Resting Regional Cerebral Blood Flow and Gambling Task Performance in Cocaine-Dependent Subjects and Healthy Comparison Subjects
Abstract
OBJECTIVE: Orbitofrontal cortex regional cerebral blood flow (rCBF) is lower in cocaine-dependent subjects than in non-cocaine-dependent subjects. Performance on the Gambling Task, a test of decision making, is a putative correlate of orbitofrontal cortex activity and is reportedly impaired in drug-dependent subjects. The authors tested the hypothesis that lower Gambling Task scores would be associated with lower resting orbitofrontal cortex rCBF in cocaine-dependent subjects. METHOD: Fifteen healthy comparison subjects and 13 abstinent cocaine-dependent subjects underwent resting single photon emission computed tomography to measure rCBF, after which they completed the Gambling Task. RESULTS: Resting anterior cingulate and left dorsolateral prefrontal cortex rCBF significantly correlated with performance on the Gambling Task, but orbitofrontal cortex rCBF did not. Left dorsolateral prefrontal cortex rCBF was lower in the cocaine-dependent subjects than in the comparison subjects. CONCLUSIONS: Resting anterior cingulate and dorsolateral prefrontal cortex rCBF is significantly related to decision making, as assessed by the Gambling Task.
The orbitofrontal cortex is involved in such critical human functions as social adjustment and the control of mood, drive, and responsibility. Deficits in these functions are typified by persons with substance dependence disorders. “Automaticity,” an impulsive style of relapse in which the return to drug use is unaccompanied by a conscious awareness of craving (1), has been suggested as a relapse style likely to be associated with deficits in orbitofrontal cortex functioning. A role for the orbitofrontal cortex in the pathophysiology of drug dependence has been strengthened by findings of lower resting orbitofrontal cortex regional cerebral blood flow (rCBF) in cocaine-dependent subjects, compared with non-cocaine-dependent subjects (2, 3), as well as increased orbitofrontal cortex activation in drug-dependent subjects after cue-induced craving (4) or procaine infusion (2).
However, a direct connection between lower orbitofrontal cortex rCBF in subjects with cocaine dependence and behaviors associated with drug dependence has been lacking. We were therefore interested in exploring whether lower orbitofrontal cortex rCBF in cocaine-dependent subjects was associated with impairment in putative cognitive measures of orbitofrontal cortex functioning. The Gambling Task was chosen as the neurocognitive measure of orbitofrontal cortex functioning because it simulates real-life experiences involving uncertainty, reward, and punishment and because performance on this task has been shown to be impaired in patients with orbitofrontal cortex damage (5) and in drug-dependent subjects (6). We hypothesized that lower Gambling Task scores would be associated with lower resting orbitofrontal cortex rCBF in cocaine-dependent subjects.
Method
Thirteen cocaine-dependent subjects (12 men and one woman; mean age=41.2 years, SD=4.3) and 15 healthy comparison subjects (seven men and eight women; mean age=35.0 years, SD=6.3) were included in the study. The subjects underwent a thorough medical history interview and psychiatric and physical assessments (2). Past and present psychiatric history was assessed with the Structured Clinical Interview for DSM-IV (SCID). Approval for the study was obtained from the institutional review boards of both the University of Texas Southwestern Medical Center and the Department of Veterans Affairs North Texas Health Care System. Written informed consent was obtained from the subjects after the procedure had been fully explained, and the subjects were financially compensated for their participation.
The subjects with cocaine dependence (as diagnosed by SCID) were recruited from residential treatment programs at the Veterans Affairs Medical Center and Homeward Bound, Inc., in Dallas and had completed 21–55 days of monitored abstinence. Exclusion criteria included present use of any CNS-active medications, a lifetime history of an axis I psychiatric disorder unrelated to drug use, a substance use disorder (other than cocaine, nicotine, or caffeine use) within the previous 12 months, and a lifetime history of withdrawal from alcohol, sedative-hypnotics, or opioids. The comparison subjects were recruited by word of mouth and advertisement. The comparison subjects were physically and psychiatrically healthy, were not taking any medication with CNS activity, and had no lifetime history of an axis I disorder.
Study sessions took place at the Nuclear Medicine Center at the University of Texas Southwestern Medical Center in Dallas. After 30 minutes of rest after intravenous insertion, subjects received 20 mCi of [99mTc] hexamethylpropyleneamine oxime (HMPAO) (Nycomed/Amersham, Princeton, N.J.). A single photon emission computed tomography (SPECT) scan was obtained 90 minutes later. SPECT images were acquired, reconstructed, and analyzed as previously described (2). Regions of interest were identified by using t-image analysis (2). Omnibus thresholds of p<0.05, p<0.01, p<0.005, and p<0.001 were used to identify voxels that differed between groups. Counts per voxel were then determined for the identified regions of interest.
