Limbic Responsiveness to Procaine in Cocaine-Addicted Subjects
Abstract
OBJECTIVE: The limbic system plays a critical role in motivation, emotional expression, and memory. The authors investigated whether a state of permanent limbic neuronal hyperexcitability, or sensitization, is present in cocaine addicts as a consequence of repeated cocaine use. METHOD: Single photon emission computed tomography (SPECT) of regional cerebral blood flow (rCBF) was used to compare the central nervous system response to the limbic stimulus procaine in 10 cocaine-dependent male patients and 10 healthy comparison male subjects. RESULTS: The cocaine-addicted subjects demonstrated bilateral activation of the orbitofrontal cortex after the procaine challenge, whereas the comparison subjects showed activation of the anterior cingulate, bilateral insular, and right amygdalar regions. After receiving placebo, the cocaine-addicted subjects showed markedly lower rCBF in the bilateral orbitofrontal cortex than the comparison subjects. CONCLUSIONS: The pattern of hypoperfusion in the placebo state followed by heightened activation with procaine in the cocaine-addicted subjects is similar to the pattern of interictal hypoperfusion and ictal hyperperfusion that has been observed in subjects with epilepsy. The findings for the cocaine-addicted subjects may thus represent evidence of localized (orbitofrontal) sensitization.
In 1912, Grode (1) administered cocaine daily to guinea pigs, rabbits, cats, and dogs. Over a period of several days, Grode found that doses of cocaine originally well tolerated eventually induced seizures in these animals. More recent studies have documented that daily administration of cocaine will produce seizures, as well as cause a progressive increase in behavioral stimulation and brain electrical activity (2–4). This phenomenon of cocaine-induced neuronal hyperexcitability has been referred to as neuronal sensitization, or “kindling.” This term refers to the gradual increasing hyperexcitability in neuronal discharge that occurs after the intermittent, repetitive application of various subthreshold stimuli. The limbic system, an integrated brain area critical to motivation, emotional expression, and memory, is particularly susceptible to sensitization. This system is generally considered to include the anterior cingulate, amygdala, hippocampus, insular cortex, and orbitofrontal cortex.
Limbic neuronal sensitization has been suggested as a causal link between chronic cocaine use and the psychological and physical sequelae of cocaine addiction. The development of seizures, panic disorder, and psychosis after the chronic use of cocaine have all been hypothesized to be sensitizing phenomena (5–10). Perhaps most intriguing and clinically important, the experience of craving by cocaine-dependent patients may be a manifestation of limbic neuronal hyperexcitability (11).
Despite the supportive animal data, the suggestive human studies, the intuitive appeal of the hypothesis, and the extensive literature over the past decade about the potential importance of this phenomenon, we could find no published studies reporting convincing evidence that the limbic system becomes more neuronally responsive in cocaine addicts over time with repeated cocaine use. To investigate this phenomenon, we administered the relatively specific limbic stimulant procaine (12–15) to cocaine-addicted subjects and healthy comparison subjects as a limbic “probe” and assessed their limbic response with single photon emission computed tomography (SPECT). We hypothesized that 1) subjects with a history of cocaine dependence would demonstrate greater limbic system regional cerebral blood flow (rCBF), as measured by SPECT, in response to procaine than would age-matched healthy comparison subjects, and 2) limbic system rCBF response after procaine administration would be positively correlated with the amount of lifetime cocaine use.
Method
Subjects
Ten cocaine-addicted and 10 healthy male subjects, 25–45 years old, participated in the study. The subjects underwent a thorough medical history interview and physical examination, assessment with the Structured Clinical Interview for DSM-IV (SCID), clinical laboratory tests, urine drug screening, ECG, and electroencephalogram. Data on lifetime history of cocaine and other substance use was obtained from the cocaine-dependent subjects by using the Timeline Followback (16). After complete description of the study to the subjects, written informed consent was obtained. The subjects were financially compensated for their participation.
The cocaine-addicted subjects (mean age=39.4 years, SD=3.4) were recruited among patients requesting treatment for cocaine dependence at the Veterans Affairs Medical Center in Dallas. The cocaine-addicted subjects reported a total lifetime use costing a mean of $340,043 (SD=$574,243) and a mean total duration of use of 2,381 days (SD=1,719), met DSM-IV criteria for cocaine dependence, and had been abstinent between 14 and 28 days (mean=18.6 days, SD=4.9) on the first study day. The addicted subjects were hospitalized as soon as possible after their last reported use of cocaine and remained in a structured residential unit until at least 3 days after completion of the study. Urine drug screens were obtained three times weekly. Exclusion criteria included present use of any medications active in the central nervous system; a lifetime history of affective, anxiety, or schizophrenic disorder or organic brain syndrome experienced before the onset of a substance abuse diagnosis or after a period of at least 3 months of abstinence; a substance use disorder (other than cocaine, nicotine, or caffeine use) within the previous 12 months; or a lifetime history of withdrawal from alcohol, sedative-hypnotics, or opioids.
Exclusion criteria for the healthy comparison subjects (mean age=32.9 years, SD=7.1) included the medical criteria as noted for the cocaine-addicted subjects, as well as a lifetime history of any other axis I disorder (except nicotine or caffeine abuse or dependence). Healthy comparison subjects with a first-degree relative with an axis I disorder or two or more second-degree relatives with a substance use disorder were also excluded.
