Yohimbine Challenge in Children With Anxiety Disorders
Abstract
OBJECTIVE: The authors evaluated the neurohormonal and subjective mood response of children with anxiety disorders who were challenged with yohimbine. METHOD: Seventeen children with DSM-IV diagnoses of anxiety disorders and 15 normal comparison children were given yohimbine orally (0.1 mg/kg). Neurohormonal measures and visual analog self-reports of tenseness were recorded over a 150-minute period. RESULTS: Yohimbine was uniformly well tolerated, and it behaviorally differentiated children with anxiety disorders from normal comparison children with higher maximum change (Δmax) ratings of anxiety in the patients (mean=17.4 mm, SD=29.8) than in the comparison subjects (mean=0.3 mm, SD=4.4). Yohimbine-stimulated Δmax growth hormone (GH) for children with anxiety disorders (mean=–1.5 ng/ml, SD=5.9) was significantly reduced compared to that of normal comparison children (mean=2.7 ng/ml, SD=4.5). CONCLUSIONS: Yohimbine selectively elevates self-rated anxiety in children with anxiety disorders and is associated with the blunting of GH in those children relative to that of comparison children. Presence of a blunted GH response to yohimbine in children with anxiety disorders is reminiscent of findings in adults with anxiety disorders, particularly panic disorder. These findings support enhanced central adrenergic sensitivity in children with anxiety disorders, as demonstrated by yohimbine-exacerbated anxiety. The findings should be reconciled with the absence of clonidine-related GH blunting in the same cohort.
Children suffer from generalized anxiety disorder, social phobia, obsessive-compulsive disorder (OCD), and panic disorder, which are recognizable by syndromal characteristics and phenomenology equivalent to those found in adult disorders and meet DSM-IV criteria (1, 2). Anxiety disorders persist throughout childhood (3, 4), may worsen over time (5), and may become essentially stable during early adolescence to young adulthood (6). Separation anxiety disorder, the most common anxiety disorder of childhood (7), often proceeds the onset of adult anxiety disorders, particularly panic disorder (8, 9). It is thought that a childhood history of separation anxiety disorder identifies a particularly heritable early-onset form of panic disorder (10). Evidence for childhood separation anxiety disorder as an antecedent to adult panic disorder includes 1) retrospective reports of childhood anxiety from adults with panic disorder (11); 2) longitudinal studies of childhood separation anxiety disorder documenting abnormalities of ventilatory physiology similar to those of adults with panic disorder and predicting the onset of panic attacks (12); and 3) the responsiveness of separation anxiety disorder to pharmacotherapies recommended for panic disorder (8). Alternative views of this proposed linkage cite the high prevalence rate of separation anxiety disorder coupled with selective recall among adults (13), such that separation anxiety disorder may be a nonspecific precursor to adult anxiety disorders (14). Neurobiologic diatheses of childhood anxiety disorders have recently been elucidated by using adult experimental paradigms following the most robust of findings in adults with panic disorder as a guide (12, 15). The most productive paradigms for this strategy, although controversial, have been either physiologic (e.g., CO2) (12) or pharmacologic (e.g., clonidine) challenges in children with anxiety disorders (15) uncovering biologic abnormalities implicated in adult panic disorder. The noradrenergic system, central to the monitoring and interpretation of suffocation cues (16) in both adult panic disorder and childhood anxiety disorders, exhibits heightened sensitivity to noradrenergic agents (17, 18). This enhanced sensitivity suggests dysregulation of noradrenergic pathways and may play a role in the pathophysiology of childhood anxiety disorders and may be a biological precursor to panic disorder.
Preclinical (19) and clinical studies (20–22) have provided evidence of dysregulation in the noradrenergic pathway in the development of anxiety disorders. Stimulation of central a2 adrenergic receptors with clonidine normally results in elevated growth hormone (GH) output, but this response appears to be blunted in adults with panic disorder (20), generalized anxiety disorder (23), and perhaps OCD (24). The response of patients with anxiety disorders to a clonidine challenge is cited as evidence of the subsensitivity of central a2 adrenergic postsynaptic receptors. This subsensitivity is potentially the result of long-term locus ceruleus activity, which, over time, leads to a down-regulation of the hypothalamic a2 adrenergic postsynaptic receptors that mediate GH release. Our laboratory was the first to demonstrate that a clonidine challenge in children with anxiety disorders is not characterized by GH blunting (15). This finding implies that the early onset of anxiety disorders may not involve a2 adrenoceptor down-regulation and argues for further exploration.