Immediately after the SPECT scan, subjects were administered a computerized version of the Gambling Task (6). The task is a card game that assesses the ability of subjects to evaluate immediate gains and long-term losses. Subjects are instructed that the task was to accumulate as much (play) money as possible by picking a card from any of four decks (A, B, C, and D). Decks A and B yield a larger short-term payoff than decks C and D, but the accumulated penalties in decks A and B are larger than in decks C and D. The optimal long-term strategy, therefore, is to choose more cards from decks C and D than from decks A and B in order to minimize overall loss in deference to more immediate short-term gains. Scores for the Gambling Task were obtained by subtracting individual scores for decks A and B from scores for decks C and D.
Results
Scores on the Gambling Task did not significantly differ between the cocaine-dependent subjects (mean=1.1, SD=29.9) and the comparison subjects (mean=13.7, SD=25.4) (t=0.85, df=26, n.s.). As expected, however, resting rCBF in the right (Talairach coordinates: x=38, y=40, z=–6) and left (x=–38, y=20, z=–8) orbitofrontal cortex was lower in the cocaine-dependent subjects than in the comparison subjects (unpaired t-image, p<0.005). There was no relationship between the counts per voxel in these two regions of interest and the Gambling Task score (right orbitofrontal cortex: r=0.13, df=27, n.s.; left orbitofrontal cortex: r=0.09, df=27, n.s.).
To examine whether rCBF in other regions was correlated with Gambling Task performance, we performed exploratory, post hoc t-image analysis using data for subjects who scored more than 0 (N=13) and less than 0 (N=13) on the Gambling Task (two subjects scored 0). At p<0.001, the anterior cingulate (Brodmann’s area 32; x=4, y=14, z=36) and left dorsolateral prefrontal cortex (Brodmann’s areas 6, 9, and 44; x=–40,y=–2, z=34) showed higher resting rCBF in the subjects with high Gambling Task scores than in those with low scores. Multiple regression analysis revealed that rCBF counts per voxel in these two regions of interest accounted for a significant amount of the variability in Gambling Task scores across groups (R2=0.44, F=6.19, df=3, 24, p=0.003) (Figure 1). The interaction of group and region of interest was not significant (R2=0.45, F=0.26, df=2, 22, n.s.), suggesting that Gambling Task performance is related to anterior cingulate and left dorsolateral prefrontal cortex rCBF irrespective of drug history. Neither region alone offered additional predictive power beyond that contributed by the two areas combined. This result may be partly accounted for by the high correlation between the counts per voxel for these two regions of interest (r=0.69, df=27, p<0.01). Of interest was a region of the left dorsolateral prefrontal cortex (Brodmann’s areas 9 and 44; x=–36, y=8, z=36) that was identified in the rCBF comparison (p<0.01) between the patients and the comparison subjects and that was in close proximity to the dorsolateral prefrontal cortex area identified earlier. The counts per voxel of the two left dorsolateral prefrontal cortex regions were highly correlated (r=0.86, df=27, p<0.5×10–10), and the counts per voxel of the latter dorsolateral prefrontal cortex region correlated with Gambling Task performance (r=0.51, df=27, p<0.005).
Discussion
Our findings do not support a relationship between resting orbitofrontal cortex rCBF and Gambling Task score, nor do they confirm previous reports demonstrating impaired performance on the Gambling Task in cocaine-dependent subjects. On the other hand, our exploratory analyses suggest that the anterior cingulate and left dorsolateral prefrontal cortex play an important role in Gambling Task performance. In addition, the high correlation between the counts per voxel for the anterior cingulate and dorsolateral prefrontal cortex suggests that the role of these two regions in Gambling Task performance is strongly integrated. The anterior cingulate is involved in problem solving and adaptive responses to changing conditions, and thus its involvement in Gambling Task performance is not unexpected. Given that Bechara et al. (5) have reported that subjects with lesions of the dorsolateral prefrontal cortex (a region critical for working memory) are not impaired in performance on the Gambling Task, the relationship between the dorsolateral prefrontal cortex and Gambling Task performance was not predicted. The presence of decreased resting dorsolateral prefrontal cortex rCBF in cocaine-dependent subjects (as previously observed by others [3]) and its relationship with Gambling Task performance suggest that cocaine-related changes in dorsolateral prefrontal cortex rCBF are associated with impaired decision making in this population.
