in the tricyclic antidepressant studies. For example, the mean imipramine dose at week 6 was 279 mg (SD=38) for younger men, 257 mg (SD=53) for younger women, 279 mg (SD=57) for older men, and 269 mg (SD=46) for older women. In the fluoxetine study, all patients received 20 mg/day.
Discussion
Despite the theoretical potential for differences between men’s and women’s response to antidepressants, we found few such differences. A higher proportion of women than men in this study group benefited from MAOIs. Women age 50 and older and those younger than age 50 had similar MAOI and fluoxetine response rates. Women age 50 and older had a superior response to tricyclic antidepressants.
Several studies have suggested that postmenopausal women are less likely to benefit from antidepressants and that hormone replacement therapy improves drug response (1, 5). The recent Women’s Health Initiative study reports increased risk of breast cancer, myocardial infraction, and stroke associated with replacement therapy (25). A 2002 editorial in JAMA concluded that even these small risks contraindicated long-term hormone therapy (26). Our data suggest that menopause, even in the absence of hormone replacement therapy, does not appear to affect antidepressants’ benefit. Depressed postmenopausal women should receive antidepressants regardless of whether they are also receiving hormone replacement therapy. It is unclear if adding hormone replacement therapy will be beneficial for women who are unresponsive to an antidepressant.
These findings appear to contradict those of Kornstein et al. (1), who found sex differences in response to antidepressants. We examined studies frequently cited as providing evidence for sex differences in antidepressant response and also did a MEDLINE literature review (1, 3–5, 11, 27–37). Hamilton (32) reviewed 205 imipramine studies published through 1991. In 35 studies (19%), a judgment about sex effect was possible. Six were reported to show a statistically significant difference favoring women. Six others suggested that men’s imipramine response was superior to women’s. Two of those six studies included schizophrenic patients (27, 28). In a third study, women with an adequate plasma level had a good response to imipramine (N=6 of 7) (33). In a fourth study, which included 13 men and 21 women and three treatment arms, the report that “interaction of sex and active treatment favored men” does not seem plausible, given the small number of subjects (35). We conclude that only two of these six studies supported an advantage of imipramine for men (34, 36).
Several other studies demonstrate a more favorable response to tricyclic antidepressants in men (1, 30, 31). One study of dothiepin maintenance suggests that time to relapse was longer for men than women (29). One study found that men had a better response to tricyclics than to MAOIs and that women had the reverse response, but the differences did not exceed chance because less than 50 comparisons were done (30). Two studies suggest superior imipramine response for men (1, 31).
When data for all patients included in the 35 studies reviewed by Hamilton were combined, 62% of the men and 51% of the women responded to imipramine. Many studies had a small number of subjects and were underpowered. Four studies clearly show an advantage of imipramine for men (1, 31, 34, 36), and none show such an advantage for women. Taken together, these data suggest a modest advantage of imipramine for men.
Kornstein et al. (1) suggested that women have a superior response to SSRIs compared to imipramine. Lewis-Hall et al. (4) analyzed data from several studies of women treated with fluoxetine and imipramine and found the drugs equally efficacious. Martenyi et al. (37) found women had a better response to fluoxetine than to maprotiline, and men had an equal response to the two drugs. However, men’s improvement while taking fluoxetine was equal to women’s. In a brief abstract, Steiner noted that women had equal benefit with imipramine and paroxetine (both superior to placebo) (3). In the fluoxetine study included in this analysis, men and women had an equivalent response in the acute open phase. Although this study had an open treatment design, equivalent relapse rates for men and women in the double-blind discontinuation phase suggest that ratings in the open phase favored neither men nor women (19). Thus, it is unclear if women’s response to SSRIs is superior to men’s; one study has suggested that it is (1) and two that it is not (37 and the current study). Whether women have a superior response to SSRIs compared to tricyclic antidepressants is also unclear; superior response to SSRIs is supported by one study (1) and not supported by two studies (3, 4).
What are the clinical and heuristic implications of these data? Men may have a slightly better outcome with imipramine, but it is unclear whether this small advantage is a result of pharmacokinetics, pharmacodynamics, or diagnostic differences. Perhaps men are more likely to have an imipramine-responsive subtype of depression with a distinctive neurophysiology that is as yet unidentified. Similar parameters should be considered in attempting to explain the slight advantage of MAOIs for women.