Study Sessions
Because of the strong subjective responsive to procaine, a fixed-order, single-blind design was employed. Two study procedures were performed 48 hours apart at approximately 12:00 p.m., with saline always administered on the first study day. Study sessions took place at the Nuclear Medicine Center at the University of Texas Southwestern Medical Center at Dallas. After the subjects rested for 30 minutes following insertion of an intravenous apparatus, they completed the SCL-90-R (17). Two minutes later, either saline or procaine (1.38 mg/kg, 100 mg/ml) was administered over 60 seconds by slow push, followed by 3 ml saline flush over 45 seconds, 20 mCi of [99mTc]hexamethylpropyleneamine oxime ([99mTc]HMPAO) (Nycomed/Amersham, Princeton, N.J.) over 30 seconds, and 10 ml saline flush over 30 seconds. Four minutes after the final infusion, the Drug Assessment Questionnaire and the SCL-90-R were given. The Drug Assessment Questionnaire consisted of five questions, rated on a scale of 0 (no effect) to 6 (strongest effect). The five questions concerned whether the subject 1) felt any drug effect, 2) felt a good effect, 3) felt a bad effect, 4) liked the effect, and 5) disliked the effect. Subjects who endorsed a “drug effect” of 2 or greater were asked how similar the effect of the drug was to their experience with other specific psychoactive substances. The SCL-90-R was used to assess cognitive, mood, and sensory changes after administration of procaine. Ninety minutes after procaine or placebo infusion, the SPECT scan was obtained.
SPECT Imaging
SPECT images were acquired with a PRISM 3000S three-headed SPECT camera (Picker International, Cleveland) by using ultra-high-resolution fan-beam collimators (reconstructed resolution of 6.5 mm) in a 128 × 128 matrix in three° increments. Twenty mCi of [99mTc]HMPAO was administered for each scan, and the total scan duration was 20 minutes. Image reconstruction was performed in the transverse domain by using back-projection with a ramp filter. For our system, voxels in reconstructed images were 1.9 mm3. Reconstructed images were smoothed with a fourth-order Butterworth postreconstruction filter, attenuation corrected by using a Chang first-order method with ellipse size adjusted for each slice, normalized for count density, and spatially coregistered (18).
Intra- and intersubject normalization of image count density was accomplished by expressing each pixel as a value relative to the average pixel value for the whole brain. All analyses of changes in rCBF were measured relative to global cerebral blood flow (ratio data). Data were automatically resliced to 2 mm3 voxels, normalized, and coregistered. Coordinate transformation was set to reformat images into Talairach space (19). Finally, images were smoothed from their original resolution of 6.5 mm full width at half maximum to a final resolution of 10 mm full width at half maximum.
Statistical Analyses
Image analysis
We employed a three-dimensional implementation of the t statistic (paired for within-group pre-/postprocaine comparisons or unpaired for between-group comparisons) as representative of the change in level of the different group means. Data for each voxel in each image were used to compute a t value. The total distribution of t values across all voxels was then mapped, and an omnibus threshold of p<0.01 based on the t value for the experiment’s degrees of freedom was used to identify voxels participating in the response to procaine. Voxels with a t value exceeding this threshold were those with increased rCBF after procaine, and voxels with a t value below this threshold were those with decreased rCBF after procaine. Since these voxels represented both areas of real response and random parts of the null set t distribution, we searched the “t-image” voxels for a neighborhood association (i.e., a cluster analysis where increased statistical weight is given to voxels with neighbors that are also significant). It was assumed that voxels from the null set t distribution would be randomly distributed in space, and so could be removed by requiring that “acceptable” voxels have neighbors that also met the selected t threshold. Remaining significant voxels were next mapped onto a model SPECT brain to produce a parametric statistical image that identified response location. This t image revealed those voxels whose relative rCBF differed most between the placebo and procaine states relative to each voxel’s inherent variability.
Regions of significant activation identified on the t images were corrected for the large number of t tests performed, the lack of independence between voxels, and the resolution of the processed images by using a modification of a statistical technique based on Gaussian random field theory (20) suggested by Worsley et al. (21). The images included about 157,000 gray matter voxels, representing approximately 1,250 resolution elements. The degrees of freedom (number of resolution elements × [number of subjects – 1]) were very large (df>20,000), leading to uncorrected p<0.0004 for an omnibus cutoff of p<0.05. However, as recommended by Worsley et al. (21), we chose a cutoff of p<0.01 both to be conservative in our identification of areas of response to procaine and to account for the three comparisons conducted (procaine versus placebo for the addicted subjects and for the healthy comparison subjects as well as a contrast of these two differences between groups [see Results]). Further, to omit isolated outlying clusters, only areas that exceeded 50 contiguous voxels were evaluated, unless the structure identified was itself of small volume (e.g., caudate head). The net effect of this process was to identify as significant only voxels with a minimum t value of 2.43 for paired analyses and 2.74 for unpaired analyses. Maximum t values for specific voxels within clusters ranged as high as 15.
In addition to determining the effect of procaine on rCBF within each group (addicted subjects or comparison subjects), we computed the voxels whose response to cocaine differed between groups (a measure of the group-by-procaine interaction). Note that such direct comparisons between the cocaine-dependent subjects and the comparison subjects demonstrate only relative differences between the two groups. Thus, a relative increase in rCBF after procaine infusion in the cocaine-addicted subjects compared to the healthy subjects may suggest increased regional activation in response to procaine in the addicted subjects and no response in the comparison subjects, no response to procaine in the addicted subjects and a decreased response in the comparison subjects, or any combination of responses in between (see Kowatch et al. [22]).