The lack of differences in Gambling Task scores between the cocaine-dependent subjects and the comparison subjects in this study may be due to our inclusion of only medically healthy, abstinent cocaine-dependent individuals who had no other substance use or psychiatric disorders. Other explanations include the wide variability in the scores of the cocaine-dependent subjects, the small number of subjects, the higher number of women in the comparison group (6), the presence or absence of coexisting gambling problems, and/or the low Gambling Task scores in the comparison group relative to comparison subjects in other studies (5, 7).
The strong relationship between anterior cingulate and dorsolateral prefrontal cortex rCBF and Gambling Task performance supports the role of these regions, in addition to the orbitofrontal cortex, in decision making and impulsivity. The relevance of these regions (as assessed by rCBF), performance on neurocognitive measures of impulsivity and decision making, and treatment outcomes of drug-dependent subjects should be explored in future studies.
Presented at the 64th Annual Scientific Meeting of the College on Problems of Drug Dependence, Quebec, June 8–13, 2002. Received May 22, 2002; revision received Feb. 4, 2003; accepted March 14, 2003. From the Departments of Psychiatry, Radiology, Family Practice, and Neurology and the Nuclear Medicine Center, University of Texas Southwestern Medical Center, Dallas; the Department of Veterans Affairs North Texas Health Care System, Dallas; the Department of Physical Medicine and Rehabilitation, Baylor College of Medicine, Houston; and the Institute for Rehabilitation and Research, Houston. Address reprint requests to Dr. Adinoff, 116A5, Dallas Veterans Affairs Medical Center, 4500 S. Lancaster Rd., Dallas, TX 75216; [email protected] (e-mail). Supported by grants DA-10218 and DA-11434 from the National Institute on Drug Abuse. The authors thank Drs. Steven Grant and Antoine Bechara for conceptual and instructional assistance, and the staffs of the Dallas Veterans Affairs Medical Center Substance Abuse Team and Homeward Bound for assistance and support.
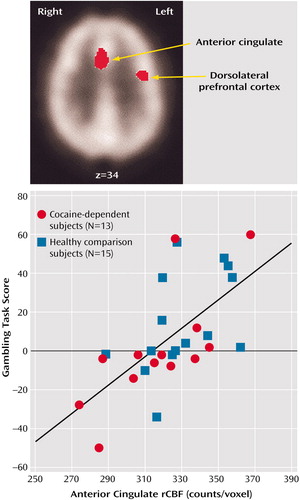
Figure 1. Areas of Increased Resting Regional Cerebral Blood Flow (rCBF) in Subjects With High Scores on the Gambling Taska and Total Scores on the Gambling Task of Cocaine-Dependent and Healthy Comparison Subjects, by Resting Anterior Cingulate rCBFb
aAreas of increased rCBF in high scorers (score >0) (N=13) relative to low scorers (score <0) (N=13) were identified by using unpaired tests (p<0.005) for between-group means computed in voxel-by-voxel analyses of images obtained with single photon emission computed tomography. The z coordinate in Talairach space is noted below the image. Both cocaine-dependent subjects and healthy comparison subjects were included in both the high-scorer and low-scorer groups.
bSignificant correlation between Gambling Task scores and resting anterior cingulate rCBF values (r=–0.64, df=27, p<0.0003).
1. Tiffany ST: A cognitive model of drug urges and drug-use behavior: role of automatic and nonautomatic processes. Psychol Rev 1990; 97:147–168Crossref, Medline, Google Scholar
2. Adinoff B, Devous MD Sr, Best SE, George MS, Alexander D, Payne K: Limbic responsiveness to procaine in cocaine-addicted subjects. Am J Psychiatry 2001; 158:390–398; correction, 158:1952Link, Google Scholar
3. Volkow ND, Hitzemann R, Wang G-J, Fowler JS, Wolf AP, Dewey SL, Handlesman L: Long-term frontal brain metabolic changes in cocaine abusers. Synapse 1992; 11:184–190Crossref, Medline, Google Scholar
4. Wang GJ, Volkow ND, Fowler JS, Cervany P, Hitzemann RJ, Pappas NR, Wong CT, Felder C: Regional brain metabolic activation during craving elicited by recall of previous drug experiences. Life Sci 1999; 64:775–784Crossref, Medline, Google Scholar
5. Bechara A, Damasio H, Tranel D, Anderson SW: Dissociation of working memory from decision making within the human prefrontal cortex. J Neurosci 1998; 18:428–437Crossref, Medline, Google Scholar
6. Reavis R, Overman WH: Adult sex differences on a decision-making task previously shown to depend on the orbital prefrontal cortex. Behav Neurosci 2001; 115:196–206Crossref, Medline, Google Scholar
7. Grant S, Contoreggi C, London ED: Drug abusers show impaired performance on a test of orbitofrontal function. Neuropsychologia 2000; 38:1180–1187Crossref, Medline, Google Scholar