Even if we assume a real but small sex-related difference in tricyclic antidepressant and MAOI outcomes, the clinical relevance of the difference is minimal. SSRIs and other second-generation antidepressants have a more favorable side effect profile than the tricyclics and MAOIs, and most often clinicians choose to prescribe them first (38). MAOIs and tricyclic antidepressants have become second-line drugs, and the slight sex advantages (or disadvantages) should not alter their use. The current data do not allow a firm algorithm of choices, although a collaborative study may permit better informed decisions in the future (39).
One limitation of this study is that there were too few patients to be sure that 10% differences in outcome between the sexes exist. Given the large number of studies that have been done, relatively few data about female-male differences in outcome are available. To help clarify the extent of sex-specific differences in antidepressant response, we recommend testing for treatment-by-sex interactions in all studies of antidepressants and reporting both the positive and negative findings.
Received April 4, 2001; revisions received Dec. 5, 2001, and May 29, 2002; accepted June 6, 2002. From the Department of Therapeutics, New York State Psychiatric Institute; and Columbia University College of Physicians and Surgeons, New York. Address reprint requests to Dr. Quitkin, New York State Psychiatric Institute, 1051 Riverside Dr., New York, NY 10032; [email protected] (e-mail).
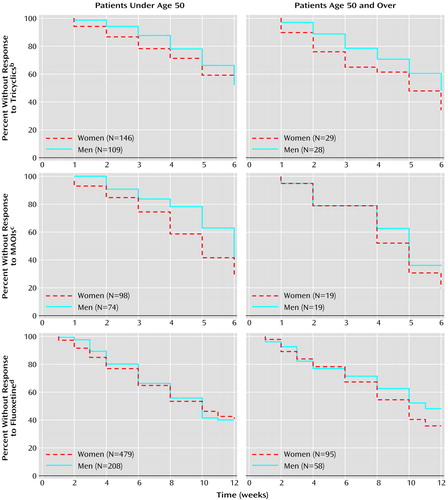
Figure 1. Kaplan-Meier Survival Analysis of Time to Onset of Response to Three Types of Antidepressant Medications for Women and Men Under Age 50 and Age 50 and Oldera
aSubjects were patients in an outpatient depression research clinic who participated in nine different studies over a 20-year period (12–19, unpublished 2002 manuscript of Stewart et al.). Eight studies were double-blind, randomized 6-week trials with fixed, flexible-dose schedules (12–18, unpublished 2002 manuscript of Stewart et al.), all but one of which (12) was placebo controlled. The ninth (19) was an open-label study.
bNo significant difference between women and men under age 50 (χ2=0.02, df=1, p=0.88, log-rank test) and age 50 and over (χ2=1.56, df=1, p=0.21, log-rank test).
cSignificant difference between women and men under age 50 (χ2=4.97, df=1, p<0.03, log-rank test); no significant difference between women and men age 50 and older (χ2=0.33, df=1, p=0.61, log-rank test).
dNo significant difference between women and men under age 50 (χ2=0.22, df=1, p=0.70, log-rank test) and age 50 and over (χ2=0.95, df=1, p=0.34, log-rank test).
1. Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, Gelenberg AJ, Davis SM, Harrison W, Keller MB: Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry 2000; 157:1445-1452Link, Google Scholar
2. Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, Gelenberg AJ, Davis SM, Keller MB: Reply to FM Quitkin: Gender differences in treatment response (letter). Am J Psychiatry 2001; 158:1532-1533Link, Google Scholar
3. Steiner M, Wheadon DE, Kreider MS, Bushnell WD: Antidepressant response to paroxetine by gender, in 1993 Annual Meeting New Research Program and Abstracts. Washington, DC, American Psychiatric Association, 1990, p 176Google Scholar
4. Lewis-Hall FC, Wilson MG, Tepner RG, Koke SC: Fluoxetine vs tricyclic antidepressants in women with major depressive disorder. J Womens Health 1997; 6:337-343Crossref, Medline, Google Scholar
5. Schneider LS, Small GW, Hamilton S, Bystritsky A, Nemeroff CB, Meyer BS (Fluoxetine Collaborative Study Group): Estrogen replacement and response to fluoxetine in a multicenter geriatric depression trial. Am J Geriatr Psychiatry 1997; 5:97-106Crossref, Medline, Google Scholar
6. Wolk SI, Weissman MM: Women and depression: an update, in American Psychiatric Press Review of Psychiatry, vol 14. Edited by Oldham JM, Riba MB. Washington, DC, American Psychiatric Press, 1995, pp 227-259Google Scholar
7. Wilson MA, Dwyer KD, Roy EJ: Direct effects of ovarian hormones on antidepressant binding sites. Brain Res Bull 1989; 22:181-185Crossref, Medline, Google Scholar
8. Bruder GE, Stewart JW, Voglmaier MM, Harrison WM, McGrath PJ, Tricamo E, Quitkin FM: Cerebral laterality and depression: relations of perceptual asymmetry to outcome of treatment with tricyclics. Neuropsychopharmacology 1996; 5:1-10Google Scholar
9. Caplan P, Crawford M, Shibley Hyde J, Richardson J: Gender Differences in Human Cognition. New York, Oxford University Press, 1997Google Scholar
10. Shaywitz BA, Shaywitz SE, Pugh KR, Constable RT, Skudlarski P, Fullbright RK, Braner RH, Fletter JM, Shankeweller DP, Katz L, Gore JC: Sex differences in the functional organization of the brain for language. Nature 1995; 373:607-609Crossref, Medline, Google Scholar
11. Frank E, Carpenter LL, Kupfer DJ: Sex differences in recurrent depression: are there any that are significant? Am J Psychiatry 1988; 145:41-45Link, Google Scholar
12. Stewart JW, Quitkin FM, Liebowitz MR, McGrath PJ, Harrison W, Klein DF: Efficacy of desipramine in depressed outpatients: response according to RDC diagnosis and severity of illness. Arch Gen Psychiatry 1983; 40:202-207Crossref, Medline, Google Scholar
13. Liebowitz MR, Quitkin FM, Stewart JW, McGrath PJ, Harrison WM, Markowitz JS, Rabkin JG, Tricamo E, Goetz DM, Klein DF: Antidepressant specificity in atypical depression. Arch Gen Psychiatry 1988; 45:129-137Crossref, Medline, Google Scholar
14. Quitkin FM, Stewart JW, McGrath PJ, Liebowitz MR, Harrison WM, Tricamo E, Klein DF, Rabkin JG, Markowitz JS, Wager SG: Phenelzine versus imipramine in the treatment of probable atypical depression: defining syndrome boundaries of selective MAOI responders. Am J Psychiatry 1988; 145:306-311Link, Google Scholar
15. McGrath PJ, Stewart JW, Harrison W, Wager S, Nunes EV, Quitkin FM: A placebo-controlled trial of L-deprenyl in atypical depression. Psychopharmacol Bull 1989; 25:63-68Medline, Google Scholar
16. Quitkin FM, McGrath PJ, Stewart JW, Harrison W, Wager SG, Nunes E, Rabkin JG, Tricamo E, Markowitz J, Klein DF: Phenelzine and imipramine in mood reactive depressives: further delineation of the syndrome of atypical depression. Arch Gen Psychiatry 1989; 46:787-793Crossref, Medline, Google Scholar
17. Quitkin FM, McGrath PJ, Stewart JW, Harrison W, Tricamo E, Wager SG, Ocepek-Welikson K, Nunes E, Rabkin JG, Klein DF: Atypical depression, panic attacks, and response to imipramine and phenelzine: a replication. Arch Gen Psychiatry 1990; 47:935-941Crossref, Medline, Google Scholar