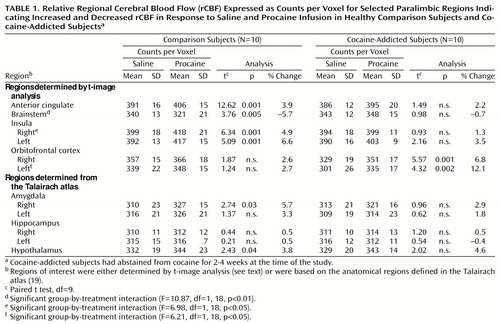
We also wished to compare rCBF changes identified by t-image analysis in one group (e.g., the cocaine-addicted subjects) to possible changes in the other group that were not evident on that group’s t images. For this purpose, cluster boundaries for six specific regions were identified (anterior cingulate, brainstem, right and left insula as identified in the comparison subjects, and right and left orbitofrontal cortex as identified in the addicted subjects), and the average counts per voxel within each cluster were determined for each subject in each condition. These values were then submitted to paired t analysis for both groups to identify whether significant changes within that region might have occurred in a group even though such changes were not significant in the voxel-based analyses.
Finally, we had a priori hypotheses concerning specific limbic or paralimbic structures. Some of these structures were not identified by t image analysis as having significant changes in either group. To further explore possible rCBF alterations in these regions after procaine administration, we used the Talairach atlas (19) to determine the anatomic boundaries of five structures (right and left amygdala, right and left hippocampus, and hypothalamus) and transposed those boundaries onto each subject’s images, extracting average counts per voxel within each of these structures for both conditions. Again, these values were submitted to paired t analysis for both groups to identify whether significant changes within that region might have occurred in a group even though such changes were not significant in the voxel-based analyses.
Subjective responses to placebo and procaine
Because the assumptions of parametric statistics (i.e., normality, homogeneity of variance) were not met, nonparametric statistics were used to examine within- and between-group differences in data from the Drug Assessment Questionnaire and the SCL-90-R. The Wilcoxon signed ranks test was used for within-group comparisons, and the Mann Whitney U Test was used for between-group comparisons.
Correlation analyses comparing changes in rCBF to subjective measures were conducted with the two different sources of rCBF values described in the previous section (t-image-based regions and anatomically drawn regions). Percent change in rCBF ([{rCBF for procaine – rCBF for placebo}/rCBF for placebo] × 100) was calculated for each region and regressed against subjective responses to procaine (all subjects) or against lifetime days of cocaine use (the cocaine-addicted subjects only). Extrinsic variables for both the cocaine-dependent subjects and the comparison subjects included the SCL-90-R anxiety score and responses to the third and fifth questions (“bad effect” and “dislike,” respectively) of the Drug Assessment Questionnaire; for the addicted subjects only, total days of and dollars spent on cocaine use were also included among the extrinsic variables.
Results
Within-Group Comparisons
Table 1 and Figure 1 show results of selected within-group comparisons of rCBF after procaine infusion, compared with rCBF after placebo infusion. Because the degrees of freedom for the t-image analyses were very large (df>20,000), the uncorrected p was less than 0.0001 for our omnibus cutoff of p<0.01. Thus, although each voxel in significant clusters had a unique t value, the minimum t value for significant voxels reported here was 2.43 for paired analyses and 2.74 for unpaired analyses; t values ranged as high as 15. The healthy comparison subjects showed significant increases (paired t image, p<0.01) in rCBF after procaine in the anterior cingulate, bilateral insula, hypothalamus, two small right inferior frontal lobe sites (Brodmann’s areas 10 and 47), and two right mesial/inferior temporal lobe sites (amygdala or Brodmann’s area 34, and Brodmann’s area 38). Decreases were noted in the right posterior parietal (Brodmann’s area 39), right lateral temporal (Brodmann’s area 37), bilateral occipital (Brodmann’s area 18), brainstem, and bilateral cerebellar regions. The cocaine-addicted subjects showed no activation of the right and only a small area of activation of the left insula after procaine infusion, but showed dramatically increased rCBF in the bilateral orbitofrontal cortex (Brodmann’s areas 11 and 47 on the right; Brodmann’s areas 11 and 25 on the left). Additional areas of increased rCBF were seen in the right parietal lobe (white matter adjacent to Brodmann’s area 40) and in the left lateral temporal (Brodmann’s area 21) and right inferior temporal (Brodmann’s area 38) cortices. Reduced rCBF was noted subcortically in an area between the left striatum and thalamus (overlapping putamen) and in the left thalamus and cortically in the right lateral parietal cortex (Brodmann’s areas 40 and 39), left hippocampus, right inferior occipital cortex (Brodmann’s areas 30 and 18), and left superior occipital cortex (Brodmann’s area 19).
Between-Group Comparisons
After saline infusion, large areas of lower relative rCBF in the cocaine-addicted subjects than in the comparison subjects (unpaired t image, p<0.01) were observed in the bilateral orbitofrontal (Brodmann’s areas 10 and 11) and right inferior temporal (Brodmann’s areas 20, 21, and 38) lobes (Figure 2). Higher relative rCBF in the cocaine-dependent subjects was observed in bilateral cerebellum and the right occipital lobe (Brodmann’s area 17) and in smaller areas in the left putamen, brainstem, and thalamus and in an area slightly superior to the right insula/putamen.
A comparison between groups of the change in rCBF with procaine infusion (rCBF in response to procaine – rCBF in response to saline) showed that the cocaine-addicted subjects had a relatively greater increase in rCBF than the comparison subjects primarily in the right orbitofrontal cortex as well as in the brainstem and the right midtemporal, midfrontal, and parietal cortex. Areas of relatively greater increase in rCBF in the comparison subjects relative to the addicted subjects were observed in the right insula and left hippocampal gyrus.