Yohimbine, an a2 adrenergic antagonist, increases the release of norepinephrine in the hippocampus and other brain areas through increased firing of the locus ceruleus, resulting in behavioral and biologic correlates of anxiety in patients with panic disorder. Interpretation of data from yohimbine challenges is complicated by mixed drug effects due to blockade of both pre- and postsynaptic a2 adrenergic receptors in the hypothalamus and presynaptic D2 dopaminergic autoreceptors in the pituitary (25, 26). The quick onset and limited duration of yohimbine effects on plasma concentrations of the norepinephrine metabolite 3-methoxy-4-hydroxyphenylglycol (MHPG), diastolic blood pressure, and patient ratings of anxiety suggest that the sum total of effect is largely reflective of presynaptic norepinephrine activity. It is hypothesized that diminished presynaptic sensitivity of the a2 adrenergic autoreceptor is related to presynaptic norepinephrine hyperactivity (evidenced by increased sensitivity to yohimbine) (27). Yohimbine in children with anxiety disorders should be consistent with presynaptic norepinephrine overactivity, but to our knowledge, such studies are absent from the literature.
Response to a norepinephrine challenge may vary not only by anxiety diagnosis but also by developmental stage. Pine et al. (18) performed clonidine challenges in a heterogenous group of children with anxiety disorders and found enhanced MHPG response, the opposite of exaggerated decreases in MHPG reported in adult panic disorder. Sallee et al. (15), using the same challenge in a similar population, documented hypersecretion of GH in relation to normal age-matched comparison subjects where blunting would be expected in adults. Mindful of developmental influences, we set out to evaluate children with anxiety disorders who had previously been characterized by an intravenous clonidine challenge (15) by means of oral yohimbine and in comparison to age-matched comparison children. Although the importance of adrenergic dysregulation is assumed to vary across adult anxiety disorders (i.e., panic disorder versus OCD), for this exploratory study, no a priori assumptions were made, and broad inclusion criteria covering many common childhood anxiety disorders were used. Separate analyses across childhood anxiety disorders were then performed to determine the importance of norepinephrine sensitivity in the pathogenesis of specific disorders of interest (i.e., separation anxiety disorder). The hypothesis to be tested was whether children with anxiety disorders would demonstrate yohimbine responses similar to those of adults with anxiety disorders (i.e., panic disorder), either by enhanced self-ratings of anxiety and/or attenuated neurohormonal output (e.g., GH blunting).
Method
Subjects
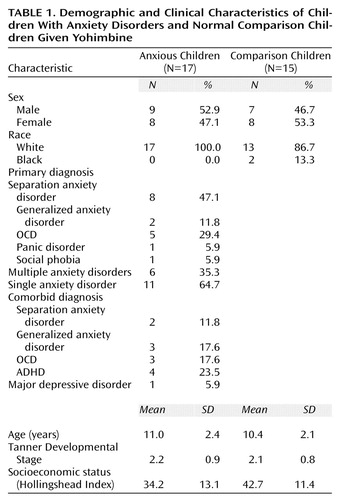
Children seen at a tertiary psychiatric outpatient service of the Medical University of South Carolina from 1994 to 1996 with a primary DSM-IV diagnosis of an anxiety disorder (N=17) were matched by sex and age to normal comparison children (N=15) who were chosen from a general clinical research center pool from which they had been selected for use as comparison subjects for metabolism and endocrinologic evaluations. Patients with anxiety disorders represented a subset of children (17 of 24) who were previously evaluated by means of a clonidine challenge (15). Demographic characteristics of children with anxiety disorders and comparison children are presented in Table 1. Patients received a structured clinical interview (Schedule for Affective Disorders and Schizophrenia for School-Age Children) modified for enhanced recognition of anxiety disorders (28), which involved both parent and child interviews. Normal comparison subjects received a brief screening for lifetime psychiatric disorders. Comparison subjects were physically healthy and without anxiety, depression, or substance abuse and had a negative family history for affective or anxiety disorders. Diagnoses were derived from all available clinical information by consensus between a clinical psychologist and child psychiatrist.
Inclusion criteria were as follows: 1) male and female outpatients ranging in age from 7 to 14 years; 2) DSM-IV primary diagnosis of an anxiety disorder (e.g., separation anxiety disorder, panic disorder, generalized anxiety disorder, OCD, social phobia); 3) normal baseline laboratory results, negative urine drug screening, and normal physical examination; and 4) medication free for at least 2 weeks before the challenge (4 weeks for fluoxetine).
Exclusion criteria were as follows: 1) diagnosis of primary posttraumatic stress disorder or specific phobia; 2) meeting DSM-IV criteria for bipolar disorder, schizophrenia, anorexia or bulimia nervosa, or autism; 3) currently (within 6 months) known to abuse or to be dependent on any drug, including alcohol, or testing positive on a urine drug screening; 4) obese (weight for height greater than 95% on the National Center for Health Statistics curve) or severely malnourished (weight for height less than third percentile); or 5) unable to complete the required self-report measures or visual analog mood scale ratings.