18. McGrath PJ, Stewart JW, Janal MN, Petkova E, Quitkin FM, Klein DF: A placebo-controlled study of fluoxetine versus imipramine in the acute treatment of atypical depression. Am J Psychiatry 2000; 157:344-350Link, Google Scholar
19. McGrath PJ, Stewart JW, Petkova E, Quitkin FM, Amsterdam JD, Fawcett J, Reimherr FW, Rosenbaum JF, Beasley C: Predictors of relapse during fluoxetine continuation or maintenance treatment of major depression. J Clin Psychiatry 2000; 61:518-524Crossref, Medline, Google Scholar
20. Spitzer RL, Endicott J, Robins E: Research Diagnostic Criteria (RDC) for a Selected Group of Functional Disorders, 3rd ed. New York, New York State Psychiatric Institute, Biometrics Research, 1977Google Scholar
21. Derogatis LR, Lipman RS, Covi L: SCL-90: an outpatient psychiatric rating scale—preliminary report. Psychopharmacol Bull 1973; 9:13-28Medline, Google Scholar
22. Stanford JL, Hartge P, Brinton LA, Hoover RN, Brookmeyer R: Factors influencing the age at natural menopause. J Chronic Dis 1987; 40:995-1002Crossref, Medline, Google Scholar
23. Cramer DW, Xu H: Predicting age at menopause. Maturitas 1996; 23:319-326Crossref, Medline, Google Scholar
24. Lee ET: Statistical Methods for Survival Data Analysis. New York, John Wiley & Sons, 1992Google Scholar
25. Women’s Health Initiative Study Group: Risks and benefits of estrogen plus progestin in healthy postmenopausal women. JAMA 2002; 288:321-333Crossref, Medline, Google Scholar
26. Fletcher SW, Colditz GA: Failure of estrogen plus progestin therapy for prevention (editorial). JAMA 2002; 288:366-368Crossref, Medline, Google Scholar
27. Raskin A: Age-sex differences in response to antidepressant drugs. J Nerv Ment Dis 1974; 159:120-130Crossref, Medline, Google Scholar
28. Raskin A, Crook TH: Antidepressants in black and white inpatients. Arch Gen Psychiatry 1975; 32:643-649Crossref, Medline, Google Scholar
29. Old Age Depression Interest Group: How long should the elderly take antidepressants? Br J Psychiatry 1993; 162:175-182Crossref, Medline, Google Scholar
30. Davidson J, Pelton S: Forms of atypical depression and their response to antidepressant drugs. Psychiatry Res 1986; 17:87-95Crossref, Medline, Google Scholar
31. Clinical Psychiatry Committee: Clinical trial of the treatment of depressive illness: report to the Medical Research Council by its Clinical Psychiatry Committee. Br Med J 1965; 3:881-886Google Scholar
32. Hamilton JA: Sex and gender as critical variables in psychotropic drug research, in Mental Health, Racism, and Sexism. Edited by Willie CV, Rieker PP, Kramer BM, Brown BS. Pittsburgh, University of Pittsburgh Press, 1995, pp 297-350Google Scholar
33. Glassman AH, Perel JM, Shostak M, Kantor SJ, Fleiss JL: Clinical implications of imipramine plasma levels for depressive illness. Arch Gen Psychiatry 1977; 34:197-204Crossref, Medline, Google Scholar
34. Wilson IC, Rabon AM, Merrick HA, Knox AE, Taylor JP, Buffaloe WJ: Imipramine pamoate in the treatment of depression. Psychosomatics 1966; 7:251-253Crossref, Medline, Google Scholar
35. Gerner R, Estabrook W, Steuer J, Jarvik L: Treatment of geriatric depression with trazodone, imipramine, and placebo: a double-blind study. J Clin Psychiatry 1980; 41:216-220Medline, Google Scholar
36. Flemminger JJ, Groden BM: Clinical features of depression and the response to imipramine (“Tofranil”). J Ment Sci 1962; 108:101-104Crossref, Medline, Google Scholar
37. Martenyi F, Dossenbach M, Mraz K, Metcalfe S: Gender differences in the efficacy of fluoxetine and maprotiline in depressed patients. Eur Neuropsychopharmacol 2001; 11:227-232Crossref, Medline, Google Scholar
38. Olfson M, Klerman GL: Trends in the prescription of antidepressants by office-based psychiatrists. Am J Psychiatry 1993; 150:571-577Link, Google Scholar
39. Epidemiology Data Center, University of Pittsburgh: Sequenced Treatment Alternatives to Relieve Depression. http://www.edc. gsph.pitt.edu/stard/Google Scholar