Region-of-Interest Analysis of the Orbitofrontal Region
T images revealed marked differences in orbitofrontal rCBF between groups after both saline and procaine challenges. Therefore, we did post hoc comparisons (see Methods) using regions whose boundaries were based on the right and left orbitofrontal clusters identified in the t-image analysis comparing rCBF between procaine and saline in the addicted subjects. Within-group comparisons (paired t tests) showed that the addicted subjects had significantly higher rCBF in both the right (t=5.57, df=9, p<0.0004) and left (t=4.32, df=9, p<0.002) orbitofrontal lobes after procaine infusion compared to placebo, confirming the t image results. As expected, the comparison subjects showed no difference in this analysis (t=1.87, df=9, n.s., and t=1.24, df=9, n.s., respectively). Between-group comparisons (unpaired t tests) showed that the addicted subjects had a significantly lower rCBF response after saline infusion in both the right (t=3.70, df=18, p<0.002) and left (t=3.57, df=18, p<0.003) orbitofrontal regions, compared with the healthy subjects. The difference between groups in the change in rCBF (rCBF in response to procaine – rCBF in response to saline) was also significantly different for both the right (t=2.23, df=18, p<0.04) and left (t=2.56, df=18, p<0.03) orbitofrontal lobes (Table 1).
Procaine-Induced rCBF and Lifetime Cocaine Use
In the cocaine-addicted subjects, there was a strong, although not statistically significant, relationship between the number of days of cocaine use and the percent change in rCBF (rCBF in response to procaine – rCBF in response to saline infusion) only for the right orbitofrontal cortex (r=0.55, df=9, p<0.09).
Subjective Responses to Procaine
Within-group comparisons found that both the healthy comparison subjects (N=10) and the cocaine-addicted subjects (N=10) endorsed a significant “drug effect” of procaine compared to saline (mean=5.7, SD=0.5, z=–2.92, p=0.004, and mean=5.1, SD=1.0, z=–2.84, p=0.005, respectively), a significant “bad effect” of procaine (mean=5.4, SD=0.7, z=–2.86, p=0.004, and mean=3.0, SD=2.1, z=–2.55, p<0.02), and a significant “dislike” of procaine (mean=5.8, SD=0.4, z=–2.41, p<0.003, and mean=3.4, SD=2.8, z=–2.97, p<0.02). The cocaine-addicted subjects also reported a significant “good effect” after procaine infusion (mean=1.9, SD=2.2, z=–2.21, p<0.03). After procaine infusion, the healthy comparison subjects reported higher levels of “bad effect” compared to the cocaine-addicted subjects (z=–2.92, p<0.003, N=20). The cocaine-addicted subjects reported that the effect of procaine was only slightly similar to the effect of cocaine (mean=2.1 on a scale from 0, not similar, to 6, strongest level of similarity, SD=2.0).
Basal measures of SCL-90-R were very low in both groups before each study session, suggesting the absence of anticipatory anxiety. There were no significant within- or between-group differences after saline infusion. Compared to saline (Δprocaine response – Δsaline response), procaine administered to the comparison subjects (N=10) produced significant increases in scores for somatization (mean increase=1.33, SD=0.54, z=–2.80, p<0.005), obsessive-compulsive symptoms (mean increase=0.40, SD=0.35, z=–2.40, p<0.02), interpersonal sensitivity (mean increase=0.16, SD=0.16, z=–2.21, p<0.03), depression (mean increase=0.40, SD=0.41, z=–2.40, p<0.02), and anxiety (mean increase=1.73, SD=0.72, z=–2.80, p<0.005) and in the positive symptom total score (frequency of response) (mean increase=22.10, SD=9.53, z=–2.80, p<0.005), positive symptom distress index (severity of response) (mean increase=1.71, SD=0.14, z=–2.80, p<0.005), and global severity index (severity × frequency of response) (mean increase=0.55, SD=0.26, z=–2.80, p<0.005). After procaine infusion, compared to saline, the cocaine-addicted subjects (N=10) reported significant increases in somatization (mean increase=0.58, SD=0.56, z=–2.80, p<0.005), obsessive-compulsive symptoms (mean increase=0.40, SD=0.33, z=–2.67, p<0.02), depression (mean increase=0.42, SD=0.46, z=–2.67, p<0.008), anxiety (mean increase =1.07, SD=0.74, z=–2.67, p<0.008), and psychoticism (mean increase=0.33, SD=0.46, z=–2.12, p<0.04) and in the positive symptom total score (mean increase=18.90, SD=11.08, z=–2.67, p<0.008), positive symptom distress index (mean increase=0.74, SD=0.86, z=–2.80, p<0.005), and global severity index (mean increase=0.42, SD=0.35, z=–2.80, p<0.005). In between-group comparisons, the healthy subjects reported higher levels of somatization (z=–2.57, p<0.005, N=20) and a higher positive symptom distress index (z=–2.42, p<0.02, N=20) than the cocaine-addicted subjects after procaine exposure.