Parents and/or guardians gave consent, and the children gave their assent in written form, as approved by the institutional review board of the Medical University of South Carolina, before study participation.
Assessments
Patients and comparison subjects received at baseline a battery of mood and anxiety ratings, including the Hamilton Anxiety Rating Scale (29), the Revised Children’s Manifest Anxiety Scale (30), the Childhood Anxiety Sensitivity Index (31), and the Fear Survey Schedule for Children (32). Subjects at baseline also received a physical examination (including Tanner staging) and an ECG. Immediately before and during the yohimbine challenge, children were instructed to complete a visual analog rating of mood states. The visual analog measure of mood states has the subject place a mark on a 100-millimeter line at a point between two opposing descriptors (i.e., not at all anxious to extremely anxious). Scores are measured in millimeters and range from 0 to 100. The measures of visual analog mood states used in this study were happy/cheerful, calm/relaxed, sad/depressed, energized/restless, and tense/anxious. The visual analog measure of mood states is highly reliable over short time frames, with correlations of repeated baseline measures ranging from 0.69 to 0.82 (p<0.0001) (15).
Yohimbine Challenge
Children were admitted to a general clinical research center and maintained on a low monoamine diet for standardization before the initiation of the study. Meals were standardized and served at noon and 6:00 p.m. Activity was limited to bed rest during yohimbine administration, from 2:00 p.m. to 4:30 p.m. The evening before the yohimbine challenge, a 20–22-gauge cannulae was inserted into the child’s nondominant arm. Beginning at 2:00 p.m. and at 30-minute intervals through 4:30 p.m., 4.5 cc of blood was sampled (four samples) for hormonal analysis. At the same times, heart rate and blood pressure were recorded. Children completed the visual analog measure of mood states at 2:30 p.m. (just before yohimbine administration) and at 60, 90, and 120 minutes (after yohimbine administration). At 2:30 p.m., in the attendance of a parent or guardian, children were orally administered yohimbine, 0.1 mg/kg, in tablet form with 100 cc water. Dose was to the nearest 2.7 mg, rounding up to a maximum dose of 8.1 mg.
Hormone Determinations
Neuroendocrine blood samples were taken with disposable polypropylene syringes and immediately transferred to EDTA-containing tubes on ice. Those samples were transferred within 5 minutes to a polypropylene tube containing 650 trypsin inhibitor units of aprotinin and centrifuged at 1000 g for 5 minutes at 4°C; the plasma was transferred to a 1.5 ml tube containing n-ethylmaleimide and stored at –70°C until assay. (Aprotinin and n-ethylmaleimide were provided by Sigma, St. Louis). Plasma prolactin, GH, and cortisol were all obtained by using commercially available reagents from Nichols Institute Diagnostics (San Juan Capistrano, Calif.) by means of a chemiluminescence immunometric procedure. High, low, and mid-range external controls from Bio-Rad (Hercules, Calif.) were used in each run. The sensitivities of the prolactin, GH, and cortisol assays were 0.1 ng/ml, 0.02 ng/ml, and 0.8 mg/dl, respectively. The intraassay variabilities for prolactin, GH, and cortisol were 4%, 5%, and 8%, respectively; the interassay variances were 7.5%, 8.3%, and 7.8%, respectively.
Data Analysis
The visual analog measures of mood states and physiological and hormone data were analyzed by means of repeated measures analysis of variance (ANOVA) evaluating the effects of group, time, and interaction of group and time (incorporating the Greenhouse-Geisser adjustment for autocorrelation [33]). This analysis was applied directly to the postyohimbine change scores from baseline. In addition, for the visual analog measures of mood states and hormonal values, the index of maximum change (Δmax) from baseline was compared by using one-way ANOVA. Baseline for all analyses was considered as the last value collected before the administration of yohimbine. Type 1 errors for all analyses were controlled at 5%. Also, Bonferroni corrections for multiple comparisons (34) were used for repeated measures ANOVA when the groups were broken down by primary diagnostic category. The Newman-Keuls test for multiple comparisons (35) was used when comparing Δmax scores between groups broken down by primary diagnosis.