Change in rCBF and Subjective Response to Procaine
The healthy comparison subjects demonstrated positive relationships between aversive subjective response (responses to the third question [“bad effect”] and fifth question [“disliked the effect”] on the Drug Assessment Questionnaire) and the percent change in rCBF (rCBF in response to procaine – rCBF in response to saline) in the left (r=0.82, df=9, p=0.004) and right (r=0.59, df=9, p=0.07) orbitofrontal regions and between the SCL-90-R anxiety score and the percent change in rCBF in the anterior cingulate (r=0.67, df=9, p=0.03). In sharp contrast, the cocaine-addicted subjects showed an inverse correlation between the aversive response to procaine and percent change in rCBF in the left orbitofrontal (r=–0.71, df=9, p=0.02), left hippocampal (r=–0.63, df=9, p<0.05), and left amygdalar (r=–0.56, df=9, p<0.09) regions as well as between procaine-induced anxiety and percent change in rCBF in the right hippocampus (r=–0.67, df=9, p<0.03) and brainstem (r=–0.75, df=9, p<0.01).
Discussion
Our findings demonstrate that 1) cocaine-addicted subjects show significantly increased rCBF after procaine infusion in different limbic areas than do healthy subjects; 2) cocaine-addicted subjects, compared to healthy subjects, exhibit marked differences in rCBF after placebo administration, presumably reflecting basal differences; and 3) relative to healthy comparison subjects, addicted subjects show decreased rCBF after placebo infusion and increased rCBF after procaine infusion in the orbitofrontal region in particular. We hypothesize that the alterations in orbitofrontal activity are most consistent with the “sensitization hypothesis” and may play a role in the addictive process.
Both the cocaine-addicted subjects and the comparison subjects demonstrated limbic and paralimbic activation after administration of procaine, although specific areas of rCBF activation differed between the two groups. Confirming the findings of previous studies (14, 15, 23), we found that the healthy comparison subjects demonstrated significant relative within-group rCBF increases in the anterior cingulate, insular cortex, hypothalamus, and right amygdala after procaine infusion, compared to rCBF after saline, with few areas of relative deactivation (the brainstem being the most noteworthy). We did not find orbitofrontal activation after procaine administration in the comparison subjects, a finding similar to that of Ketter et al. (14), but not that of Servan-Schreiber et al. (23). In contrast, the cocaine-addicted subjects demonstrated marked bilateral orbitofrontal activation after procaine administration, but increased activation in the anterior cingulate, bilateral insular cortex, or right amygdala was not found. When the within-group changes in rCBF from saline to procaine were directly compared between group with voxel-by-voxel analyses, right orbitofrontal and brainstem rCBF after procaine infusion was significantly greater in the addicted subjects than in the comparison subjects, whereas right insular activation was lower in the addicted subjects than in the comparison subjects. Post hoc analyses using region-of-interest methods demonstrated significantly greater change in rCBF after procaine infusion in both the right and left orbitofrontal lobes in the addicted subjects compared to the healthy subjects.
Decreased relative rCBF in the cocaine-addicted subjects’ bilateral orbitofrontal and right temporal regions after administration of placebo may be due to group differences in the response to placebo or to genuine differences in resting state rCBF. Others have reported similar (although smaller) areas of decreased rCBF in abstinent cocaine-addicted subjects at rest (24, 25). Our larger areas of differences may be a result of our tight exclusion criteria and the confined period of abstinence required (2–4 weeks abstinence) and may also be related to differences in neuroimaging methods and analyses.
Our findings, therefore, suggest that bilateral orbitofrontal rCBF in cocaine-addicted subjects is decreased after placebo infusion and increased after procaine, compared to that in healthy subjects. Coupled with the observation that days of lifetime cocaine use positively correlated with right orbitofrontal activation (although this relationship was not statistically significant), these preliminary observations suggest that the orbitofrontal area may be “sensitized” after persistent cocaine use. Volkow and Fowler (26) recently noted that the decreased basal activity, with heightened stimulation following provocation, in the orbitofrontal cortex of cocaine-addicted subjects is similar to that observed in animal models of kindling (27, 28) and temporal lobe epilepsy (29, 30) (e.g., interictal hypoperfusion and ictal hyperperfusion).
The suggestion that there is sensitization of the orbitofrontal regions, and not other limbic areas, is intriguing. Modell et al. (31) earlier discussed the potential role of a frontothalamic neuronal loop in craving, and several other investigators have recently reviewed the potential relevance of the orbitofrontal cortex to the addictive process (26, 32, 33). The orbitofrontal region integrates and modulates the internal response to the external environment, establishing a motivational value of a stimulus on the basis of an estimation of its potential reward. When the orbitofrontal cortex is lesioned, perseveration occurs because of a lack of error detection (34). Unrewarded or punished behaviors, therefore, are not extinguished. On the other hand, a pathologically activated orbitofrontal cortex is associated with obsessive-compulsive behaviors (35). These findings suggest a pattern of orbitofrontal dysfunction in which attenuation of orbitofrontal activity at rest is associated with an impulsive, “automatic” pattern of relapse (36), whereas an environment stimulating orbitofrontal activation, perhaps through conditioned stimuli, would induce relapse by the induction of obsessive craving (37). Neuroimaging studies, for example, have demonstrated activation of the orbitofrontal cortex during craving in cocaine-addicted subjects (38–40).
Activation of the orbitofrontal and/or other limbic regions is observed during obsessive stimulation in patients with obsessive-compulsive disorder (41) as well as during other anxiety-inducing paradigms in various populations (42–45). With respect to procaine, we and others (14, 23) have described positive correlations between procaine-induced fear or anxiety and increases in limbic rCBF. The cocaine-addicted subjects, however, showed an absent or inverse correlation between limbic rCBF activation and subjective responses. Left orbitofrontal, hippocampal, and brainstem rCBF were all inversely correlated with aversion or anxiety in the addicted subjects. These findings suggest that heightened visceral and affective arousal associated with limbic activation is not correctly interpreted as a primitive danger warning. The inverse relationship between regional brain activation and negative affect in the cocaine-addicted subjects may suggest an active inhibition of brain circuitry involved in aversion, allowing drug taking to persist despite negative life events.
Several methodologic issues may limit the significance of our findings. All subjects were administered saline first. Since the effects of procaine are profound, administering procaine first would have forewarned the subjects that the second infusion would be saline, thus breaking the blind and removing any potential placebo effect of the second (saline) infusion. To our knowledge, all subjects remained blinded to the order in which the substances were administered. Our measure of previous “sensitizing” episodes—self-reported days of cocaine use—is a relatively crude and possibly inaccurate measure, but it is probably the best there is. Furthermore, magnetic resonance imaging scans were not obtained, so we were unable to coregister SPECT images with anatomical structures. We do not believe that this is a severe limitation, because the literature suggests that, at most, small lacunae would have been expected in a population such as the one from which the study subjects were drawn (46), particularly given their lack of symptoms of stroke or transient ischemic attack. The extensive placebo-state orbitofrontal deficits and subsequent procaine-induced activation are therefore unlikely to be related to any consistent within-group structural abnormalities. Limits of spatial resolution and the ability to determine only relative measures of limbic activation were inherent in SPECT methods and the camera we used. However, many of the reported differences were quite large and well within these limits of resolution, and previous studies of limbic-induced procaine activation with positron emission tomography have not demonstrated marked differences in relative versus absolute measures of rCBF (14). Thus, these limitations do not significantly detract from our findings. As others have reported gender differences in cerebral perfusion (47), our results may not generalize to female subjects with cocaine addiction. Finally, group differences may reflect premorbid genetic or environmental differences rather than the results of chronic cocaine use.
Our findings suggest partial, and perhaps more realistic, support for the sensitization hypothesis. Our findings of altered rCBF in the orbitofrontal region after administration of both placebo and procaine, coupled with the altered relationship between subjective responses and rCBF and the importance of this region in decision making and impulsivity, suggest that the orbitofrontal region should be a more focused target for future studies exploring cocaine addiction. Demonstrating a meaningful clinical connection between the development and/or expression of the addictive process and orbitofrontal disruption will be a critical step in assessing the role of this region.
 |
Presented at the 31st Winter Conference on Brain Research, Snowbird, Utah, Jan. 24–31, 1998, the New York Academy of Sciences Conference, Charlottesville, Va., Oct.18–21, 1998, the 54th Convention of the Society of Biological Psychiatry, Washington, D.C., May 13–15, 1999, the 39th annual meeting of the American College of Neuropsychopharmacology, San Juan, P.R., Dec. 10–14, 2000, and the 34th annual Winter Conference on Brain Research, Steamboat Springs, Colo., Jan. 20–24, 2001. Received April 26, 2000; revision received Sept. 26, 2000; accepted Oct. 3, 2000. From the Departments of Psychiatry and Radiology and the Nuclear Medicine Center, University of Texas Southwestern Medical Center, Dallas; the VA North Texas Health Care System, Dallas; the Departments of Psychiatry, Radiology, and Neurology, Medical University of South Carolina, Charleston; and the Ralph H. Johnson VA Medical Center, Charleston, S.C. Address reprint requests to Dr. Adinoff, 116A5, Dallas VAMC, 4500 S. Lancaster Rd., Dallas, TX 75216; [email protected] (e-mail). Supported by grants DA10218 and DA11434 from the National Institute on Drug Abuse and by the Sarah and Charles E. Seay Center for Research in Psychiatric Illness.The authors thank Mark Williams for assistance with statistics and Charlotte Tenebackand for assistance with the neuroimaging analysis.
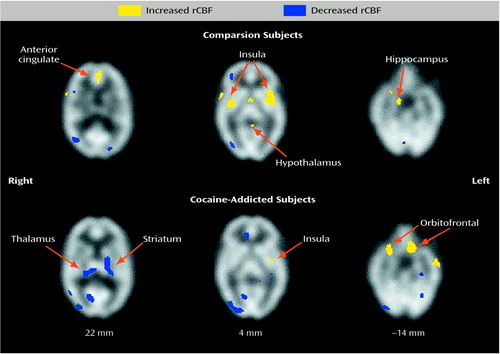
Figure 1. Areas of Increased and Decreased Regional Cerebral Blood Flow (rCBF) in Response to Procaine Infusion, Relative to the Response to Saline Infusion, in Healthy Comparison Subjects (N=10) and Cocaine-Addicted Subjects (N=10)a,b
aAreas of significant changes were identified by using paired t tests (p<0.01) for within-group means computed in voxel-by-voxel analyses of images obtained with single photon emission computed tomography. Regions of significant activation were corrected for the large number of t tests performed, the lack of independence between voxels, and the resolution of the processed images. The z coordinate in Talairach space (19) for each pair of transverse images is noted below the images.
bCocaine-addicted subjects had abstained from cocaine for 2-4 weeks at the time of the study.
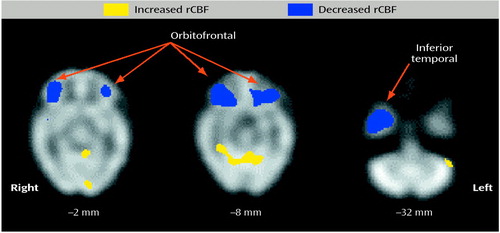
Figure 2. Areas in Which Regional Cerebral Blood Flow (rCBF) in Response to Placebo Infusion Was Higher or Lower in Cocaine-Addicted Subjects (N=10) Than in Healthy Comparison Subjects (N=10)a,b
aAreas of significant differences were identified by using unpaired t tests (p<0.01) for between-group means computed in voxel-by-voxel analyses of images obtained with single photon emission computed tomography. Regions of significant activation were corrected for the large number of t tests performed, the lack of independence between voxels, and the resolution of the processed images. The z coordinate in Talairach space (19) for each transverse image is noted below the image.
bCocaine-addicted subjects had abstained from cocaine for 2-4 weeks at the time of the study.