Results
Subject Baseline
The group with anxiety disorders, largely composed of children with a primary diagnosis of separation anxiety disorder, was similar to the group of comparison subjects in age, sex ratio, racial composition, and pubertal status (Table 1). The comparison group, however, scored higher on the Hollingshead Index of socioeconomic status (mean=42.7, SD=11.4) than the group with anxiety disorders (mean=34.2, SD=13.1), although this difference did not approach statistical significance. Distinguishing features between the children with anxiety disorders and comparison children were primarily the clinical and self-report measures of anxiety (Table 2). The clinician-rated Clinical Global Impression (CGI) of anxiety severity and Hamilton anxiety scale scores differed markedly between children with anxiety disorders and comparison children. Self-ratings on the Revised Children’s Manifest Anxiety Scale also differentiated the two groups. Other self-ratings such as the Fear Survey Schedule for Children and the Childhood Anxiety Sensitivity Index, although elevated in the children with anxiety disorders, failed to approach statistical significance. Physiologic measures such as resting heart rate and blood pressure were not significantly different between groups. Baseline morning hormone plasma concentrations were not different between the groups for any hormone. Prolactin level for children with anxiety disorders (mean=7.7 ng/ml, SD=5.2) was comparable to that of comparison children (mean=7.6 ng/ml, SD=5.1). There was also no significant difference in baseline cortisol level for comparison children (mean=6.5 mg/ml, SD=2.8) compared to children with anxiety disorders (mean=9.1 mg/ml, SD=8.4). GH plasma concentration for comparison children (mean=1.9 ng/ml, SD=2.9) was also similar to that of children with anxiety disorders (mean=2.0 ng/ml, SD=3.4).
Yohimbine Challenge
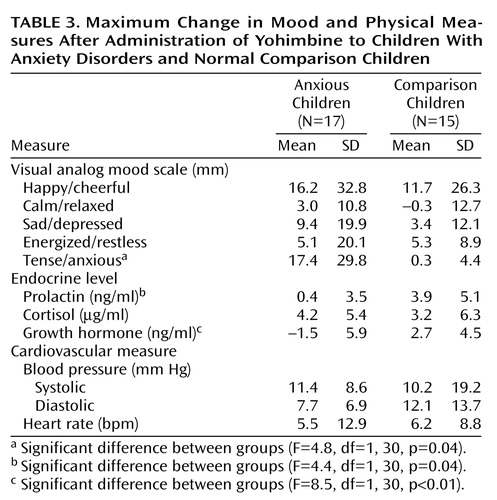
The mean administered dose of yohimbine was 4.4 mg (SD=1.6), with a median dose of 5.4 mg. Five subjects (three patients and two comparison subjects) experienced adverse events of dizziness (N=2) and stomachache (N=4) attributed to yohimbine. No child experienced a panic attack, but children with anxiety disorders did report increases in self-rated anxiety. The repeated measures ANOVA for tense/anxious ratings showed a significant group effect (F=4.1, df=1, 30, p=0.05, Greenhouse-Geisser corrected) (Figure 1). Neither the time effect nor the interaction of group and time was significant. The Δmax for tense/anxious ratings was also significantly different between groups (F=4.8, df=1, 30, p=0.04), with the children with anxiety disorders exhibiting greater Δmax than the comparison subjects (Table 3). These yohimbine-related anxiety change ratings were not influenced by anxiety symptom severity (i.e., CGI and Hamilton anxiety scale scores). Loading for multiple anxiety disorders (N=6) did produce tense/anxious Δmax ratings twice that for children with a single anxiety disorder and tenfold higher than that of comparison subjects
Examining the largest subgroup of children with an anxiety disorder (i.e., separation anxiety disorder) by parceling out the OCD subset still demonstrated a robust group effect on tense/anxious scale scores (F=4.8, df=2, 29, p=0.02, repeated measures ANOVA, Greenhouse-Geisser corrected). Neither the time effect nor the interaction of group and time was significant. The group with separation anxiety disorder exhibited higher tense/anxious ratings than the comparison subjects (F=4.8, df=1, 20, p=0.04), but this was not significant after use of the Bonferroni correction for multiple comparisons. The tense/anxious Δmax value for children with separation anxiety disorder (mean=28.1 mm, SD=36.8) was also dramatically higher than that found in children with OCD (mean=3.6 mm, SD=4.9) (Newman-Keuls post hoc test p<0.05).
The repeated measures ANOVA of GH response to yohimbine for children with anxiety disorders versus that of comparison children revealed a significant group effect (F=4.2, df=1, 30, p=0.05, Greenhouse-Geisser corrected) (Figure 2). Neither the time effect nor the interaction of group and time was significant. The associated Δmax GH levels were also significantly different between groups (F=4.1, df=1, 30, p=0.05), with the children with anxiety disorders demonstrating blunting relative to the comparison children (Table 3). Blunting of GH was not different between subgroups with separation anxiety disorder (mean=–1.8 ng/ml, SD=6.5) and OCD (mean=–1.1 ng/ml, SD=5.5). Neither anxiety symptom severity nor loading for anxiety disorders had any effect on yohimbine-related GH response. Yohimbine-related cortisol output did not differentiate between groups. Prolactin Δmax did distinguish the children with anxiety disorders (mean=0.4 ng/ml, SD=3.5) from comparison children (mean=3.9 ng/ml, SD=5.1) (F=4.4, df=1, 30, p=0.04) (Table 3), but the repeated measures analysis did not demonstrate a significant difference between the groups.