1. Grode J: Ueber die Wirkung langerer Cocainedarrechung bei Tieren. Arch Exp Pathol U Pharmakol 1912; 67:172–179Crossref, Google Scholar
2. Post RM, Kopanda RT: Cocaine, kindling and reverse tolerance. Lancet 1975; 1:409–410Crossref, Medline, Google Scholar
3. Stripling JS, Ellinwood EH Jr: Augmentation of the behavioral and electrophysiologic response to cocaine by chronic administration in the rat. Exp Neurol 1977; 54:546–564Crossref, Medline, Google Scholar
4. Marley RJ, Witkin JM, Goldberg SR: A pharmacogenetic evaluation of the role of local anesthetic actions in the cocaine kindling process. Brain Res 1991; 562:251–257Crossref, Medline, Google Scholar
5. Pascual-Leone A, Dhuna A, Anderson DC: Longterm neurological complications of chronic, habitual cocaine abuse. Neurotoxicology 1991; 12:393–400Medline, Google Scholar
6. Baxter LR Jr, Schwartz JM, Phelps ME, Mazziotta JC, Barrio J, Rawson RA, Engel J, Guze BH, Selin C, Sumida R: Localization of neurochemical effects of cocaine and other stimulants in the human brain. J Clin Psychiatry 1988; 49:S23–S26Google Scholar
7. Aronson TA, Craig TJ: Cocaine precipitation of panic disorder. Am J Psychiatry 1986; 143:643–645Link, Google Scholar
8. Louie AK, Lannon RA, Ketter TA: Treatment of cocaine-induced panic disorder. Am J Psychiatry 1989; 146:40–44Link, Google Scholar
9. Bartlett E, Hallin A, Chapman B, Angrist B: Selective sensitization to the psychosis-inducing effects of cocaine: a possible marker for addiction relapse vulnerability. Neuropsychopharmacology 1997; 16:77–82Crossref, Medline, Google Scholar
10. Satel SL, Edell WS: Cocaine-induced paranoia and psychosis proneness. Am J Psychiatry 1991; 148:1708–1711Google Scholar
11. Halikas JA, Kuhn KL: A possible neurophysiological basis of cocaine craving. Ann Clin Psychiatry 1990; 2:79–83Crossref, Google Scholar
12. Adamec RE, Stark-Adamec C, Saint-Hilaire JM, Livingston KE: Basic science and clinical aspects of procaine HCI as a limbic system excitant. Prog Neuropsychopharmacol Biol Psychiatry 1985; 9:109–119Crossref, Medline, Google Scholar
13. Ususbiaga JR, Wikinski J, Ferrero T, Usubiaga LE, Wikinski R: Local anesthetic induced convulsions in man: an electroencephalographic study. Anesth Analg Curr Res 1966; 45:611–620Crossref, Medline, Google Scholar
14. Ketter TA, Andreason PJ, George MS, Lee C, Gill DS, Parekh PI, Willis MW, Herscovitch P, Post RM: Anterior paralimbic mediation of procaine-induced emotional and psychosensory experiences. Arch Gen Psychiatry 1996; 53:59–69Crossref, Medline, Google Scholar
15. Parekh PI, Spencer JW, George MS, Gill DS, Ketter TA, Andreason P, Herscovitch P, Post RM: Procaine-induced increases in limbic rCBF correlate positively with increases in occipital and temporal EEG fast activity. Brain Topogr 1995; 7:209–216Crossref, Medline, Google Scholar
16. Sobell MB, Sobell LC: Behavioral Treatment of Alcohol Problems. New York, Plenum, 1978Google Scholar
17. Derogatis L, Kickels K, Rock A: The SCL-90 and the MMPI: a step in the validation of a new self-report scale. Br J Psychiatry 1976; 128:280–289Crossref, Medline, Google Scholar
18. Devous MS: SPECT functional brain imaging: technical considerations. J Neuroimaging 1995; 5:S2–S13Google Scholar
19. Talairach J, Tournoux P: Co-Planar Stereotaxic Atlas of the Human Brain. New York, Thieme Medical, 1988Google Scholar
20. Adler RJ: The Geometry of Random Fields. New York, John Wiley & Sons, 1981Google Scholar
21. Worsley KJ, Evans AC, Marrett S, Neelin P: A three-dimensional statistical analysis for CBF activation studies in human brain. J Cereb Blood Flow Metab 1992; 12:900–918Crossref, Medline, Google Scholar
22. Kowatch RA, Devous MD Sr, Harvey DC, Mayes TL, Trivedi MH, Emslie GJ, Weinberg WA: A SPECT HMPAO study of regional cerebral blood flow in depressed adolescents and normal controls. Prog Neuropsychopharmacol Biol Psychiatry 1999; 23:643–656Crossref, Medline, Google Scholar
23. Servan-Schreiber D, Perlstein WM, Cohen JD, Mintun M: Selective pharmacological activation of limbic structures in human volunteers: a positron emission tomography study. J Neuropsychiatry Clin Neurosci 1998; 10:148–159Crossref, Medline, Google Scholar
24. Flowers DL, Wood FB, Garrett AS, Porrino LJ, Keyes JW: Clusters of regional activation across time of abstinence in cocaine users. Abstracts of the Society for Neuroscience 1994; 20:221Google Scholar
25. Volkow ND, Hitzemann R, Wang G-J, Fowler JS, Wolf AP, Dewey SL, Handlesman L: Long-term frontal brain metabolic changes in cocaine abusers. Synapse 1992; 11:184–190Crossref, Medline, Google Scholar
26. Volkow ND, Fowler JS: Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex 2000; 10:318–325Crossref, Medline, Google Scholar
27. Engel J Jr, Wolfson L, Brown L: Anatomical correlates of electrical and behavioral events related to amygdaloid kindling. Ann Neurol 1978; 3:538–544Crossref, Medline, Google Scholar
28. Goodman JH, Homan RW: Interictal metabolic deficits in the kindling model of epilepsy (abstract). Epilepsia 1989; 25:660Google Scholar
29. Duncan R, Patterson J, Roberts R, Hadley DM, Bone I: Ictal/postictal SPECT in the pre-surgical localization of complex partial seizures. J Neurol Neurosurg Psychiatry 1993; 56:141–148Crossref, Medline, Google Scholar
30. Rowe C: Nuclear medicine in the management of patients with epilepsy, in Nuclear Medicine in Clinical Diagnosis and Treatment. Edited by Murray IPC, Ell PJ, Van der Wall H, Strauss HW. Edinburgh, Churchill Livingstone, 1998, pp 575–588Google Scholar
31. Modell JG, Mountz JM, Beresford TP: Basal ganglia/limbic striatal and thalamocortical involvement in craving and loss of control in alcoholism. J Neuropsychiatry Clin Neurosci 1990; 2:123–144Crossref, Medline, Google Scholar
32. London ED, Ernst M, Grant S, Bonson K, Weinstein A: Orbitofrontal cortex and human drug abuse: functional imaging. Cereb Cortex 2000; 10:334–342Crossref, Medline, Google Scholar
33. Porrino LJ, Lyons D: Orbital and medial prefrontal cortex and psychostimulant abuse: studies in animal models. Cereb Cortex 2000; 10:326–333Crossref, Medline, Google Scholar
34. Bechara A, Damasio H, Damasio AR: Emotion, decision making and the orbitofrontal cortex. Cereb Cortex 2000; 10:295–307Crossref, Medline, Google Scholar
35. Heinz A: Neurobiological and anthropological aspects of compulsions and rituals. Pharmacopsychiatry 1999; 32:223–229Crossref, Medline, Google Scholar
36. Tiffany ST: A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev 1990; 97:147–168Crossref, Medline, Google Scholar
37. Anton RF, Moak DH, Latham PK: The Obsessive Compulsive Drinking Scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol Clin Exp Res 1995; 19:92–99Crossref, Medline, Google Scholar
38. Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A: Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci USA 1996; 93:12040–12045Google Scholar
39. Maas LC, Lukas SE, Kaufman MJ, Weiss RD, Daniels SL, Rogers VW, Kukes TJ, Renshaw PF: Functional magnetic resonance imaging of human brain activation during cue-induced cocaine craving. Am J Psychiatry 1998; 155:122–126Link, Google Scholar
40. Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, Wong CT, Felder C: Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci 1999; 64:775–784Crossref, Medline, Google Scholar
41. Cottraux J, Gerard D, Cinotti L, Froment JC, Deiber MP, Le Bars D, Galy G, Millet P, Labbe C, Lavenne F, Bouvard M, Mauguiere F: A controlled positron emission tomography study of obsessive and neutral auditory stimulation in obsessive-compulsive disorder with checking rituals. Psychiatry Res 1996; 60:101–112Crossref, Medline, Google Scholar
42. Benkelfat C, Bradwejn J, Meyer E, Ellenbogen M, Milot S, Gjedde A, Evans A: Functional neuroanatomy of CCK4-induced anxiety in normal healthy volunteers. Am J Psychiatry 1995; 152:1180–1184Google Scholar
43. Rauch SL, Savage CR, Alpert NM, Fischman AJ, Jenike MA: The functional neuroanatomy of anxiety: a study of three disorders using positron emission tomography and symptom provocation. Biol Psychiatry 1997; 42:446–452Crossref, Medline, Google Scholar
44. Reiman EM, Lane RD, Ahern GL, Schwartz GE, Davidson RJ, Friston KJ, Yun L-S, Chen K: Neuroanatomical correlates of externally and internally generated human emotion. Am J Psychiatry 1997; 154:918–928Link, Google Scholar
45. Fredrickson M, Wik G, Annas P, Ericson K, Stone-Elander S: Functional neuroanatomy of visually elicited simple phobic fear: additional data and theoretical analysis. Psychophysiology 1995; 32:43–48Crossref, Medline, Google Scholar
46. Strickland TL, Mena I, Villanueva-Meyer J, Miller BL, Cummings J, Mehringer CM, Satz P, Myers H: Cerebral perfusion and neuropsychological consequences of chronic cocaine use. J Neuropsychiatry Clin Neurosci 1993; 5:419–427Crossref, Medline, Google Scholar
47. Levin JM, Holman BL, Mendelson JH, Teoh SK, Garada B, Johnson KA, Springer S: Gender differences in cerebral perfusion in cocaine abuse: technetium-99m-HMPAO SPECT study of drug-abusing women. J Nucl Med 1994; 35:1902–1909Google Scholar