Heart rates revealed an effect of time by repeated measures analysis, with all subjects showing an increased heart rate (F=3.6, df=2, 60, p=0.03). Neither the group effect nor the interaction of group and time was significant for heart rate. An effect of time on diastolic blood pressure was also evident, with elevations in all subjects (F=3.3, df=2, 60, p=0.03). Neither the group effect nor the interaction of group and time was significant for diastolic blood pressure. Repeated measures analysis of heart rate and blood pressure and Δmax relative to baseline did not distinguish between groups. High intersubject variability in both systolic and diastolic blood pressure change after yohimbine administration was encountered, however, in the comparison group.
Discussion
Previous findings related to a clonidine challenge in children with anxiety disorders (15) are now supplemented with data from this yohimbine challenge, which demonstrated increased sensitivity in the same patients. This increased responsivity to yohimbine is further magnified in separation anxiety disorder relative to other anxiety disorders such as OCD. Prepubertal children with separation anxiety disorder exhibited an enhanced response to a range of unconditional stimuli, including yohimbine, before the developmental period encompassing risk for spontaneous panic attacks (6, 12). The response of children with anxiety disorders to yohimbine is consistent with the overactivity of noradrenergic outflow. This overactivity has been attributed to decreased sensitivity of the a2 adrenergic autoreceptor, which may leave the system less adaptable to inhibitory feedback of norepinephrine in the synapse (22). The present finding of yohimbine-related GH blunting in children with anxiety disorders is consistent with the literature for the same challenge in adults with anxiety disorders (22, 36). The apparent absence of GH blunting on challenge with clonidine in childhood anxiety disorders, however, differs substantially from the findings in adult anxiety disorders (21, 23, 27, 37). The two challenge findings together suggest that presynaptic norepinephrine sensitivity is present in early-onset anxiety disorders, but an overactive noradrenergic system is not necessarily accompanied by a2 adrenoceptor down-regulation. Speculation regarding the developmental trajectory for a2 adrenoceptor down-regulation cannot be supported, however, by any of the child challenge studies because of their cross-sectional design.
Studies profiling a wide variety of challenges, including those using clonidine, yohimbine, caffeine, glucose, GH-releasing factor, and thyrotropin-releasing hormone, have suggested a hypothalamo-GH dysfunction in adult panic disorder (36, 38). Uhde et al. (36) have raised the possibility that panic disorder may be associated with previously unrecognized disturbances of growth in prepubertal children. The authors cite the convergence of childhood panic disorder with disturbances of stature. From another perspective, adults who had been GH deficient as children demonstrated an increased incidence of anxiety disorders such as social phobia (39). Pine et al. (40) have provided some support for this concept from a prospective study in which various childhood anxiety disorders predicted a 1–2-inch decrement in adult stature in women but not in men. The present yohimbine-related GH data in childhood anxiety disorders is also consistent with hypothalamo-GH dysfunction, but clonidine challenge data (15) do not support this hypothesis.
From a developmental perspective, characteristic behavioral and neuroendocrine responses to a yohimbine challenge appear to be present early in the onset of anxiety disorders. These responses do not appear to be state related because neither is correlated with symptom severity or Hamilton anxiety scale scores. The behavioral response to yohimbine administration does demonstrate diagnostic specificity because the response in children with separation anxiety disorder is far more robust than in children with OCD. Furthermore, yohimbine sensitivity reflected by self-reported anxiety is heightened in the presence of multiple anxiety disorder diagnoses. Loading for anxiety disorder (i.e., multiple anxiety diagnoses) tends to track with genetic loading and family history of anxiety disorders. Fully 33% of the group with anxiety disorders had a first-degree relative (i.e., mother, father, or sibling) with an anxiety disorder diagnosis. Just as CO2 sensitivity varies by family loading, demonstrates diagnostic specificity for panic disorder, and may confer risk for the development of panic disorder in children with separation anxiety disorder (15), yohimbine appears to define another physiologic continuum by which this phenotype can be characterized. Because the most frequent diagnosis in the anxiety group was separation anxiety disorder, characteristic enhanced sensitivity to yohimbine and other unconditioned stimuli in separation anxiety disorder may antedate the onset of panic disorder and potentially constitute a necessary but not sufficient precursor for the development of panic disorder. It should be reiterated that no child in this study, including the lone subject with panic disorder, experienced a panic attack. Yohimbine sensitivity, however, was indicated by the perception in even prepubertal children of increased tension/anxiety.
Several factors may account for protection from panic attacks in our study group, the most obvious of which is prepubertal status (1). Another protective factor is attendance of an attachment figure during the challenge (i.e., proximity to a “safe person” [41]). Finally, the stage of cognitive development appears to be critical (1, 42). The construct of “anxiety sensitivity” (fear of bodily sensations perceived as heralding a catastrophic event), a risk factor for adult anxiety disorders, is only evident in children 12 years and older in concert with cognitive developmental predictions (31). Although norepinephrine sensitivity in children with anxiety disorders, demonstrated by a yohimbine challenge, is a biological vulnerability, interaction with environmental and constitutional factors such as attachment milieu (43) and temperament (1) must be present for the development of panic disorder or anxiety disorders.
Although challenge strategies are controversial, the performance of the yohimbine challenge was well tolerated in both the children with anxiety disorders and the comparison children. Subjects and their guardians who elected to participate in the yohimbine challenge had the advantage of extensive informational meetings, prior positive experience by the child in a clonidine infusion paradigm, and close monitoring as well as supervision during and after the challenge. Challenge studies remain vitally important in defining a phenotype, in elucidating biological diatheses amenable to therapeutic manipulation, and for identifying risk factors potentially influencing developmental progression of the disease from separation anxiety disorder to panic disorder. From this perspective, intervention and prevention strategies may be directed at those factors necessary and specific to this progression. If we speculate with the present yohimbine findings along with clonidine challenge data in children with anxiety disorders, it would be those factors crucial to the development of a2 adrenoceptor down-regulation that should be the focus of future studies.
 |
 |
 |
Received Jan. 6, 1998; revisions received Oct. 13, 1999, and Jan. 21, 2000; accepted March 9, 2000. From the Department of Psychiatry, Medical University of South Carolina. Address reprint requests to Dr. Sallee, Department of Psychiatry, University of Cincinnati, 231 Bethesda Ave., P.O. Box 670559, Cincinnati, OH 45267-0559; [email protected] (e-mail).Supported in part by NIMH grant MH-46673 to Dr. Sallee, the L. Vernon Maddox Investigator Award from the National Alliance for Research on Schizophrenia and Depression, and grant RR-01070 from the Medical University of South Carolina.
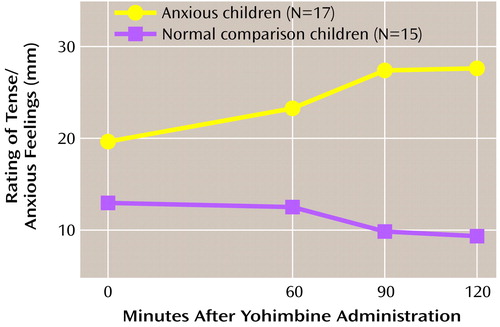
Figure 1. Visual Analog Ratings of Tense/Anxious Feelings for Children With Anxiety Disorders and Normal Comparison Children Given Yohimbinea
a0 mm=not at all anxious; 100 mm=extremely anxious. Repeated measures analysis of variance demonstrated that tense/anxious ratings were significantly higher in children with anxiety disorders than in normal comparison children.
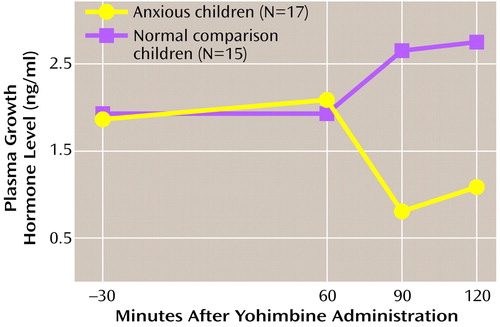
Figure 2. Plasma Concentration of Growth Hormone Level Before and After Administration of Yohimbine in Children With Anxiety Disorders and Normal Comparison Childrena
aA dose of yohimbine, 0.1 mg/kg, was administered orally at 0 minutes. Repeated measures analysis of variance demonstrated that growth hormone output was significantly lower for children with anxiety disorders than for normal comparison children.
1. Ollendick TH: Panic disorder in children and adolescents: new developments, new directions. J Clin Child Psychol 1998; 27:234–245Crossref, Medline, Google Scholar
2. Beidel DC, Turner SM, Morris TM: Psychopathology of childhood social phobia. J Am Acad Child Adolesc Psychiatry 1999; 38:643–650Crossref, Medline, Google Scholar
3. Beidel DC, Fink CM, Turner SM: Stability of anxious symptomatology in young children. J Abnorm Child Psychol 1996; 24:257–269Crossref, Medline, Google Scholar
4. Keller MB, Lavori PW, Wunder J, Beardslee WR, Schwartz CE, Roth J: Chronic course of anxiety disorders in children and adolescents. J Am Acad Child Adolesc Psychiatry 1992; 31:595–599Crossref, Medline, Google Scholar
5. Strauss CC, Lease CA, Last CG, Francis G: Overanxious disorder: an examination of developmental differences. J Abnorm Child Psychol 1988; 16:433–443Crossref, Medline, Google Scholar
6. Pine DS, Grun J: Childhood anxiety: integrating developmental psychopathology and affective neuroscience. J Child Adolesc Psychopharmacol 1999; 9:1–12Crossref, Medline, Google Scholar
7. Bernstein GA, Borchardt CM, Perwien AR: Anxiety disorders in children and adolescents: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry 1996; 35:1110–1119Google Scholar
8. Klein RG: Is panic disorder associated with childhood separation anxiety disorder? Clin Neuropharmacol 1995; 18:S7–S14Google Scholar
9. Warner V, Mufson L, Weissman MM: Offspring at low and high risk for depression and anxiety: mechanisms of psychiatric disorder. J Am Acad Child Adolesc Psychiatry 1995; 34:786–797Crossref, Medline, Google Scholar
10. Battaglia M, Bertella S, Politi E, Bernardeschi L, Perna G, Gabriele A, Bellodi L: Age at onset of panic disorder: influence of familial liability to the disease and of childhood separation anxiety disorder. Am J Psychiatry 1995; 152:1362–1364Google Scholar
11. Pollack MH, Otto MW, Sabatino S, Majcher D, Worthington JJ, McArdle ET, Rosenbaum JF: Relationship of childhood anxiety to adult panic disorder: correlates and influence on course. Am J Psychiatry 1996; 153:376–381Link, Google Scholar
12. Pine DS, Coplan JD, Papp LA, Klein RG, Martinez JM, Kovalenko P, Tancer N, Moreau D, Dummit ES, Shaffer D, Klein DF, Gorman JM: Ventilatory physiology of children and adolescents with anxiety disorders. Arch Gen Psychiatry 1998; 55:123–129Crossref, Medline, Google Scholar
13. Angold A, Erkanli A, Costello EJ, Rutter M: Precision, reliability, and accuracy in the dating of symptom onsets in child and adolescent psychopathology. J Child Psychol Psychiatry 1996; 37:657–666Crossref, Medline, Google Scholar
14. Moreau D, Follett C: Panic disorder in children and adolescents. Child Adolesc Psychiatr Clin North Am 1993; 2:581–602Crossref, Google Scholar
15. Sallee FR, Richman H, Sethuraman G, Dougherty D, Sine L, Altman-Hamamdzic S: Clonidine challenge in childhood anxiety disorders. J Am Acad Child Adolesc Psychiatry 1998; 37:655–662Crossref, Medline, Google Scholar
16. Klein DF: False suffocation alarms, spontaneous panics, and related conditions: an integrative hypothesis. Arch Gen Psychiatry 1993; 50:306–317Crossref, Medline, Google Scholar
17. Goddard AW, Charney DS: Toward an integrated neurobiology of panic disorder. J Clin Psychiatry 1997; 58:4–11Medline, Google Scholar
18. Pine DS, Coplan JD, Papp LA, Moreau D, Tancer M, Dummit ES, Shaffer D, Gorman JM, Klein DF: Noradrenergic function in youth and adults with anxiety disorders, in Scientific Proceedings of the 42nd Annual Meeting of the American Academy of Child and Adolescent Psychiatry. Washington, DC, AACAP, 1995Google Scholar
19. Bremner JD, Krystal JH, Southwick SM, Charney DS: Noradrenergic mechanisms in stress and anxiety, I: preclinical studies. Synapse 1996; 23:28–38Crossref, Medline, Google Scholar
20. Charney DS, Heninger GB: Abnormal regulation of noradrenergic function in panic disorders. Arch Gen Psychiatry 1986; 43:1042–1054Google Scholar
21. Uhde TW, Vittone BJ, Siever LJ, Kaye WH, Post RM: Blunted growth hormone response to clonidine in panic disorder patients. Biol Psychiatry 1986; 21:1081–1085Google Scholar
22. Bremner JD, Krystal JH, Southwick SM, Charney DS: Noradrenergic mechanisms in stress and anxiety, II: clinical studies. Synapse 1996; 23:39–51Crossref, Medline, Google Scholar
23. Abelson JL, Glitz D, Cameron OG, Lee MA, Bronzo M, Curtis GC: Blunted growth hormone response to clonidine in patients with generalized anxiety disorder. Arch Gen Psychiatry 1991; 48:157–162Crossref, Medline, Google Scholar
24. Brambilla F, Perna G, Bellodi L, Arancio C, Betani A, Perini G, Carraro C, Gava F: Noradrenergic receptor sensitivity in obsessive-compulsive disorders, I: growth hormone response to clonidine stimulation. Psychiatry Res 1997; 69:155–162Crossref, Medline, Google Scholar
25. Gold MS, Denabedian RK, Redmond DE Jr: Further evidence for alpha2-adrenergic receptor mediated inhibition of prolactin secretion: the effect of yohimbine. Psychoneuroendocrinology 1979; 3:253–260Crossref, Google Scholar
26. Meltzer HY, Miljana S, Gudelsky GA: Effect of yohimbine on rat prolactin secretion. J Pharmacol Exp Ther 1983; 224:21–27Medline, Google Scholar
27. Charney DS, Woods SW, Krystal JH, Nagy LM, Heninger GR: Noradrenergic neuronal dysregulation in panic disorder: the effects of intravenous yohimbine and clonidine in panic disorder patients. Acta Psychiatr Scand 1992; 86:273–282Crossref, Medline, Google Scholar
28. Last CG: Schedule for Affective Disorders and Schizophrenia for School-Age Children (Present Episode Revised). Pittsburgh, University of Pittsburgh, 1986Google Scholar
29. Hamilton M: Diagnosis and rating of anxiety. Br J Psychiatry 1969; 3:76–79Google Scholar
30. Reynolds CR, Richmond BO: What I Think and Feel: a revised measure of children’s manifest anxiety. J Abnorm Child Psychol 1978, 6:271–280Google Scholar
31. Silverman WK, Fleisig W, Rabian B, Peterson RA: Childhood Anxiety Sensitivity Index. J Clin Child Psychol 1991; 20:162–168Crossref, Google Scholar
32. Ollendick TH: Reliability and validity of the revised Fear Survey Schedule for Children (FSSC-R). Behav Res Ther 1983; 21:395–399Crossref, Google Scholar
33. Greenhouse SW, Geisser S: On methods in the analysis of profile data. Psychometrika 1959; 24:95–112Crossref, Google Scholar
34. Miller RG: Simultaneous Statistical Inference. New York, Springer-Verlag, 1981, pp 67–70Google Scholar
35. Keuls M: The use of the studentized range in connection with an analysis of variance. Euphytica 1952; 1:112–122Crossref, Google Scholar
36. Uhde TW, Tancer ME, Rubinow DR, Roscow DB, Boulenger JP, Vittone B, Gurguis G, Geraci M, Back B, Post RM: Evidence for hypothalamo-growth hormone dysfunction in panic disorder: profile of growth hormone (GH) responses to clonidine, yohimbine, caffeine, glucose, GRF and TRH in panic disorder patients versus healthy volunteers. Neuropsychopharmacology 1992; 6:101–118Medline, Google Scholar
37. Nutt DJ: Altered central alpha2-adrenoceptor sensitivity in panic disorder. Arch Gen Psychiatry 1989; 46:165–169Crossref, Medline, Google Scholar
38. Tancer ME, Stein MB, Black B, Uhde TW: Blunted growth hormone responses to growth hormone-releasing factor and to clonidine in panic disorder. Am J Psychiatry 1993; 150:336–337Link, Google Scholar
39. Stabler B, Tancer JR, Underwood LE: Evidence for social phobia and other psychiatric disorders in adults who were growth hormone deficient during childhood. Anxiety 1996; 2:86–89Crossref, Medline, Google Scholar
40. Pine DS, Cohen P, Brook J: Emotional problems during youth as predictors of stature during early adulthood: results from a prospective epidemiologic study. Pediatrics 1996; 97:856–863Medline, Google Scholar
41. Carter MM, Hollan SD, Carson R, Shelton RC: Effects of a safe person on induced distress following a biological challenge in panic disorder with agoraphobia. J Abnorm Psychol 1995; 104:156–161Crossref, Medline, Google Scholar
42. Nelles WB, Barlow DH: Do children panic? Clin Psychol Rev 1988; 8:359–372Google Scholar
43. Shear MK: Factors in the etiology and pathogenesis of panic disorder: revisiting the attachment-separation paradigm. Am J Psychiatry 1996; 153:125–136Medline, Google Scholar



